Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Chemicals
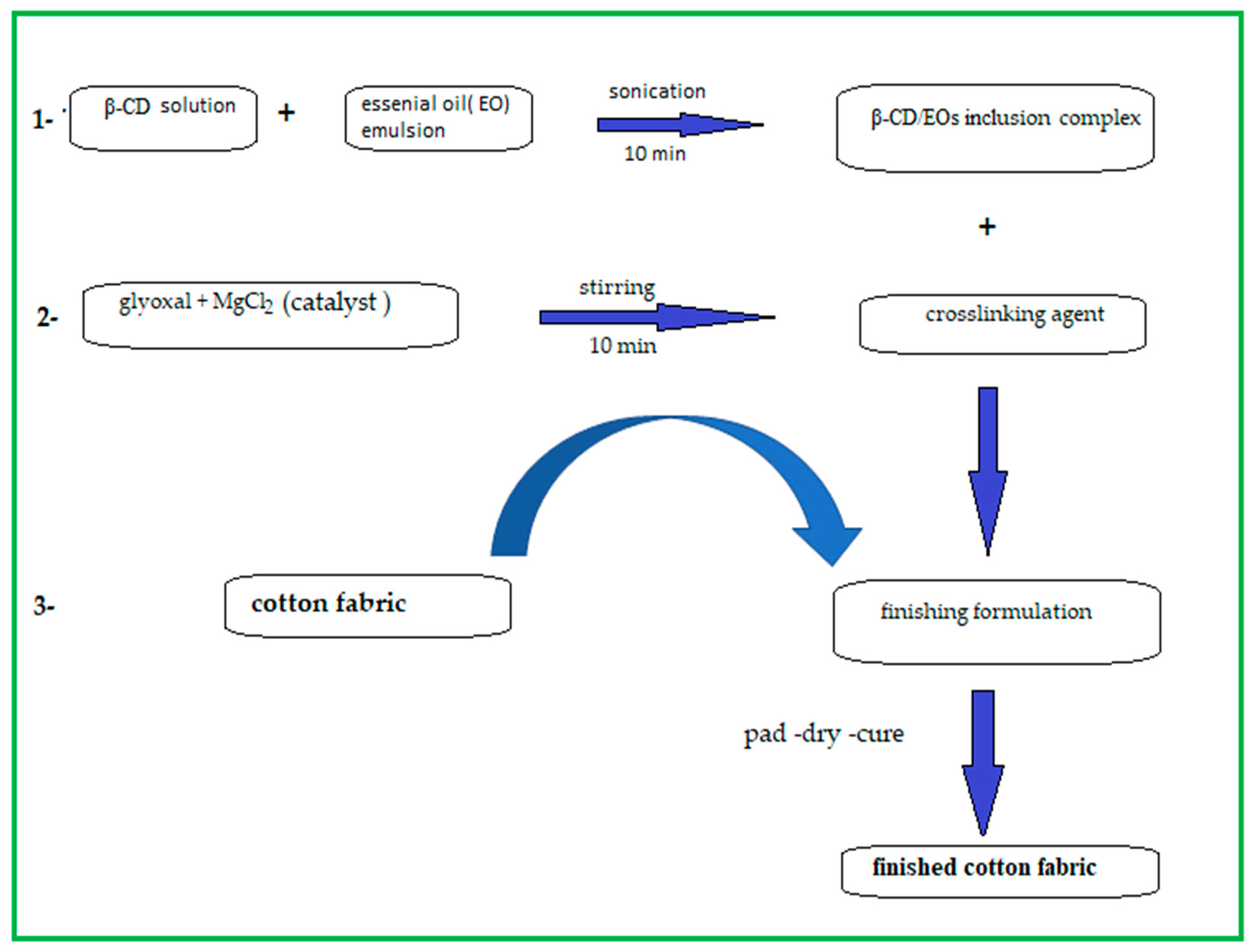
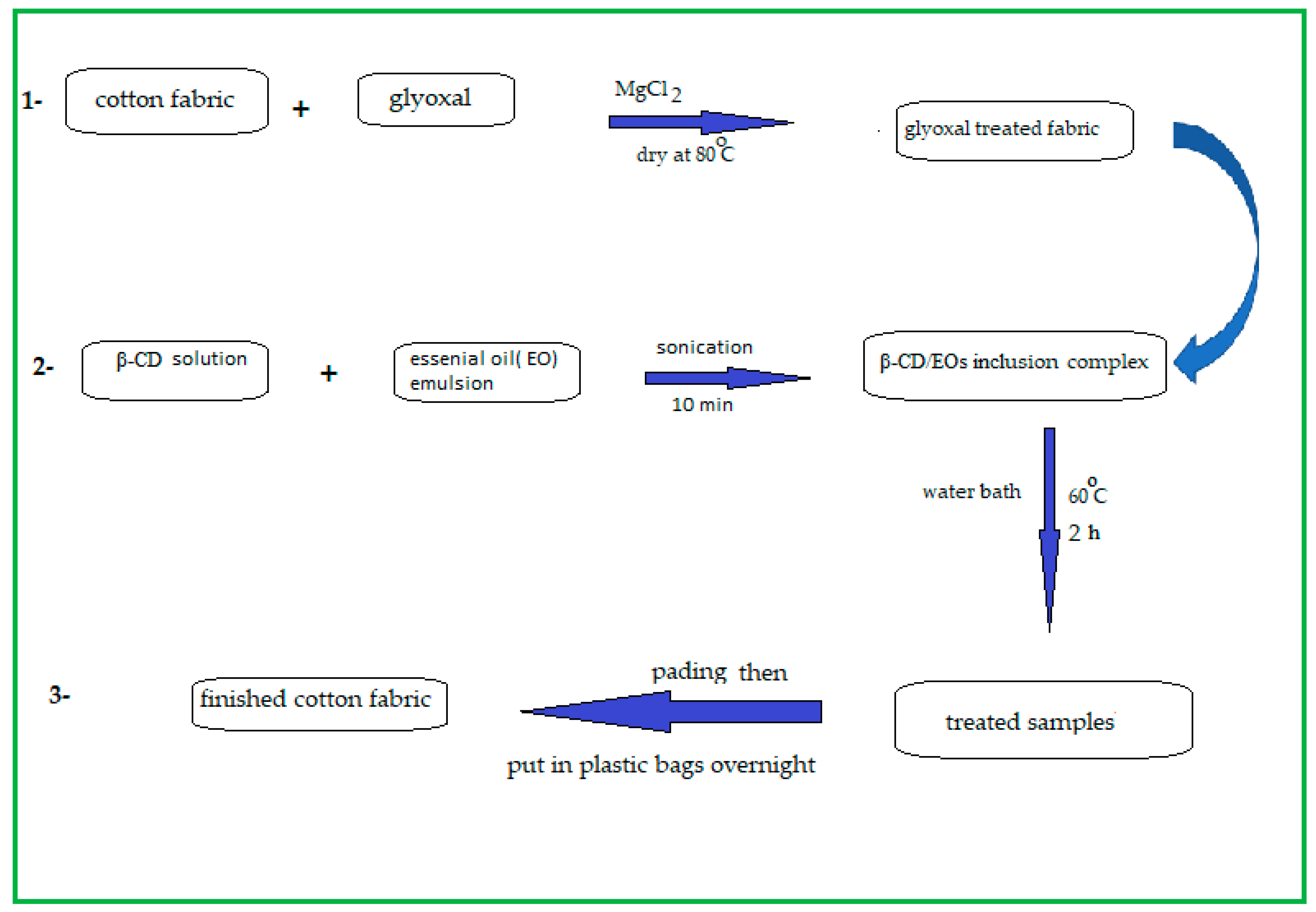
2.3. Preparation of Fabrics-Coated β-CD/Eos Inclusion Complex
2.3.1. Method (1) (Pad-Dry–Cure)
2.3.2. Method (2) (Pad-Batch)
3. Characterization
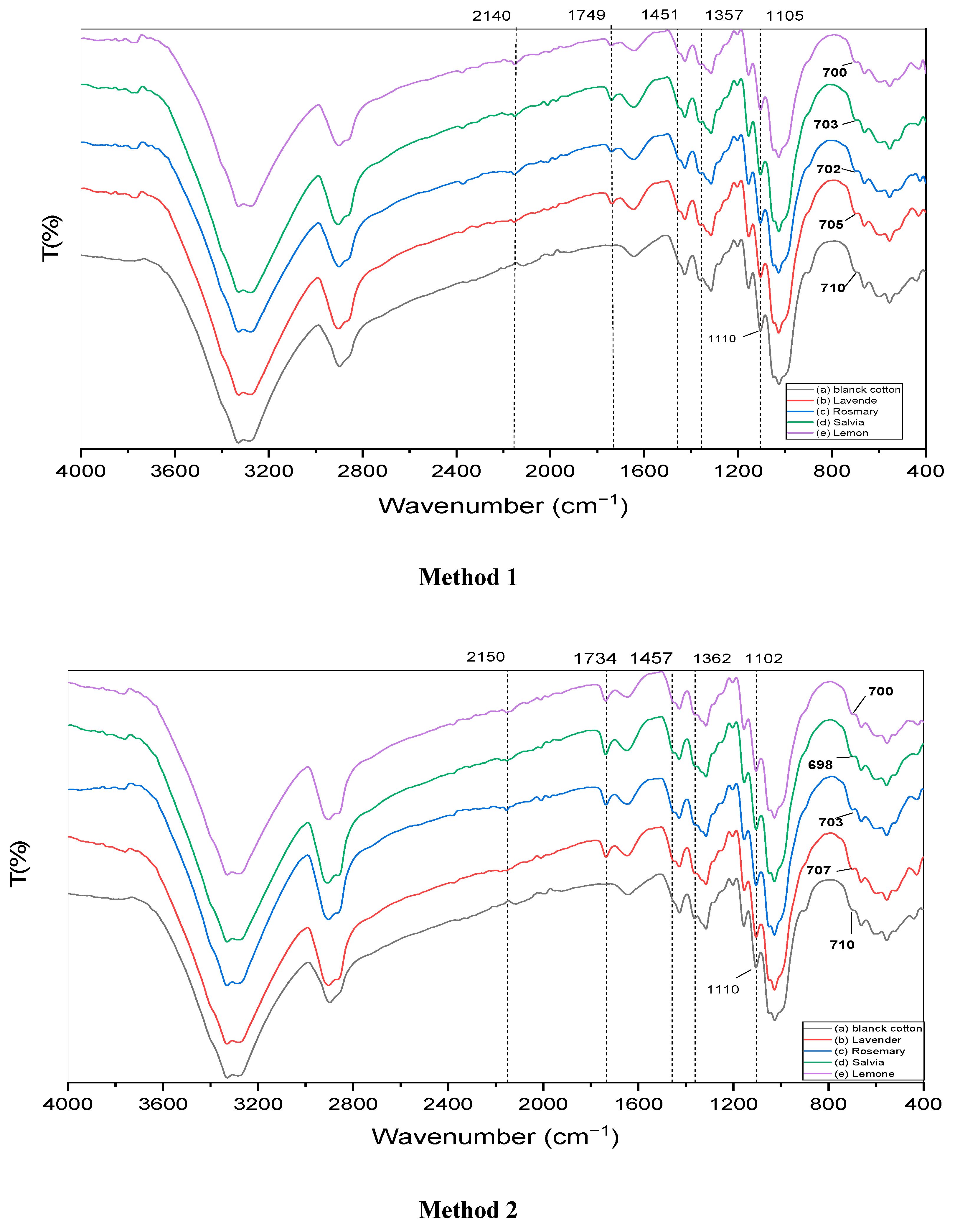
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
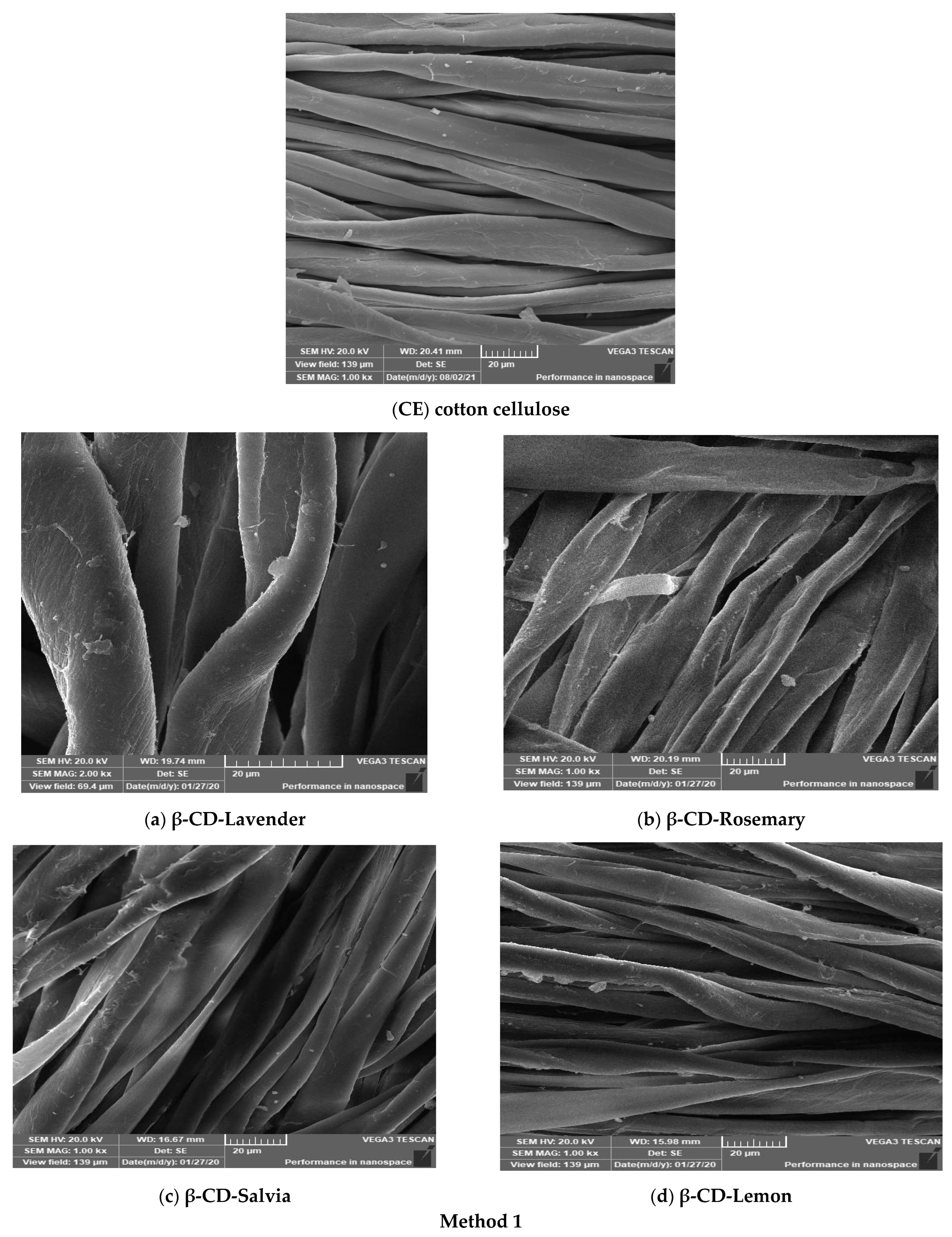
3.2. Scanning Electron Microscopy (SEM)/EDX Analysis and Visual Color
3.3. Antibacterial Analysis
3.4. Weight Gain
3.5. Durability Test
3.6. Scent Intensity
3.7. Air Permeability
3.8. Tensile Strength
3.9. Statistical Analysis
4. Results and Discussion
4.1. FTIR Analysis
4.2. SEM Analysis
4.3. Antibacterial Activity
- All cotton fabric treated by either method 1 or method 2 exhibited high antibacterial activities.
- The cotton samples treated by pad batch (method 2) showed higher antibacterial properties. This may have been due to the effect of the high temperature used in pad-dry-cure (method 1) on the encapsulation process, and the rapid release of EOs from the fabric, consequently reducing its bioactivity to some extent. This is in agreement with the results obtained in a previous study [36].
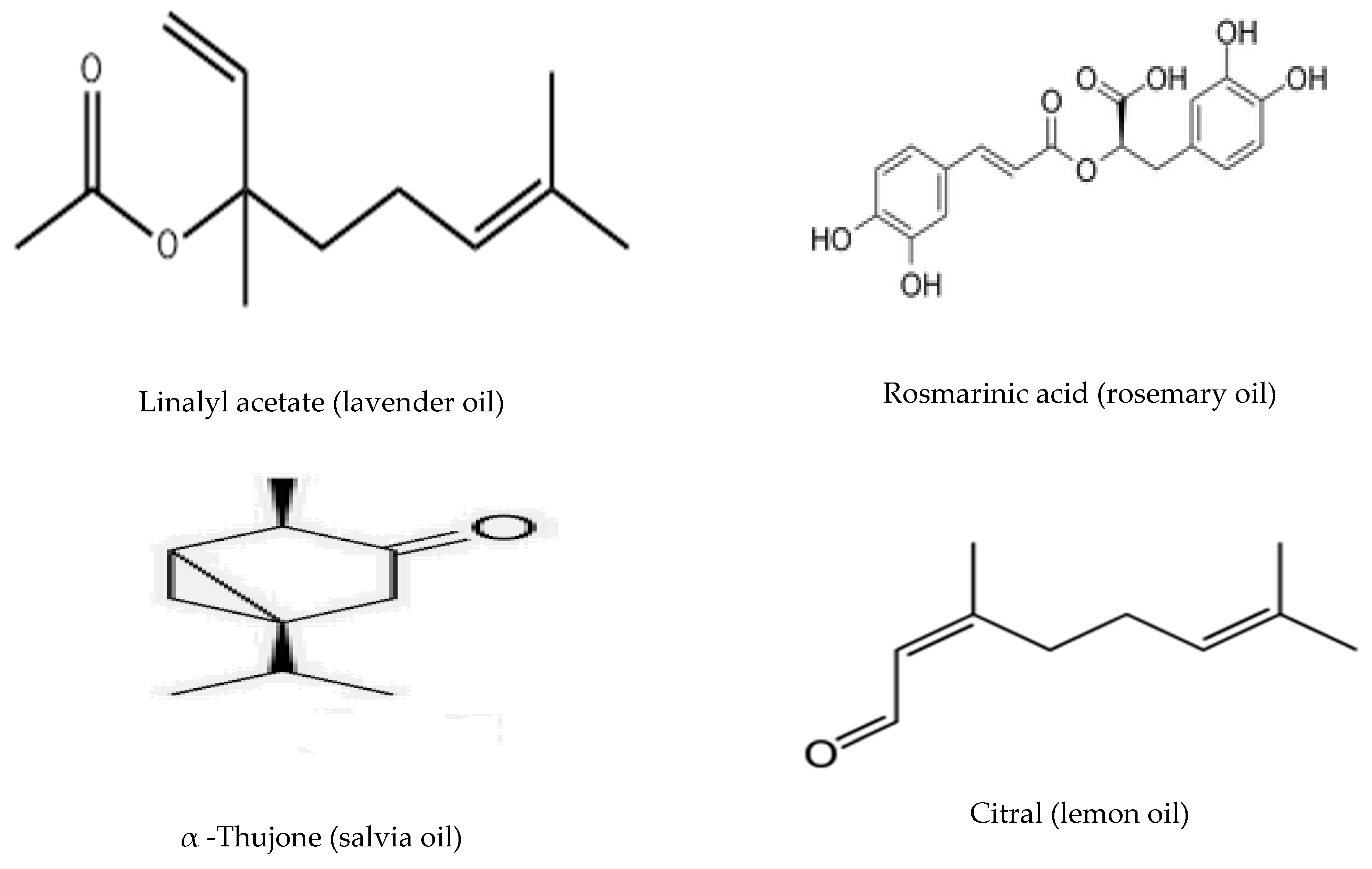
- The antibacterial activities of the treated samples varied according to the nature of EOs. They followed the order: β-CD/Lavender ˃ β-CD/Rosemary~β-CD/Lemon ˃ β-CD/Salvia.
- The bioactive constituents of EOS, such as terpenes and their oxygenated derivatives, are responsible for their biological properties.
