Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis latifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
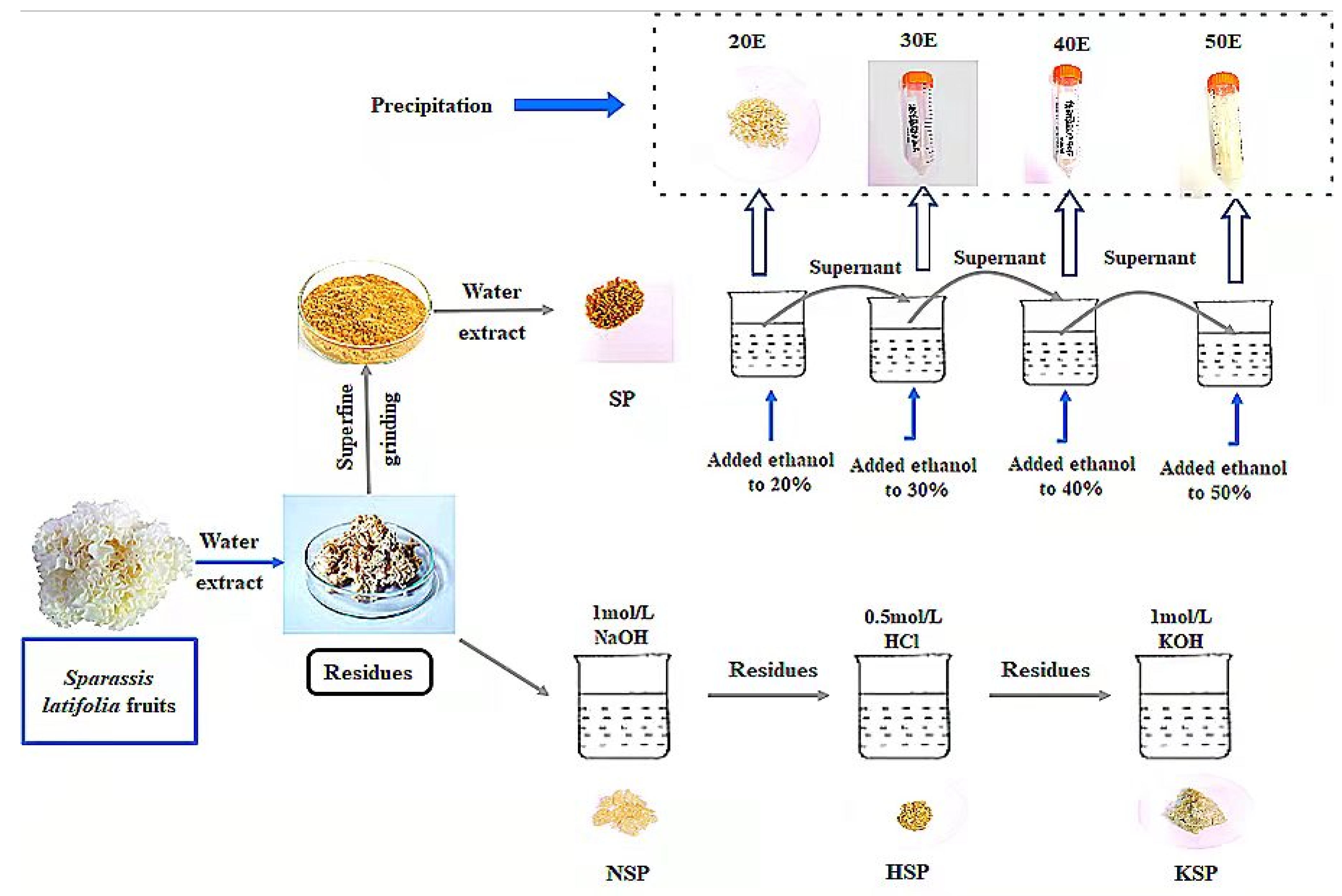
2.2. Extraction and Isolation of Cell Wall Polysaccharides
2.2.1. Alkali-Acid-Alkali Sequential Extraction
2.2.2. Superfine Grinding Assisted Extraction
2.2.3. Isolation of Cell Wall Polysaccharide
2.3. The Total Sugar and β-Glucan Content Determination
2.4. Molecular Weight Distribution Analysis
2.5. Monosaccharide Composition Analysis
2.6. Immunostimulatory Activity of Purified Fractions on Inducing NF-κB Activation via Dectin-1 Receptor
2.7. FT-IR Analysis
2.8. Methylation Analysis
2.9. NMR Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization Analysis of Cell Wall Polysaccharides Extracted with Different Methods
3.1.1. Comparison of the Yields, Sugar and β-Glucan Content
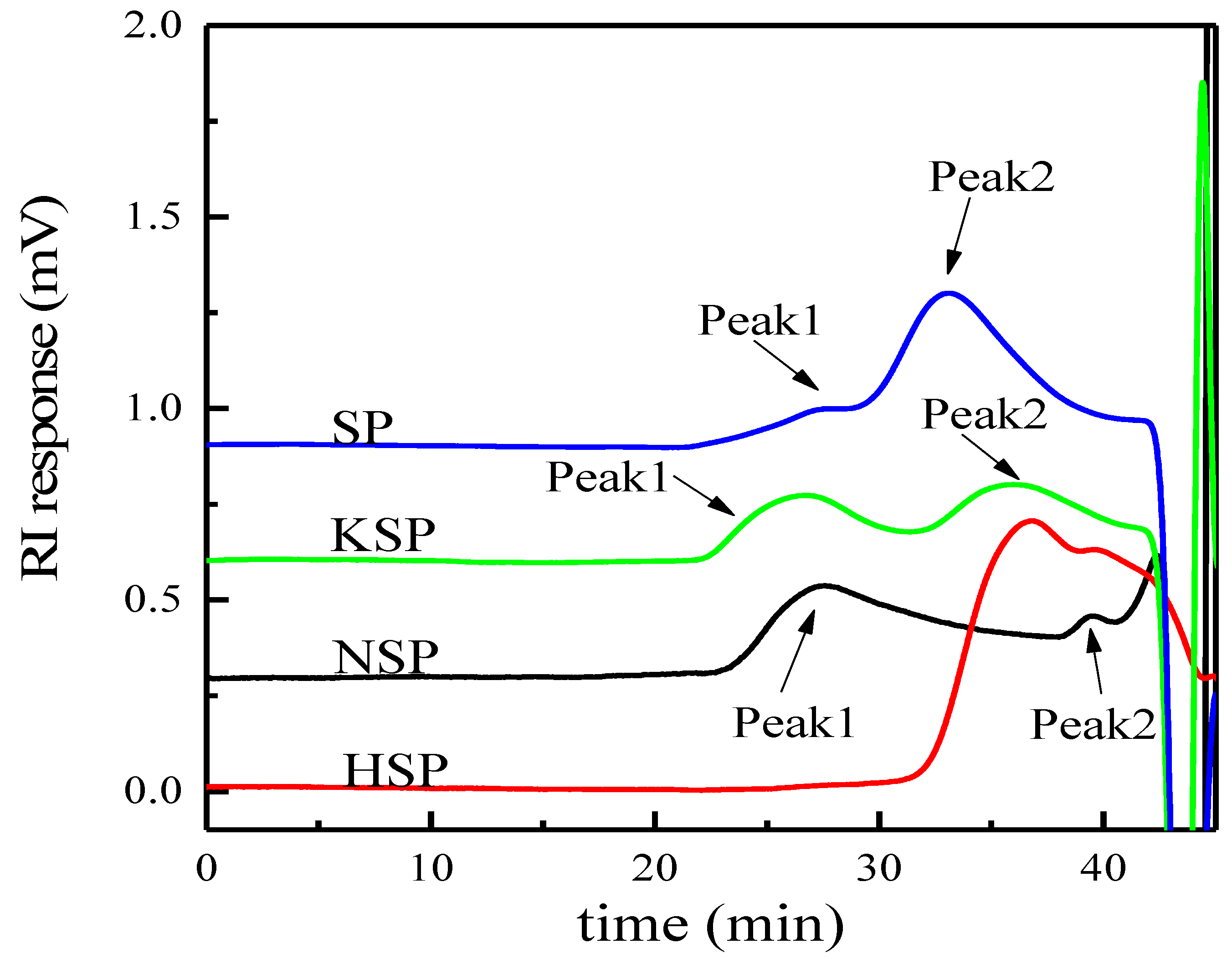
3.1.2. Molecular Weight Distribution and Monosaccharide Composition Analysis
3.2. Structure and Activity Analysis of Ethanol Precipitated Fraction
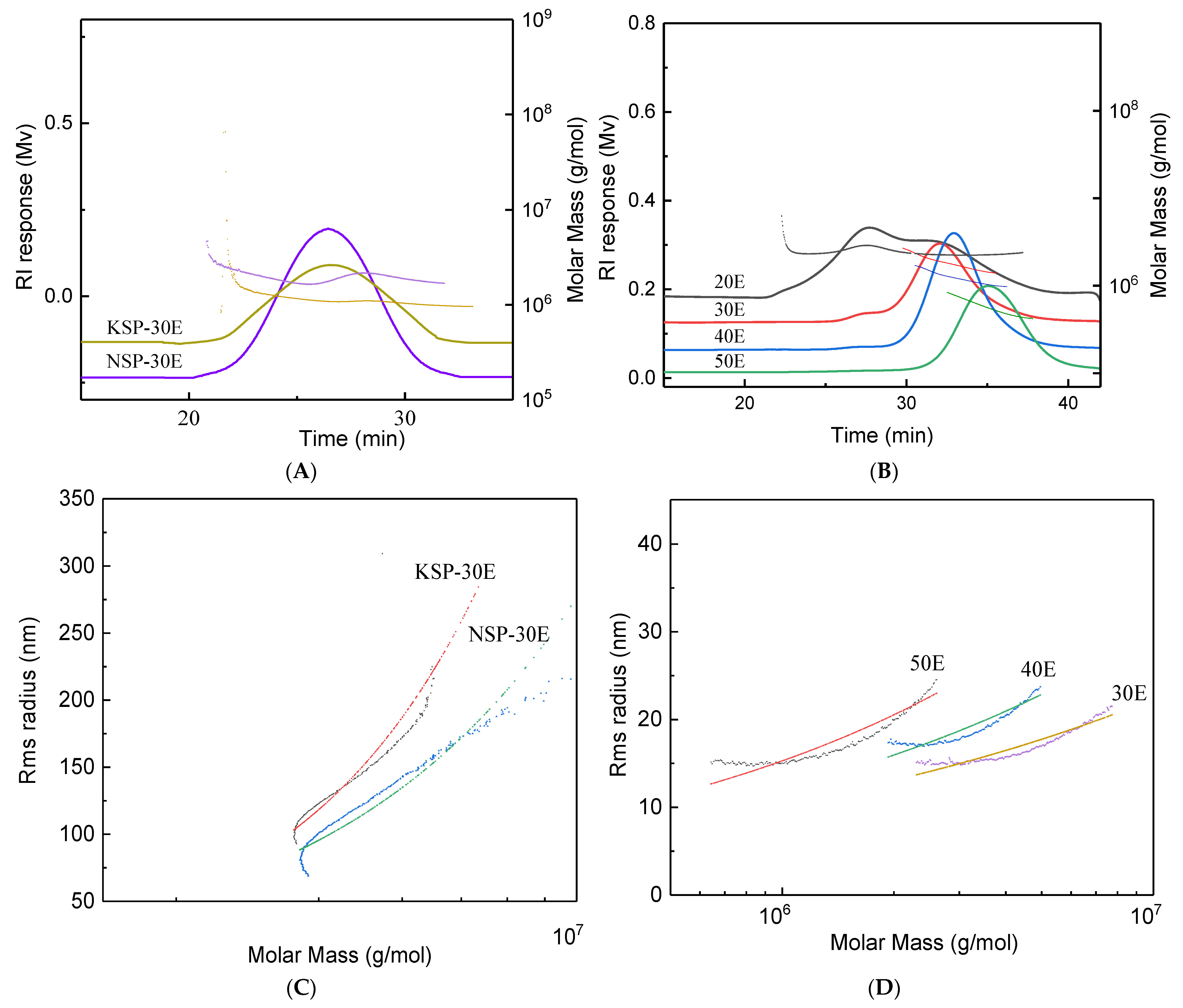
3.2.1. Comparison of Purified Polysaccharide Fractions from Different Extracts
3.2.2. Activity Determination on Activating Dectin-1 Receptor
3.2.3. FT-IR Analysis
3.2.4. Methylation Analysis
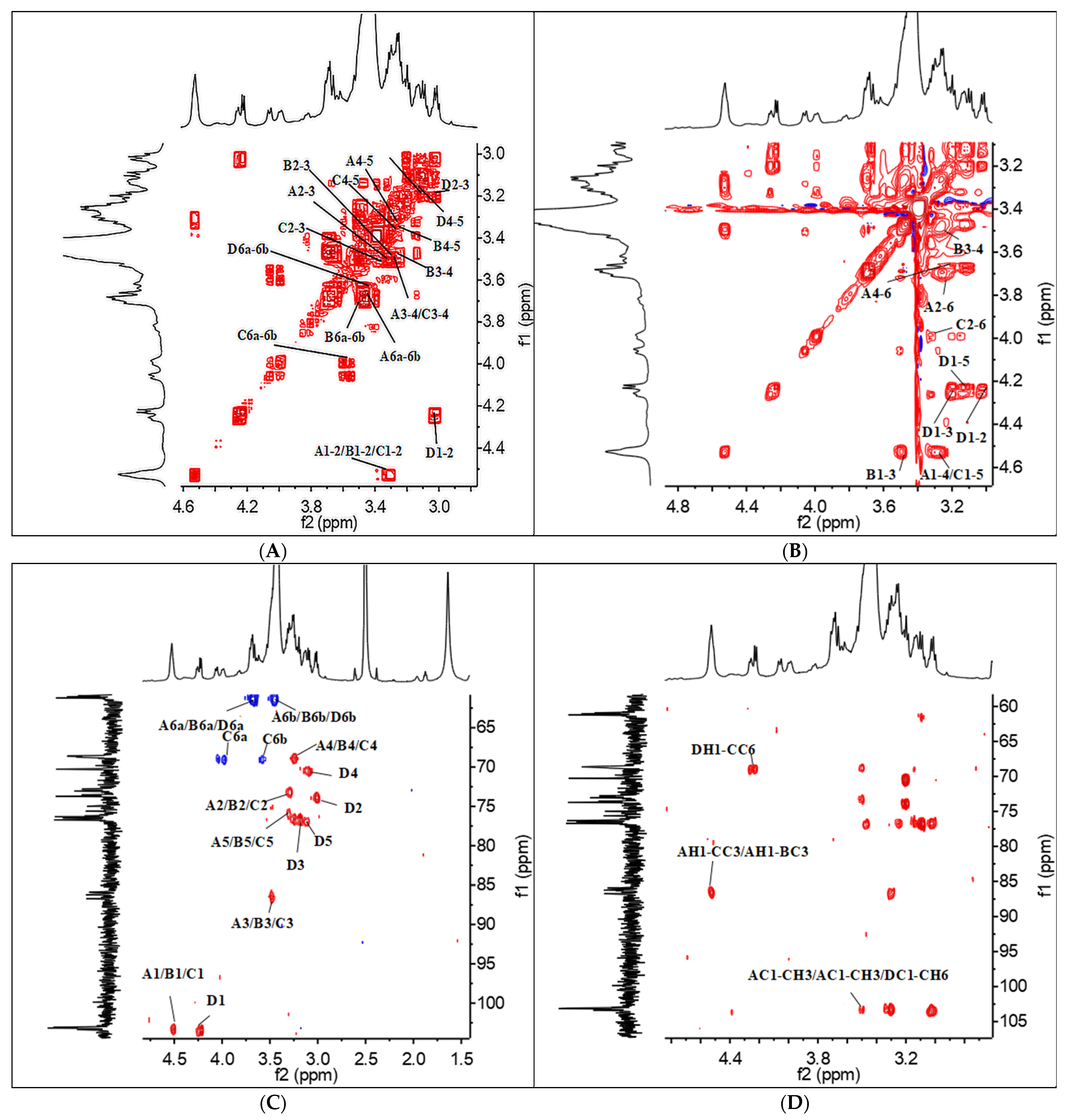
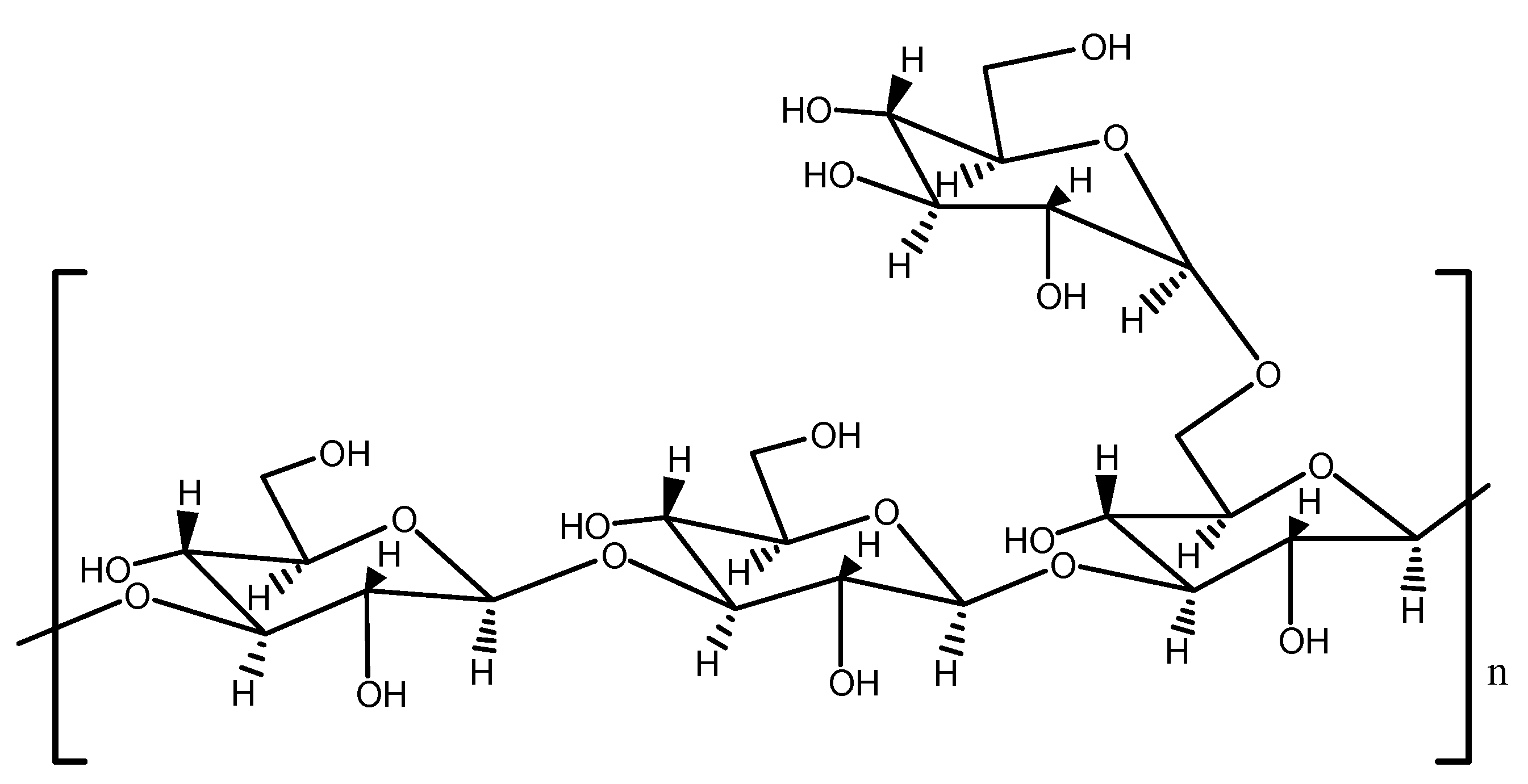
3.2.5. NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Feng, B.; Yang, Z.L.; Dai, Y.-C.; Wang, Z.; Tolgor, B. New species and distinctive geographical divergences of the genus Sparassis (basidiomycota): Evidence from morphological and molecular data. Mycol. Prog. 2013, 12, 445–454. [Google Scholar] [CrossRef]
