Surface Functionalization of Polyester Textiles for Antibacterial and Antioxidant Properties
Abstract
1. Introduction
2. Methods and Materials
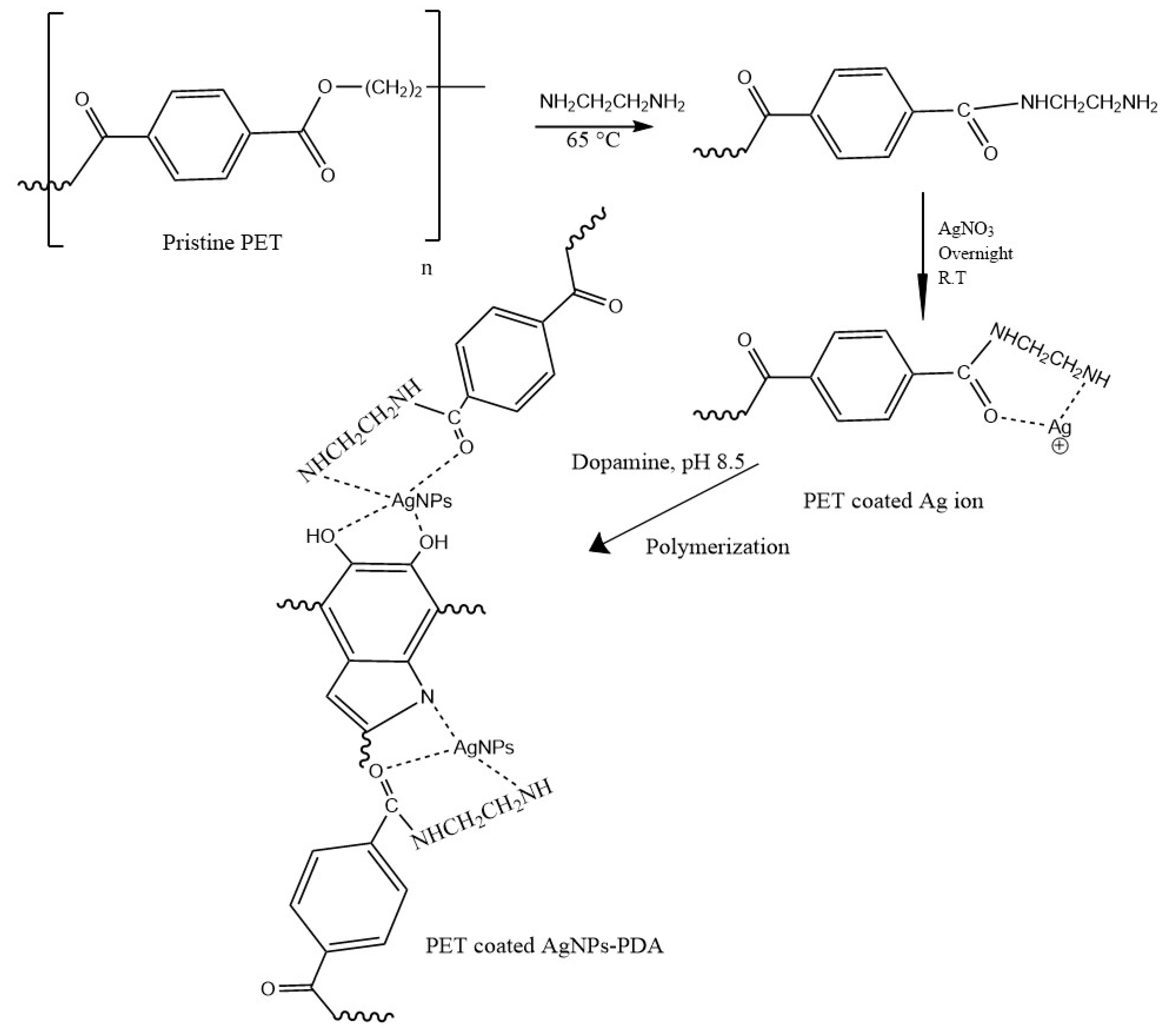
2.1. Polyester Treatment
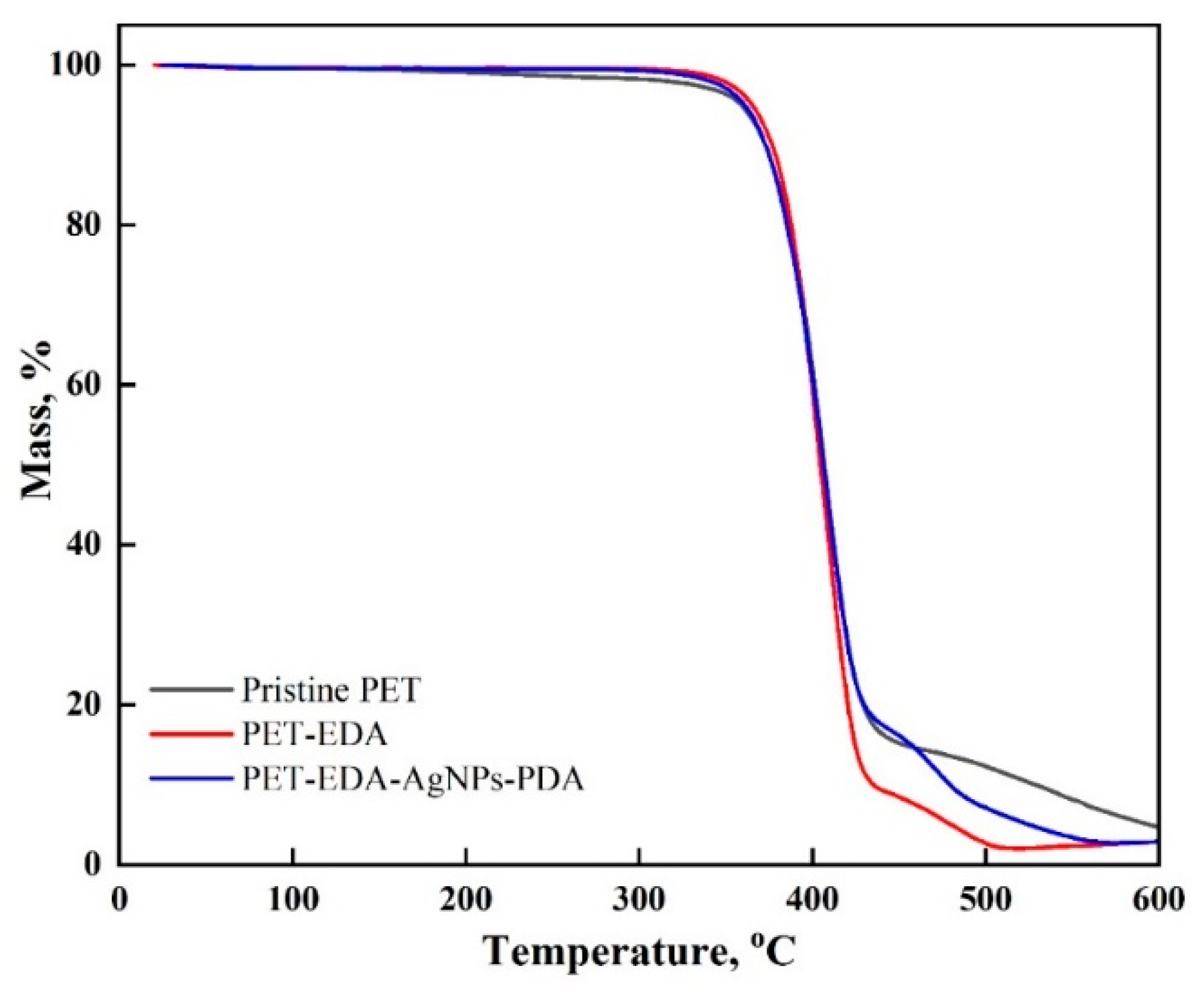
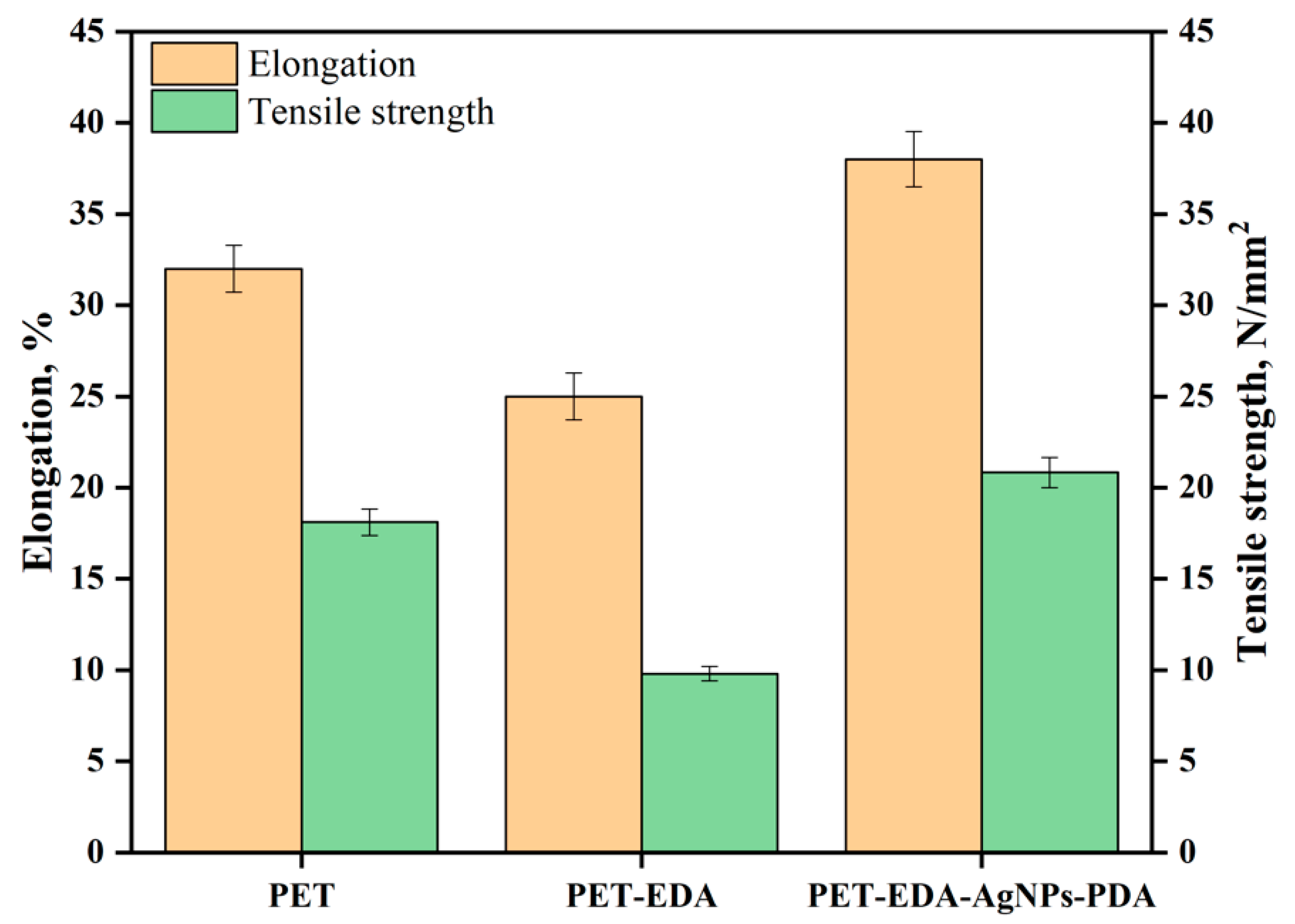
2.2. Characterization
2.3. Antioxidant Activity
2.3.1. Ferric Reducing Antioxidant Power Assay (FRAP)
2.3.2. DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
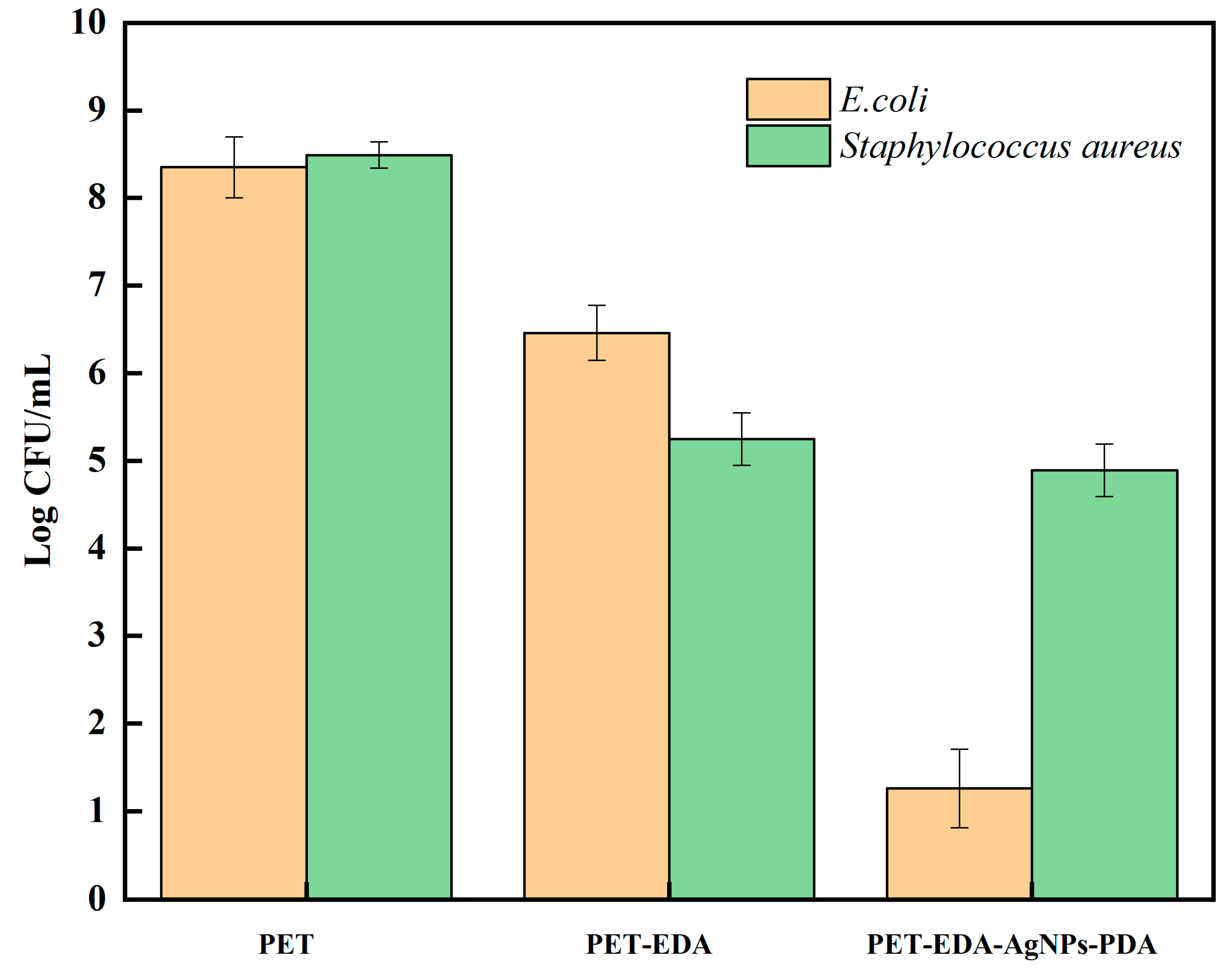
2.4. Antimicrobial Study
2.5. Broth Assay
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Pączkowski, P.; Puszka, A.; Miazga-Karska, M.; Ginalska, G.; Gawdzik, B. Synthesis, characterization and testing of antimicrobial activity of composites of unsaturated polyester resins with wood flour and silver nanoparticles. Materials 2021, 14, 1122. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Nikiforov, A.; Vujosevic, D.; Vuksanovic, V.; Mugoša, B.; Cvelbar, U.; Leys, C. Antibacterial activity of nano-silver non-woven fabric prepared by atmospheric pressure plasma deposition. Mater. Lett. 2015, 149, 95–99. [Google Scholar] [CrossRef]
- Holt, B.A.; Gregory, S.A.; Sulchek, T.; Yee, S.; Losego, M.D. Aqueous zinc compounds as residual antimicrobial agents for textiles. ACS Appl. Mater. Interfaces 2018, 10, 7709–7716. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Simončič, B.; Klemenčič, D. Preparation and performance of silver as an antimicrobial agent for textiles: A Review. Text. Res. J. 2016, 86, 210–223. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Duling, M.G.; Lawrence, R.B.; Thomas, T.A.; LeBouf, R.F.; Wade, E.E.; Abbas Virji, M. Dermal exposure potential from textiles that contain silver nanoparticles. Int. J. Occup. Environ. Health 2014, 20, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Bacciarelli-Ulacha, A.; Rybicki, E.; Matyjas-Zgondek, E.; Pawlaczyk, A.; Szynkowska, M.I. A new method of finishing cotton fabric by in situ synthesis of silver nanoparticles. Ind. Eng. Chem. Res. 2014, 53, 4147–4155. [Google Scholar] [CrossRef]
- Allehyani, E.S.; Almulaiky, Y.Q.; Al-Harbi, S.A.; El-Shishtawy, R.M. In Situ Coating of Polydopamine-AgNPs on Polyester Fabrics Producing Antibacterial and Antioxidant Properties. Polymers 2022, 14, 3794. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Nada, A.; Al-Moghazy, M.; Soliman, A.A.F.; Rashwan, G.M.T.; Eldawy, T.H.A.; Hassan, A.A.E.; Sayed, G.H. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int. J. Biol. Macromol. 2018, 107, 585–594. [Google Scholar] [CrossRef]
- Waite, J.H.; Housley, T.J.; Tanzer, M.L. Peptide repeats in a mussel glue protein: Theme and variations. Biochemistry 1985, 24, 5010–5014. [Google Scholar] [CrossRef]