- The mechanism of their activity could be attributed to their hydrophobic nature, which enables them to interact with the lipids found in the cell membranes of bacteria. This increases susceptibility by disrupting cell structures as they bind to the cell’s surface and subsequently penetrate the cell membrane’s phospholipid bilayer. Their accumulation affects the cell membrane’s structural stability, which can have a severe effect on cell metabolism and cause cell death. This finally leads to the death of the bacteria cell due to the significant leaking of its essential components and ions [37].
4.4. Scent Intensity
4.5. Weight Gain, Tensile Strength, and Air Permeability Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guerra-Rosas, M.; Morales-Castro, J.; Cubero-Márquez, M.; Salvia-Trujillo, L.; Martín-Belloso, O. Antimicrobial activity of nanoemulsions containing essential oils and high methoxyl pectin during long-term storage. Food Control 2017, 77, 131–138. [Google Scholar] [CrossRef]
- Abd El-Hady, M.M.; Farouk, A.; Saeed, S.E.S.; Zaghloul, S. Antibacterial and UV Protection Properties of Modified Cotton Fabric Using a Curcumin/TiO2 Nanocomposite for Medical Textile Applications. Polymers 2021, 13, 4027. [Google Scholar] [CrossRef]
- Islam, S.; Shahid, M.; Mohammad, F. Perspectives for natural product based agents derived from industrial plants in textile applications—A review. J. Clean. Prod. 2013, 57, 2–18. [Google Scholar] [CrossRef]
- Solorzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Kavetsou, E.; Pitterou, I.; Katopodi, A.; Petridou, G.; Adjali, A.; Grigorakis, S.; Detsi, A. Preparation, Characterization, and Acetylcholinesterase Inhibitory Ability of the Inclusion Complex of β-Cyclodextrin–Cedar (Juniperus phoenicea) Essential Oil. Micro 2021, 1, 250–266. [Google Scholar] [CrossRef]
- Sell, C.S. (Ed.) The Chemistry of Fragrances: From Perfumer to Consumer, 2nd ed.; RSC Pub.: Cambridge, UK, 2006. [Google Scholar]