- Shin, J.; Oh, D.-S.; Lee, H.-D.; Kang, H.-B.; Lee, C.-W.; Cha, W.-S. Analysis of mineral, amino acid and vitamin contents of fruiting body of Sparassis crispa. J. Life Sci. 2007, 17, 1290–1293. [Google Scholar] [CrossRef]
- Huang, J.-C.; Li, K.-B.; Lin, Y.-C.; Ying, Z.-H.; Yu, Y.-R. Analysis of main nutritional components of Sparassis crispa fr. fruitbody. Acta Nutr. Sin. 2007, 29, 514–515. [Google Scholar]
- Naohito, O.; Miura, N.N.; Mitsuhiro, N.; Toshiro, Y. Antitumor 1,3-β-glucan from cultured fruit body of Sparassis crispa. Biol. Pharm. Bull. 2000, 23, 866–872. [Google Scholar]
- Wei, W.; Fang, L.; Bao, W.-R.; Ma, D.-L.; Leung, C.-H.; Nie, S.-P.; Han, Q.-B. Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 2016, 64, 881–889. [Google Scholar] [CrossRef]
- Natalia, N.; Renata, N.; Kinga, L.M.; Wojciech, R.; Urszula, G.; Nikola, S.; Zbigniew, K. Promising potential of crude polysaccharides from Sparassis crispa against colon cancer: An in vitro study. Nutrients 2021, 13, 161. [Google Scholar]
- Zhang, W.; Guo, Y.; Cheng, Y.; Zhao, W.; Zheng, Y.; Qian, H. Ultrasonic-assisted enzymatic extraction of Sparassis crispa polysaccharides possessing protective ability against H2O2-induced oxidative damage in mouse hippocampal HT22 cells. RSC Adv. 2020, 10, 22164–22175. [Google Scholar] [CrossRef]
- Li, Z.; Nie, K.; Wang, Z.; Luo, D. Quantitative structure activity relationship models for the antioxidant activity of polysaccharides. PLoS ONE 2016, 11, e0163536. [Google Scholar] [CrossRef]