- Naghili, H.; Tajik, H.; Mardani, K.; Razavi Rouhani, S.M.; Ehsani, A.; Zare, P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Vet. Res. Forum Int. Q. J. 2013, 4, 179–183. [Google Scholar]
- Chen, Y.H.; Hsu, C.C.; He, J.L. Antibacterial silver coating on poly (ethylene terephthalate) fabric by using high power impulse magnetron sputtering. Surf. Coat. Technol. 2013, 232, 868–875. [Google Scholar] [CrossRef]
- Gün Gök, Z.; Günay, K.; Arslan, M.; Yiğitoğlu, M.; Vargel, İ. Coating of modified poly (ethylene terephthalate) fibers with sericin-capped silver nanoparticles for antimicrobial application. Polym. Bull. 2020, 77, 1649–1665. [Google Scholar] [CrossRef]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly(ethyleneterephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Zhong, L.; Zhang, F.; Zhang, G. Aminolysis of polyethylene terephthalate fabric by a methodinvolving the gradual concentration of dilute ethylenediamine. Colloids and Surfaces A: Physicochem. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 146–152. [Google Scholar] [CrossRef]
- Palekar, V.S.; Shah, R.V.; Shukla, S.R. Ionic Liquid-Catalyzed Aminolysis of Poly(ethylene terephthalate) Waste. J. Appl. Polym. Sci. 2012, 126, 1174–1181. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Ahmed, N.S.E. Anionic coloration of acrylic fibre. Part 1: Efficient pretreatment and dyeing with acid dyes. Color. Technol. 2005, 121, 139–146. [Google Scholar] [CrossRef]