- Pophof, B.; Gert, S.; Leif, A. Volatile organic compounds as signals in a plant–herbivore system: Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem. Senses 2005, 30, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Mehalaine, S.; Belfadel, O. Chemical composition and antibacterial activity of essential oils of three medicinal plants from Algerian semi-arid climatic zone. Phytothérapie 2018, 16, S155–S163. [Google Scholar] [CrossRef]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Khedher, M.R.B.; Khedher, S.B. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160. [Google Scholar] [PubMed]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, P.A.P.; Buera, M.P.; Elizalde, B.E. Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in β-cyclodextrin: Effect of interactions with water on complex stability. J. Food Eng. 2010, 99, 70–75. [Google Scholar] [CrossRef]
- Stan, M.; Chirila, L.; Popescu, A.; Radulescu, D.M.; Radulescu, D.E.; Dinischiotu, A. Essential Oil Microcapsules Immobilized on Textiles and Certain Induced Effects. Materials 2019, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.F.; Valente, A.J.M.; Lobo, V.M.M. Transport properties of cyclodextrins: Intermolecular diffusion coefficients. J. Balk. Tribol. Assoc. 2008, 14, 396–404. [Google Scholar]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Green Chemistry Approaches to Develop Antimicrobial Textiles Based on Sustainable Biopolymers, A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Grgac, S.F.; Malinar, R. Application of Environment-Friendly Processes Aimed at Bonding β-Cyclodextrine onto Cellulosic Materials. Fibres Text. East. Eur. 2018, 26, 63–69. [Google Scholar] [CrossRef]
- Agrawal, P.B.; Warmoeskerken, M.M.C.G. A β-cyclodextrin based slow and controlled release system for digital finishing of cotton. In Proceedings of the Autex Textile World Conference 2008, Biella, Italy, 24–26 June 2008. [Google Scholar]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef]