- Feng, S.; Luan, D.; Ning, K.; Shao, P.; Sun, P. Ultrafiltration isolation, hypoglycemic activity analysis and structural characterization of polysaccharides from Brasenia schreberi. Int. J. Biol. Macromol. 2019, 135, 141–151. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Kimura, T.; Perazzo, F.F. Natural products and biological activity of the pharmacologically active cauliflower mushroom Sparassis crispa. Biomed. Res. Int. 2013, 2013, 982317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, T.; Toshie, H.; Noriko, N.-M.; Yoshiyuki, A.; Mitsuhiro, N.; Toshiro, Y.; Naohito, O. NMR characterization of the structure of a beta-(1→3)-D-glucan isolate from cultured fruit bodies of Sparassis crispa. Carbohydr. Res. 2007, 342, 2611–2618. [Google Scholar]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Park, H.-G.; Yong, O.K.; Kang, J.S.; Kim, H.M.; Jin, T.H.; Kim, Y.; Han, S.-B. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int. Immunopharmacol. 2010, 10, 1284–1294. [Google Scholar] [CrossRef]
- Wang, M.; Hao, Z.; Chang, M.; Meng, J.; Liu, J.; Feng, C. Characterization, antioxidant and immunity activities of Sparassis latifolia polysaccharides. Mycosystema 2019, 38, 707–716. [Google Scholar]
- Hao, Z.; Wang, R.; Feng, C.; Chang, M.; Meng, J.; Liu, J.; Cheng, H. Research of Sparassis crispa polysaccharide and its function. Edible Fungi China 2017, 36, 48–51. [Google Scholar]
- Duan, G.-L.; Yu, X.-B. Isolation, purification, characterization, and antioxidant activity of low-molecular-weight polysaccharides from Sparassis latifolia. Int. J. Biol. Macromol. 2019, 137, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, H.Y.; Xiao, D.L.; Lin, Y.Q. Chemistry and in vitro immunological activity of polysaccharides from Sparassis latifolia. Fujian J. Agric. Sci. 2019, 34, 1093–1099. [Google Scholar]