- Kan, C.W. Impact on textile properties of polyester with laser. Opt. Laser Technol. 2008, 40, 113–119. [Google Scholar] [CrossRef]
- Bora, C.; Gogoi, P.; Baglari, S.; Dolui, S.K. Preparation of polyester resin/graphene oxide nanocomposite with improved mechanical strength. J. Appl. Polym. Sci. 2013, 129, 3432–3438. [Google Scholar] [CrossRef]
- Seyhan, A.T.; Gojny, F.H.; Tanoğlu, M.; Schulte, K. Critical aspects related to processing of carbon nanotube/unsaturated thermoset polyester nanocomposites. Eur. Polym. J. 2007, 43, 374–379. [Google Scholar] [CrossRef][Green Version]
- Sârbu, C.; Casoni, D. Comprehensive evaluation of biogenic amines and related drugs’ antiradical activity using reactive 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical. Open Chem. 2013, 11, 679–688. [Google Scholar] [CrossRef]
- Shabbir, M.; Mohammad, F. Multifunctional AgNPs@Wool: Colored, UV-protective and antioxidant functional textiles. Appl. Nanosci. 2018, 8, 545–555. [Google Scholar] [CrossRef]
- Choukairi, Z.; Hazzaz, T.; José, M.F.; Fechtali, T. The cytotoxic activity of salvia officinalis L. and rosmarinus officinalis L. Leaves extracts on human glioblastoma cell line and their antioxidant effect. J. Complement. Integr. Med. 2020, 17, 20180189. [Google Scholar] [CrossRef]
