Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Vegetal Materials
2.3. Characterization of Vegetal Material
2.4. Extraction of Wall Materials
2.5. Preparation of Microcapsules
2.6. Characterization of the Guava Pulp Microcapsules
2.6.1. Carotenoids Quantification
2.6.2. Lycopene and β-Carotene Analysis by HPLC–MS
2.6.3. Trolox Equivalent Antioxidant Capacity (TEAC)
2.6.4. Color Parameters
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
2.8. Microstructural Characterization
2.8.1. Scanning Electron Microscopy (SEM)
2.8.2. Particle Size
2.9. Thermal Characterization
2.10. Dietary Fiber Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Total Carotenoid Content, Antioxidant Capacity, Dietary Fiber Content, and Color Parameters
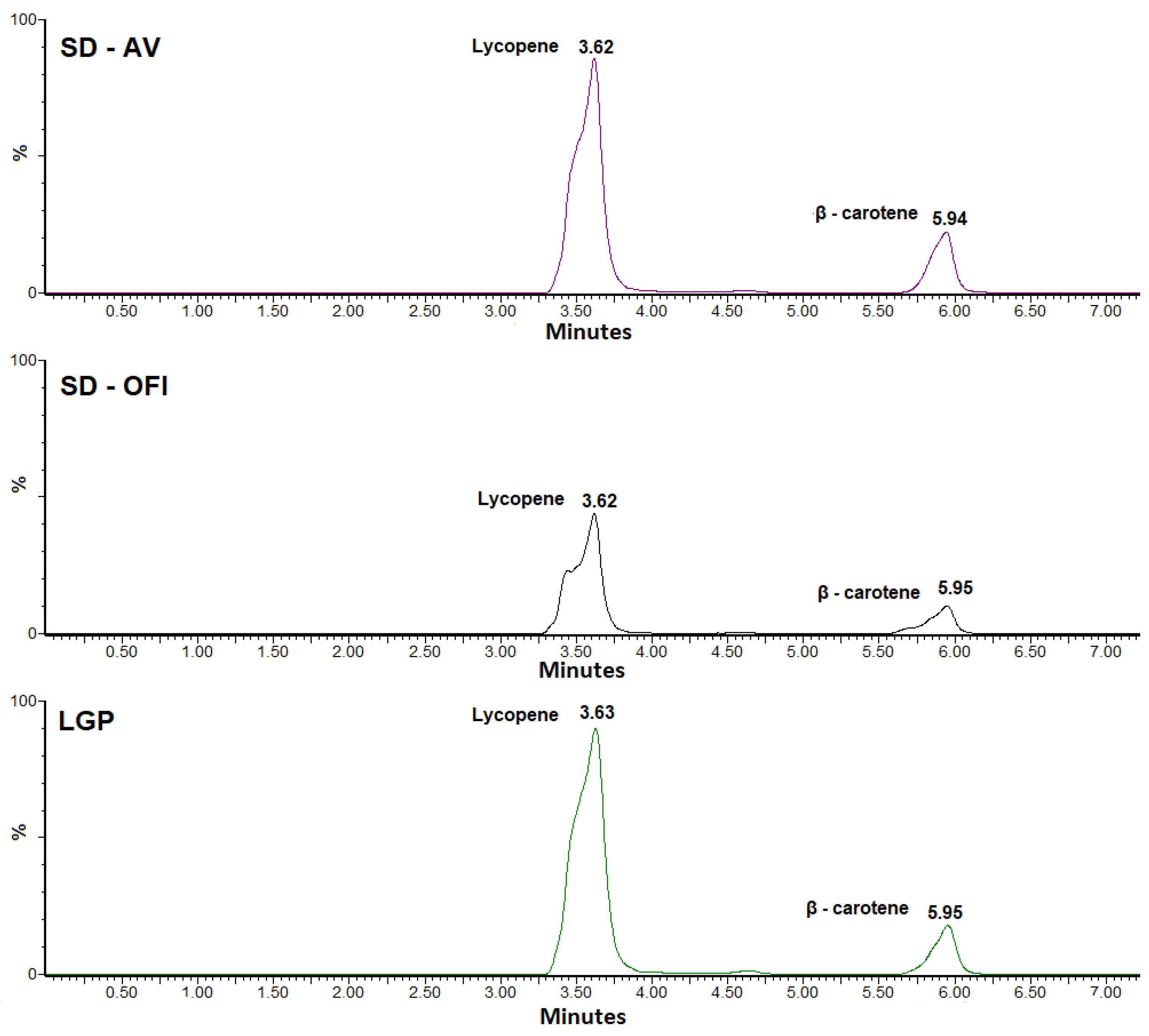
3.2. HPLC-MS Identification Analysis
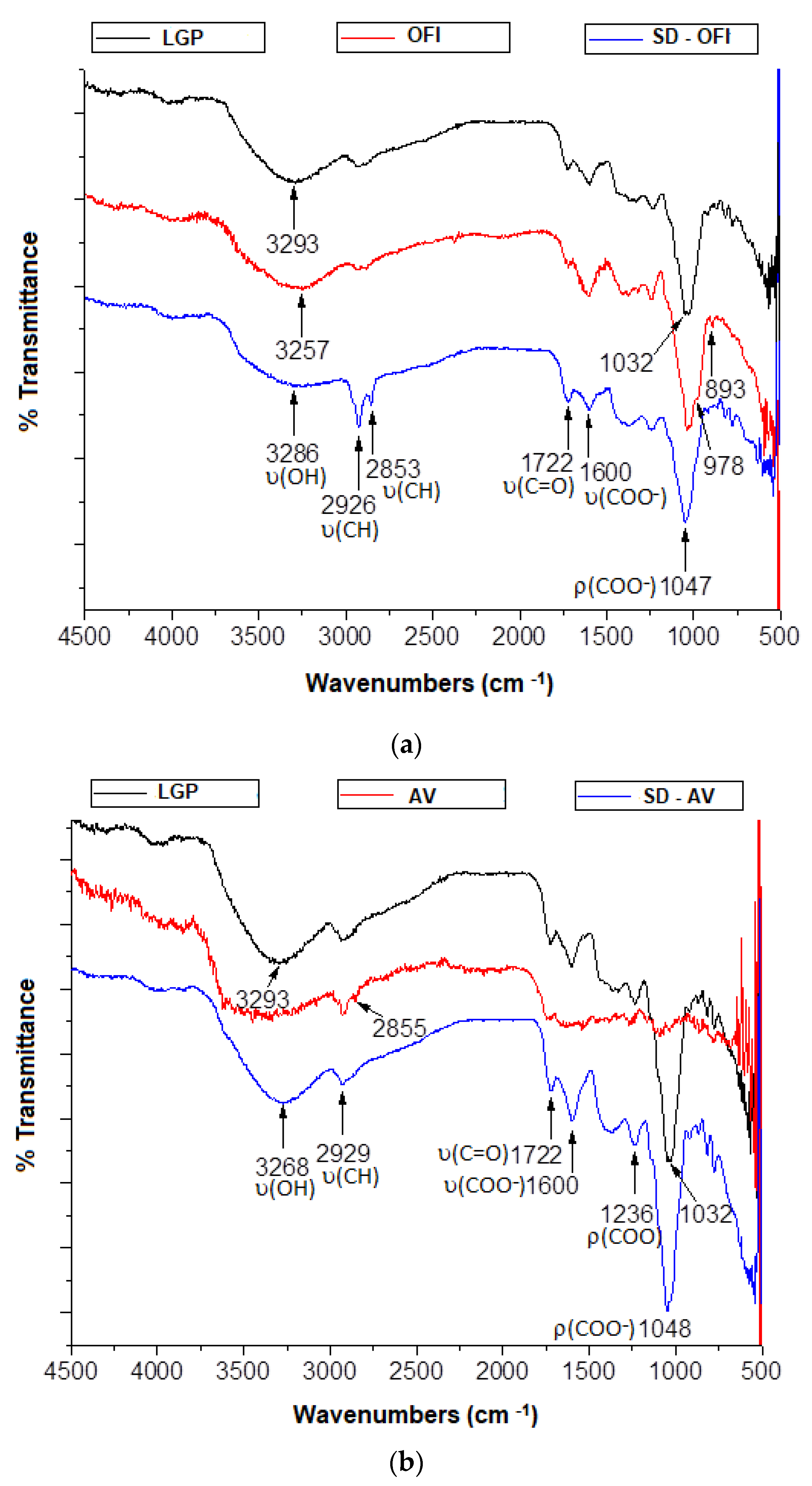
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
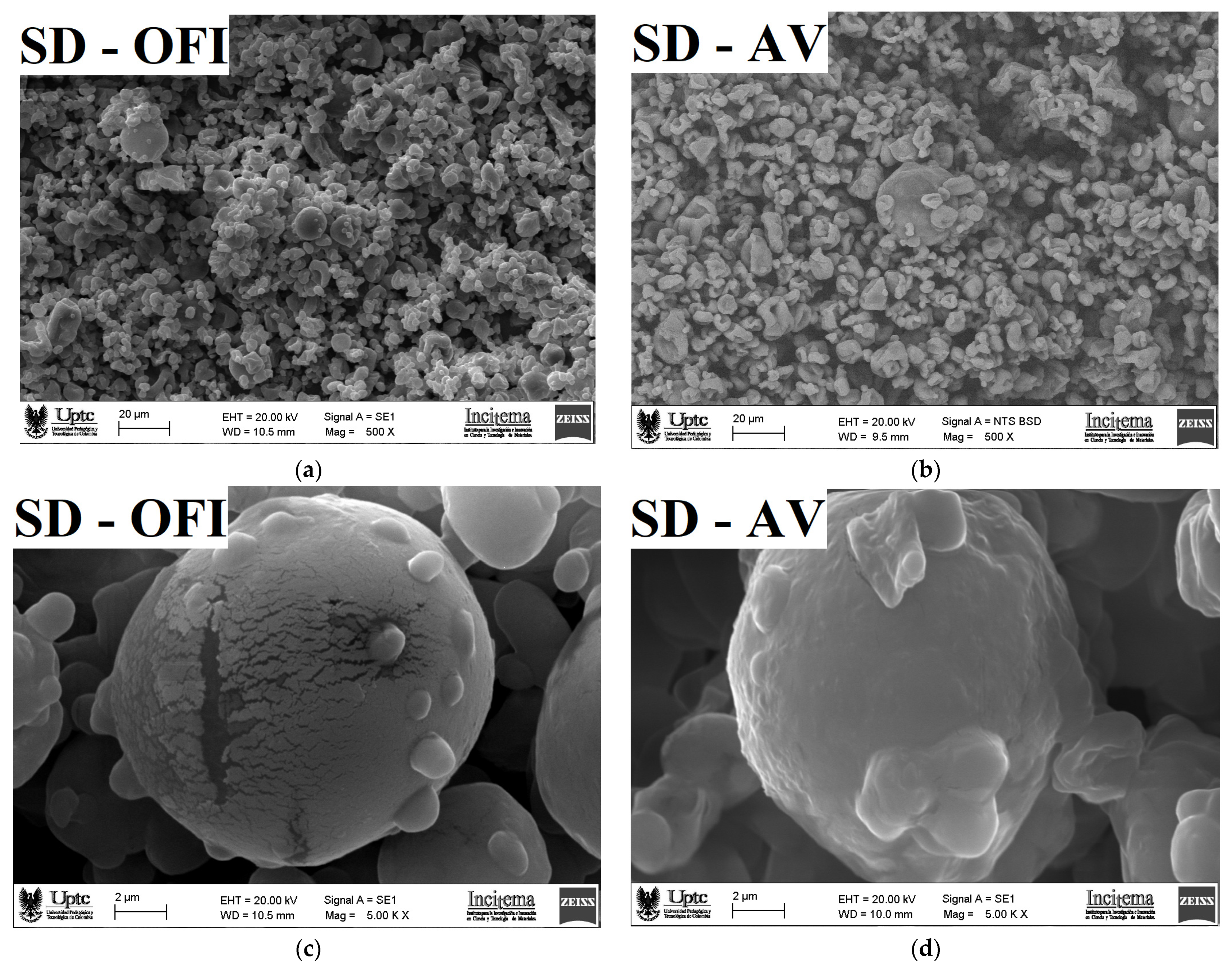
3.4. Microscopy Morphology and Particle Size
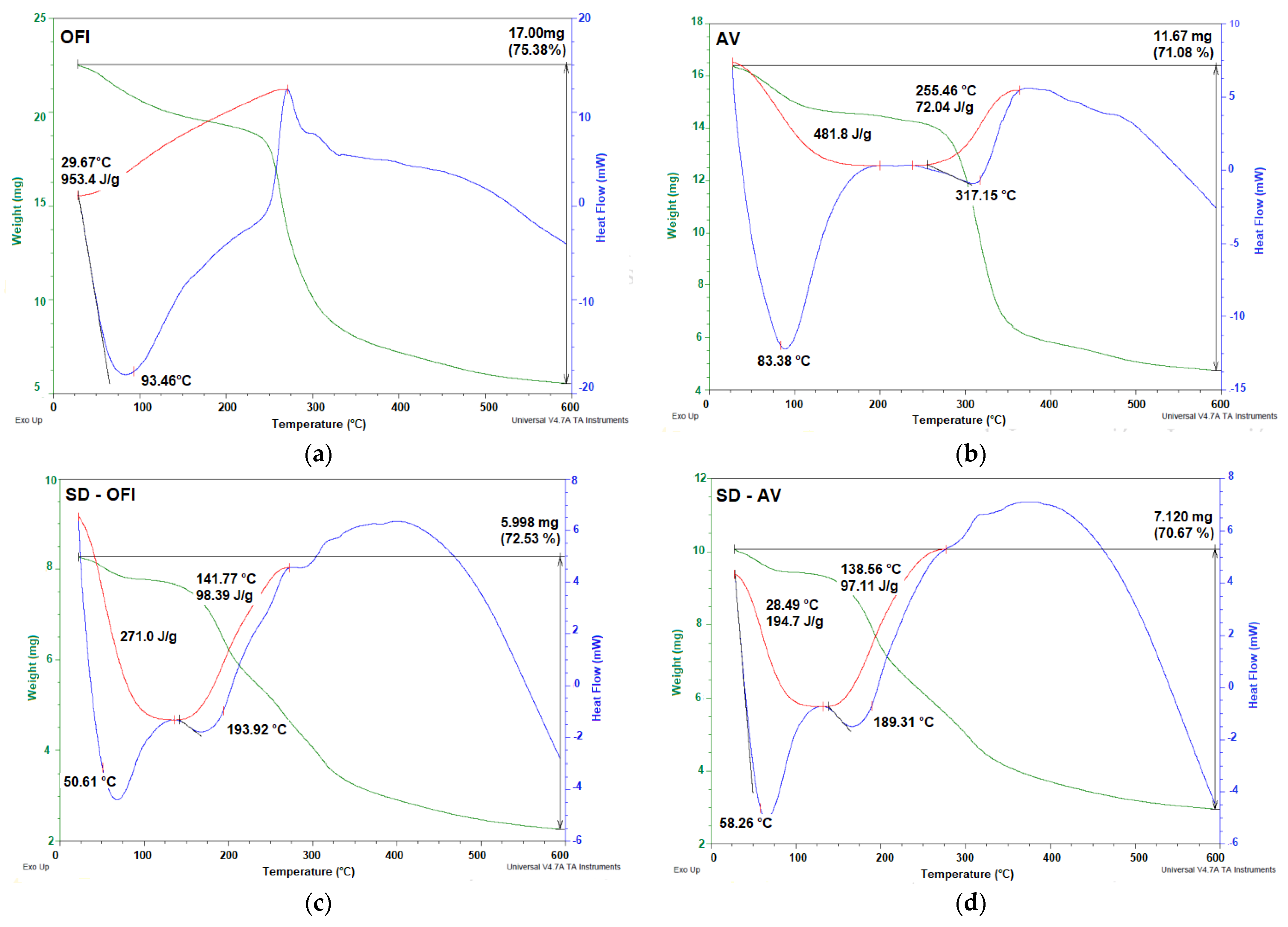
3.5. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tridge.com. Available online: https://www.tridge.com/intelligences/guava/production (accessed on 11 November 2021).
- Chang, Y.P.; Woo, K.K.; Gnanaraj, C. Pink guava. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 227–252. [Google Scholar] [CrossRef]
- Nutritiondata.self.com. Available online: https://nutritiondata.self.com/facts/fruits-and-fruit-juices/1927/2 (accessed on 11 November 2021).