- Huang, X.; Zeng, Y.; Wang, J.; Yang, F.; Hu, J. Structure and function of yeast cell wall polysaccharide. China Feed. 2014, 42–43. [Google Scholar]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zhu, C.-X.; Dai, A.-R.; Chen, L.; You, C.-P.; Zhang, B.-B. Chemical characterization and antioxidant properties of cell wall polysaccharides from Antrodia cinnamomea mycelia. Food Biosci. 2021, 41, 100932. [Google Scholar] [CrossRef]
- Li, M.; Li, T.; Yan, D.; Hu, X.; Ren, G.; Zhang, H.; Wang, Z.; Teng, Z.; Wu, R.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Extraction of Angelica sinensis polysaccharides using ultrasound-assisted way and its bioactivity. Int. J. Biol. Macromol. 2016, 88, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-R.; Sung, S.-K.; Jang, M.; Lim, T.-G.; Cho, C.-W.; Han, C.-J.; Hong, H.-D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng meyer). Int. J. Biol. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wu, D.; Yang, Y.; Li, Q.; Liu, Y.; Zhou, S.; Yan, M. Extraction and structural characteristics of cell wall polysaccharides from Hericium erinaceus. J. Food Sci. Biotechnol. 2016, 35, 871–877. [Google Scholar]
- Bao, N.; Luo, H.; Lv, T.; Zhang, X.; Zhang, D.; Duan, T.; Wang, Q.; Xia, Q. Influence of different particle sizes on exraction and antioxidant activity effect of polysaccharide from Auricularia auricula. Genom. Appl. Biol. 2020, 39, 2183–2190. [Google Scholar]
- Nitschke, J.; Modick, H.; Busch, E.; Rekowski, W.R.; Altenbach, H.-J.; Mölleken, H. A new colorimetric method to quantify β-1,3-1,6-glucans in comparison with total β-1,3-glucans in edible mushrooms. Food Chem. 2011, 127, 791–796. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ko, Y.-T.; Lin, Y.