- Hadžiabdíc, J.; Elezovíc, A.; Rahíc, O.; Mujezin, I. Effect of Cyclodextrin Complexation on the Aqueous Solubility of Diazepam and Nitrazepam: Phase- Solubility Analysis, Thermodynamic Properties. Am. J. Anal. Chem. 2012, 3, 811–819. [Google Scholar] [CrossRef]
- Lis, M.J.; Carmona, Ó.G.; Carmona, C.G.; Bezerra, F.M. Inclusion complexes of citronella oil with β-cyclodextrin for controlled release in biofunctional textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; da Silva, J.G.D.; Valle, R.d.S.C.; Valle, J.A.B.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of β-Cyclodextrin in the Textile Industry-Review. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef] [PubMed]
- Haji, A. Functional Finishing of Textiles with β-Cyclodextrin. In Frontiers of Textile Materials: Polymers, Nanomaterials, Enzymes, and Advanced Modification Techniques; Shabbir, M., Ahmed, S., Sheikh, J.N., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 87–116. [Google Scholar]
- Grgac, S.F.; Jablan, J.; Inić, S.; Malinar, R.; Kovaček, I.; Čorak, I. The Effect of Ultrasonic Treatment on the Binding of the Inclusion Complex β-Cyclodextrin-peppermint Oil with Cellulose Material. Materials 2022, 15, 470. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Sharma, S.; Chakraborty, J.N. Performance assessment of fragrance finished cotton with cyclodextrin assisted anchoring hosts. Fash. Text. 2015, 2, 1–17. [Google Scholar] [CrossRef]
- Sricharussin, W.; Sopajaree, C.; Maneerung, T.; Sangsuriya, N. Modification of cotton fabrics with β-cyclodextrin derivative for aroma finishing. J. Text. Inst. 2009, 100, 682–687. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Castillo, S.; Sánchez-García, E.; Aguilera González, C.; Galindo-Rodríguez, S.A.; Gabaldón-Hernández, J.A.; Báez-González, J.G. Inclusion complexes of concentrated orange oils and β-cyclodextrin: Physicochemical and biological characterizations. Molecules 2020, 25, 5109. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, Y.; Wang, M.; Chen, F.; Liu, J.; Meng, K.; Zhao, H. Double-Layered Microcapsules Significantly Improve the Long-Term Effectiveness of Essential Oil. Polymers 2020, 12, 1651. [Google Scholar] [CrossRef]
- Hebeish, A.; Sharaf, S.; Refaie, R.; El Shafei, A. Multifinishing of Cotton Fabrics Using Microwave Techniques. Res. J. Text. Appar. 2012, 16, 68–81. [Google Scholar] [CrossRef]
- Perremans, D.; Hendrickx, K.; Verpoest, I.; Van Vuure, A.W. Effect of chemical treatments on the mechanical properties of technical flax fibres with emphasis on stiffness improvement. Compos. Sci. Technol. 2018, 160, 216–223. [Google Scholar] [CrossRef]
- Shu, D.; Fang, K.; Liu, X.; Cai, Y.; Zhang, X.; Zhang, J. Cleaner coloration of cotton fabric with reactive dyes using a pad-batch-steam dyeing process. J. Clean. Prod. 2018, 196, 935–942. [Google Scholar] [CrossRef]
- Abd El-Hady, M.M.; Farouk, A.; Sharaf, S. Multiwalled-Carbon-Nanotubes (MWCNTs)–GPTMS/Tannic-Acid-Nanocomposite-Coated Cotton Fabric for Sustainable Antibacterial Properties and Electrical Conductivity. Coatings 2022, 12, 178. [Google Scholar] [CrossRef]
- El-Sayed Saeed, S.; Al-Harbi, T.M.; Abdel-Mottaleb, M.S.A.; Al-Hakimi, A.N.; Albadria, A.E.A.E.; Abd El-Hady, M.M. Novel Schiff base transition metal complexes for imparting UV protecting and antibacterial cellulose fabric: Experimental and computational investigations. Appl. Organomet. Chem. 2022, 36, e6889. [Google Scholar] [CrossRef]
- Suthaphot, N.; Chonsakorn, S.; Mongkholrattanasit, R. Application of Aromatherapy on Cotton Fabric by Microcapsules. In Proceedings of the RMUTP International Conference: Textile and Fashion 2012, Bangkok, Thailand, 3–4 July 2012. [Google Scholar]