- AL Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef] [PubMed]
- Afshari, S.; Montazer, M.; Harifi, T.; Mahmoudi Rad, M. A coloured polyester fabric with antimicrobial properties conferred by copper nanoparticles. Color. Technol. 2019, 135, 427–438. [Google Scholar] [CrossRef]
- Salam, M.A.; Obaid, A.Y.; El-Shishtawy, R.M.; Mohamed, S.A. Synthesis of nanocomposites of polypyrrole/carbon nanotubes/silver nano particles and their application in water disinfection. RSC Adv. 2017, 7, 16878–16884. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Asiri, A.M.; Abdelwahed, N.A.M.; Al-Otaibi, M.M. In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Ahmed, N.S.E. Color data and antibacterial properties of smart Ag/polypyrrole-nanocoated cotton fabric. Egypt. J. Chem. 2020, 63, 3547–3555. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Di Girolamo, M.; Sarret, G.; Bureau, S.; Fernandez-Martinez, A.; Lelong, C.; Eymard Vernain, E. In situ formation of silver nanoparticles (Ag-NPs) onto textile fibers. ACS Omega 2021, 6, 1316–1327. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Tkavc, T.; Vesel, A.; Šauperl, O. Chitosan coatings onto polyethylene terephthalate for the development of potential active packaging material. Appl. Surf. Sci. 2013, 265, 697–703. [Google Scholar] [CrossRef]