-L. 1,3-β-Glucan quantification by a fluorescence microassay and analysis of its distribution in foods. J. Agric. Food Chem. 2004, 52, 3313–3318. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Yang, Y.; Tang, Q.; Zhou, S.; Wu, D.; Zhang, J. Structural characteristics and hypoglycemic activity of polysaccharides from Coprinus comatus. Bioact. Carbohydr. Diet. Fibre 2013, 2, 164–169. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Tian, L.; Berg, M.v.d.; Bruggeman, G.; Bruininx, E.; Schols, H.A.; Faas, M.M.; Vos, P.d. Endo-glucanase digestion of oat β-glucan enhances Dectin-1 activation in human dendritic cells. J. Funct. Foods 2016, 21, 104–112. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Yang, Y.; Jia, W.; Tang, Q.; Tang, C.; Feng, N.; Zhang, J.S. Separation and structural elucidation of a polysaccharide CC30w-1 from the fruiting body of Coprinus comatus. Bioact. Carbohydr. Diet. Fibre. 2013, 1, 99–104. [Google Scholar] [CrossRef]
- Tada, R.; Adachi, Y.; Ishibashi, K.; Ohno, N. An unambiguous structural elucidation of a 1,3-beta-D-glucan obtained from liquid-cultured Grifola frondosa by solution NMR experiments. Carbohydr. Res. 2009, 344, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Hua, X.; Liu, J.; Wang, M.; Liu, Y.; Yang, R.; Cao, Y. Extraction of sunflower head pectin with superfine grinding pretreatment. Food Chem. 2020, 320, 126631. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Q.; Zhang, J.; Wang, C. Effect of particle size on the extraction, molecular character and in vitro immunological activity of polysaccharides from Ganoderma lucidum fruit bodies. Acta Edulis Fungi 2018, 25, 56–62. [Google Scholar]