- Sharaf, S.; El-Naggar, M.E. Eco-friendly technology for preparation, characterization and promotion of honey bee propolis extract loaded cellulose acetate nanofibers in medical domains. Cellulose 2018, 25, 5195–5204. [Google Scholar] [CrossRef]
- Azhdarzadeh, F.; Hojjati, M. Chemical composition and antimicrobial activity of leaf, ripe and unripe peel of bitter orange (Citrus aurantium) essential oils. Nutr. Food Sci. Res. 2016, 3, 43–50. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef]
- Panigrahi, D.; Kumar, S.; Dhar, A. Modulating chain conformations of polyvinyl alcohol through low cost and nontoxic glyoxal crosslinker: Application in high performance organic transistors. Org. Electron. 2019, 65, 193–200. [Google Scholar] [CrossRef]
- Sharaf, S.; Higazy, A.; Hebeish, A. Propolis induced antibacterial activity and other technical properties of cotton textiles. Int. J. Biol. Macromol. 2013, 59, 408–416. [Google Scholar] [CrossRef]
- Ghosh, S.; Chipot, N. Embedding aromatherapy essential oils into textile fabric using β-cyclodextrin inclusion compound. Indian J. Fibre Text. Res. 2015, 40, 140–143. [Google Scholar]
- Muresan, A.; Cerempei, A.; Dunca, S.; Muresan, R.; Butnaru, R. Aromatherapeutic characteristics of cotton fabrics treated with rosemary essential oil. Cellul. Chem. Technol. 2009, 43, 435. [Google Scholar]
- Wang, H.; Wei, D.; Ziaee, Z.; Xiao, H.; Zheng, A.; Zhao, Y. Preparation and properties of nonleaching antimicrobial linear low-density polyethylene films. Ind. Eng. Chem. Res. 2015, 54, 1824–1831. [Google Scholar] [CrossRef]
- Farouk, A.; Moussa, S.; Ulbricht, M.; Textor, T. ZnO Nanoparticles-Chitosan Composite as Antibacterial Finish for Textiles. Int. J. Carbohydr. Chem. 2012, 1–8. [Google Scholar] [CrossRef]