| Wavenumber, cm−1 | ||
|---|---|---|
| Peak Assignment | PET | PET-EDA-AgNPs-PDA |
| C–O stretching vibration | 1709 | 1713 |
| C–O ester vibration | 1010–1240 | 1010–1240 |
| C–H aliphatic asymmetric stretching vibration | 2967 | 2973 |
| C–H aromatic asymmetric stretching vibration | 3073 | 3082 |
| C–H out of plane vibration | 717–820 | 717–820 |
| C–N stretching vibration | - | 3436, 3206 |
| Carboxylate and amino group stretching vibration | - | 955, 2145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allehyani, E.S. Surface Functionalization of Polyester Textiles for Antibacterial and Antioxidant Properties. Polymers 2022, 14, 5512. https://doi.org/10.3390/polym14245512
Allehyani ES. Surface Functionalization of Polyester Textiles for Antibacterial and Antioxidant Properties. Polymers. 2022; 14(24):5512. https://doi.org/10.3390/polym14245512
Chicago/Turabian StyleAllehyani, Esam S. 2022. "Surface Functionalization of Polyester Textiles for Antibacterial and Antioxidant Properties" Polymers 14, no. 24: 5512. https://doi.org/10.3390/polym14245512
APA StyleAllehyani, E. S. (2022). Surface Functionalization of Polyester Textiles for Antibacterial and Antioxidant Properties. Polymers, 14(24), 5512. https://doi.org/10.3390/polym14245512