- Eugenio, S.A.; Salwa, K.; Lan, L. Extraction and characterization of cell wall polysaccharides from cranberry (Vaccinium macrocarpon var. Stevens) pomace. Carbohydr. Polym. 2021, 267, 118212. [Google Scholar]
- Harding, S.E.; Abdelhameed, A.S.; Morris, G.A. On the hydrodynamic analysis of conformation in mixed biopolymer systems. Polym. Int. 2011, 60, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Hua, F.; Cui, S.W. Triple helix conformation of β-D-glucan from Ganoderma lucidum and effect of molecular weight on its immunological activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Q.; Cui, S.W.; Kang, J.; Hu, X.; Xing, X.; Yada, R.Y. Conformational properties of high molecular weight heteropolysaccharide isolated from seeds of Artemisia sphaerocephala krasch. Food Hydrocoll. 2013, 32, 155–161. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Lin, S.; Cheung, P.C.K. Cell wall structure of mushroom sclerotium (Pleurotus tuber regium): Part 1. Fractionation and characterization of soluble cell wall polysaccharides. Food Hydrocoll. 2014, 36, 189–195. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tang, Q.; Yang, Y.; Guo, Q.; Wang, Q.; Wu, D.; Cui, S.W. Physicochemical characterization of a high molecular weight bioactive β-D-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr. Polym. 2014, 101, 968–974. [Google Scholar] [CrossRef]
- Hall, L.D.; Johnson, L.F. Chemical studies by 13C nuclear magnetic resonance spectroscopy: Some chemical shift dependencies of oxygenated derivatives. J. Chem. Soc. D 1969, 10, 509–510. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef] [PubMed]




| Extraction Methods | Samples | Yields (%) | Sugar Content (%) | β-Glucan Content (%) |
|---|---|---|---|---|