- Afzal, A.; Hussain, T.; Malik, M.H.; Rasheed, A.; Ahmad, S.; Basit, A.; Nazir, A. Investigation and modeling of air permeability of Cotton/Polyester blended double layer interlock knitted fabrics. Fibers Polym. 2014, 15, 1539–1547. [Google Scholar] [CrossRef]


| Treatment of Fabric | Inhibition Zone (mm/1 cm Sample) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| G+ | G− | ||||||||
| Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | ||||||
| No. of washing cycles | 1 | 5 | 1 | 5 | 1 | 5 | 1 | 5 | |
| Untreated cotton fabric | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Method (1) | β-CD/Lavender | 33 ± 0.57 | 24 ± 0.28 | 31 ± 0.28 | 24 ± 0.57 | 32 ± 0.28 | 29 ± 0.28 | 31 ± 0.52 | 26 ± 0.57 |
| β-CD/Rosemary | 29 ± 0.57 | 23 ± 0.57 | 28 ± 0.57 | 21 ± 0.57 | 32 ± 0.52 | 28 ± 0.28 | 30 ± 0.57 | 24 ± 0.57 | |
| β-CD/Salvia | 26 ± 0.57 | 20 ± 0.28 | 25 ± 0.57 | 19 ± 0.28 | 30 ± 0.57 | 24 ± 0.23 | 28 ± 0.25 | 22 ± 0.57 | |
| β-CD/Lemon | 30 ± 0.28 | 21 ± 0.75 | 30 ± 0.28 | 20 ± 0.5 | 31 ± 0.28 | 27 ± 0.2 | 29 ± 0.25 | 23 ± 0.25 | |
| Method (2) | β-CD/Lavender | 39 ± 0.20 | 30 ± 0.28 | 34 ± 0.76 | 26 ± 0.25 | 35 ± 0.28 | 30 ± 0.25 | 33 ± 0.20 | 29 ± 0.20 |
| β-CD/Rosemary | 36 ± 0.57 | 28 ± 0.28 | 32 ± 0.50 | 24 ± 0.28 | 33 ± 0.28 | 30 ± 0.28 | 30 ± 0.57 | 25 ± 0.20 | |
| β-CD/Salvia | 29 ± 0.25 | 22 ± 0.57 | 28 ± 0.28 | 20 ± 0.17 | 30 ± 0.28 | 27 ± 0.51 | 28 ± 0.52 | 23 ± 0.51 | |
| β-CD/Lemon | 32 ± 0.57 | 23 ± 0.75 | 31 ± 0.76 | 22 ± 0.17 | 32 ± 0.57 | 25 ± 0.20 | 31 ± 0.76 | 24 ± 0.57 | |
| Method | Evaluation Day | β-CD/Lavender | β-CD/Rosemary | β-CD/Salvia | β-CD/Lemon |
|---|---|---|---|---|---|
| Method (1) | 1 | 4.75 | 4.50 | 4.25 | 3.75 |
| 5 | 4.50 | 4.50 | 4.25 | 3.50 | |
| 10 | 4.25 | 4.00 | 4.00 | 3.25 | |