| Alkali-acid-alkali | NSP | 8.90 | 88.17 ± 2.09 | 50.10 ± 0.43 |
| HSP | 2.74 | 81.37 ± 0.28 | 16.66 ± 0.29 | |
| KSP | 8.83 | 93.36 ± 1.13 | 87.96 ± 1.03 | |
| Superfine grinding | SP | 20.80 | 78.16 ± 2.15 | 19.35 ± 1.20 |
| Extraction Methods | Samples | Peak | Mw (g/mol) | Mn (g/mol) | Polydispersity (Mw/Mn) |
|---|---|---|---|---|---|
| Alkali-acid-alkali | NSP | Peak1 | 3.32 × 106 | 2.44 × 106 | 1.36 |
| Peak2 | 6.36 × 105 | 6.15 × 105 | 1.03 | ||
| HSP | Peak | 2.59 × 105 | 4.65 × 104 | 5.57 | |
| KSP | Peak1 | 3.30 × 106 | 2.93 × 106 | 1.13 | |
| Peak2 | 5.81 × 105 | 3.73 × 105 | 1.56 | ||
| Superfine grinding | SP | Peak1 | 1.44 × 107 | 1.20 × 107 | 1.20 |
| Peak2 | 2.50 × 106 | 1.54 × 106 | 1.62 |
| Extraction Methods | Samples | Monosaccharide (mol%) | |||||
|---|---|---|---|---|---|---|---|
| Fucose | Glucosamine | Galactose | Glucose | Xylose | Mannose | ||
| Alkali-acid -alkali | NSP | 2.56 | 1.47 | 15.56 | 76.35 | 3.3 | - |
| HSP | 1.01 | - | 4.38 | 93.53 | 0.97 | - | |
| KSP | 1.18 | - | 4.20 | 91.66 | 2.95 | - | |
| Superfine grinding | SP | 0.62 | - | 4.54 | 92.58 | - | 2.25 |
| Linkage Types (Mol %) | Samples | |||||
|---|---|---|---|---|---|---|
| NSP-30E | KSP-30E | 20E | 30E | 40E | 50E | |
| Terminal-linked-Glcp | 27.86 | 20.70 | 19.91 | 20.86 | 16.12 | 13.86 |
| 1,3-linked Glcp | 43.09 | 40.17 | 17.63 | - | - | - |
| 1,3-linked Hexp | 3.12 | - | - | - | - | - |
| 1,4-linked Glcp | 3.95 | - | 40.15 | 69.04 | 71.15 | 62.68 |
| 1,6-linked Glcp | 6.19 | 5.38 | 8.56 | - | - | 1.96 |
| 1,6-linked Galp | 2.24 | - | 3.67 | - | - | - |
| 1,3,4-linked Glcp | - | - | 2.59 | - | 3.30 | 11.69 |
| 1,2,3-linked Glcp | 1.88 | 6.50 | - | - | - | - |