| 15 | 4.00 | 3.75 | 3.75 | 3.25 | |
| 20 | 3.50 | 3.50 | 3.25 | 3.00 | |
| 25 | 3.25 | 3.00 | 3.00 | 2.75 | |
| 30 | 3.00 | 2.25 | 2.00 | 2.75 | |
| Method (2) | 1 | 5.00 | 4.75 | 5.00 | 5.00 |
| 5 | 5.00 | 4.50 | 4.75 | 4.75 | |
| 10 | 4.50 | 4.25 | 4.50 | 4.75 | |
| 15 | 4.50 | 3.75 | 3.75 | 4.25 | |
| 20 | 4.00 | 3.50 | 3.25 | 3.75 | |
| 25 | 3.50 | 3.00 | 3.00 | 3.50 | |
| 30 | 3.25 | 2.50 | 2.25 | 3.00 |
| Treatment of Fabric | Weight Gain (%) | Tensile Strength (kg f) | Air Permeability (cm3/cm2/s) | |
|---|---|---|---|---|
| Untreated cotton fabric (CE) | 0 | 56 ± 0.66 | 28.56 | |
| Method (1) | β-CD/Lavender | 10.55 ± 0.1 | 56 ± 0.76 | 25.24 |
| β-CD/Rosemary | 9.55 ± 0.06 | 57 ± 1.50 | 26.22 | |
| β-CD/Salvia | 8.65 ± 0.05 | 58 ± 0.36 | 23.23 | |
| β-CD/Lemon | 9.65 ± 0.4 | 56 ± 0.55 | 27.48 | |
| Method (2) | β-CD/Lavender | 11.58 ± 0.5 | 55 ± 0.05 | 26.76 |
| β-CD/Rosemary | 10.02 ± 0.04 | 55 ± 0.21 | 26.18 | |
| β-CD/Salvia | 9.01 ± 0.02 | 54 ± 0.87 | 24.21 | |
| β-CD/Lemon | 9.32 ± 0.23 | 53 ± 0.68 | 27.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farouk, A.; Sharaf, S.; Refaie, R.; Abd El-Hady, M.M. Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex. Polymers 2022, 14, 4899. https://doi.org/10.3390/polym14224899
Farouk A, Sharaf S, Refaie R, Abd El-Hady MM. Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex. Polymers. 2022; 14(22):4899. https://doi.org/10.3390/polym14224899
Chicago/Turabian StyleFarouk, A., S. Sharaf, R. Refaie, and M. M. Abd El-Hady. 2022. "Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex" Polymers 14, no. 22: 4899. https://doi.org/10.3390/polym14224899
APA StyleFarouk, A., Sharaf, S., Refaie, R., & Abd El-Hady, M. M. (2022). Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex. Polymers, 14(22), 4899. https://doi.org/10.3390/polym14224899