| 1,4,6-linked Glcp | - | - | 3.79 | 10.10 | 9.43 | 9.81 |
| 1,3,6-linked Glcp | 20.98 | 25.45 | 7.38 | - | - | - |
| Residues | Sugar Linkage | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a-H6b/C6 |
|---|---|---|---|---|---|---|---|
| A | (1→3)-Linked | 4.53 | 3.30 | 3.50 | 3.26 | 3.32 | 3.68, 3.45 |
| -β-Glcp | 103.08 | 73.47 | 86.18 | 68.76 | 76.24 | 61.30 | |
| B | (1→3)-Linked | 4.52 | 3.30 | 3.47 | 3.24 | 3.33 | 3.7, 3.51 |
| -β-Glcp | 103.19 | 73.21 | 86.75 | 68.52 | 76.56 | 61.20 | |
| C | (1→3,1→6)-Linked | 4.51 | 3.34 | 3.50 | 3.27 | 3.33 | 3.97, 3.57 |
| -β-Glcp | 102.93 | 73.30 | 86.38 | 69.50 | 76.13 | 69.04 | |
| D | Terminal Linked | 4.24 | 3.01 | 3.19 | 3.10 | 3.14 | 3.64, 3.42 |
| β-Glcp | 103.14 | 74.15 | 76.29 | 70.86 | 76.87 | 61.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, X.; Zhang, J.; Yan, M.; Li, D.; Zhou, S.; Feng, J.; Liu, Y. Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis latifolia. Polymers 2022, 14, 549. https://doi.org/10.3390/polym14030549
Liu J, Zhang X, Zhang J, Yan M, Li D, Zhou S, Feng J, Liu Y. Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis latifolia. Polymers. 2022; 14(3):549. https://doi.org/10.3390/polym14030549
Chicago/Turabian StyleLiu, Jing, Xuemeng Zhang, Jingsong Zhang, Mengqiu Yan, Deshun Li, Shuai Zhou, Jie Feng, and Yanfang Liu. 2022. "Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis latifolia" Polymers 14, no. 3: 549. https://doi.org/10.3390/polym14030549
APA StyleLiu, J., Zhang, X., Zhang, J., Yan, M., Li, D., Zhou, S., Feng, J., & Liu, Y. (2022). Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis latifolia. Polymers, 14(3), 549. https://doi.org/10.3390/polym14030549