- Chang, S.K.; Alasalvar, C.; Shanhidi, F. Superfruits: Phytochemicals, antioxidant efficacies and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1580–1604. [Google Scholar] [CrossRef]
- Kong, K.W.; Ismail, A. Lycopene content and lipophilic antioxidant capacity of by-products from Psidium guajava fruits produced during puree production industry. Food Bioprod. Process. 2011, 89, 53–61. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprako, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Koguishi de Brito, C.A.; Becker, P.; de Souza, J.C.; André, H.M. In vitro antioxidant capacity, phenolic, ascorbic acid and lycopene content of guava (Psidium guajava L.) juices and nectars. Bol. Cent. Pesqui. Process. Aliment. 2009, 27, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Carotenoids. In Nutraceutical and Functional Food Components. Effects of Innovative Processing Techniques; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 259–296. [Google Scholar] [CrossRef]
- Santos, W.N.L.; Sauthier, M.C.S.; Santos, A.M.P.; Santana, D.A.; Azevedo, R.S.A.; Caldas, J.C. Simultaneous determination of 13 phenolic bioactive compounds in guava (Psidium guajava L.) by HPLC-PAD with evaluation using PCA and Neural Network Analysis (NNA). Microchem. J. 2017, 133, 583–592. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Amorim, A.G.N.; dos Santos, R.C.; de Souza, J.M.T.; Souza, L.K.M.; Araújo, T.S.L.; Nicolau, L.A.D.; Carvalho, L.L.; de Aquino, P.E.A.; da Silva Martins, C.; et al. Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice. Int. Food Res. J. 2017, 99, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT—Food Sci. Technol. 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, S.J.C.; et al. Cytotoxic activity of poly-ɛ-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Int. Food Res. J. 2020, 136, 109548. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, I.; Aprodu, I.; Cîrciumaru, A.; Rapeanu, G.; Bahrim, G.E.; Stanciuc, N. Microencapsulation of lycopene from tomatoes peels by complex coacervation and freeze-drying: Evidences on phytochemical profile, stability and food applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of gallic acid by spray drying with aloe vera mucilage (aloe barbadensis miller) as wall material. Ind. Crops Prod. 2019, 138, 111461. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- De Campo, C.; Dick, M.; dos Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; Rios, A.O.; Flôres, S.H. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Soto-Castro, D.; Gutiérrez Miguel Chávez, M.; León-Martínez, F.; Santiago-García, P.A.; Aragón-Lucero, I.; Antonio-Antonio, F. Spray drying microencapsulation of betalain rich extracts from Escontria chiotilla and Stenocereus queretaroensis fruits using cactus mucilage. Food Chem. 2019, 272, 715–722. [Google Scholar] [CrossRef]
- Otálora, M.C.; Gómez Castaño, J.A.; Wilches-Torres, A. Preparation, study and characterization of complex coacervates formed between gelatin and cactus mucilage extracted from cladodes of Opuntia ficus-indica. LWT—Food Sci. Technol. 2019, 112, 108234. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; González Laredo, R.F.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodríguez-Ramírez, J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT—Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Taip, F.S.; Aziz, N.A.; Talib, R.A. Physical Properties of Spray-dried Pink Guava (Psidium Guajava) Powder. Agric. Agric. Sci. Procedia 2014, 2, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Osorio, C.; Forero, D.P.; Carriazo, J.G. Characterization and performance assessment of guava (Psidium guajava L.) microencapsulates obtained by spray-drying. Int. Food Res. J. 2011, 44, 1174–1181. [Google Scholar] [CrossRef]
- Quinzio, C.; Corvalán, M.; López, B.; Iturriaga, L. Studying stability against coalescence in tuna mucilage emulsions. Acta Hortic. 2009, 811, 427–431. [Google Scholar] [CrossRef]
- B-290 Mini Spray Dryer Operation Manual 093001N. Available online: https://static1.buchi.com/sites/default/files/downloads/B290_OM_en_I_0.pdf?cf595fc09d939d0eb8f2bee907c35bca8feeee47 (accessed on 7 June 2021).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cunniff, P. Enzymatic-gravimetric method. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Santos, P.D.F.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Carmen Sílvia Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef] [PubMed]
- Quinzio, C.; Ayunta, C.; Alancay, M.; de Mishima, B.L.; Iturriaga, L. Physicochemical and rheological properties of mucilage extracted from Opuntia ficus indica (L. Miller). Comparative study with guar gum and xanthan gum. J. Food Meas. Charact. 2018, 12, 459–470. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Minjares, R.; Femenia, A.; Sanchez-Olivares, G.; Gonzalez-Laredo, F.R.; Santiago-Adame, R.; Ramirez-Nuñez, D.M.; Rodríguez-Ramírez, J.; Manero, O. Structure preservation of Aloe vera (barbadensis Miller) mucilage in a spray drying process. LWT—Food Sci. Technol. 2016, 66, 93–100. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Osorio, C.; Nazareno, M.A. Encapsulation of cactus (Opuntia megacantha) betaxanthins by ionic gelation and spray drying: A comparative study. Int. Food Res. J. 2018, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.I.; Espinal, M.; García, J.; Jiménez, L.; Silva, K.; Restrepo, P. Desarrollo de productos enriquecidos con fibra de guayaba. In Desarrollo de Productos Funcionales Promisorios a Partir de la Guayaba (Psidium guajava L.) para el Fortalecimiento de la Cadena Productiva, 1st ed.; Morales, A.L., Melgarejo, L.M., Eds.; Panamericana Formas e Impresos S.A.: Bogotá, Colombia, 2010; pp. 155–174. [Google Scholar]
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of powder particle structure on the oxidation stability and color of encapsulated crystalline and emulsified carotenoids in carrot concentrate powders. J. Food Eng. 2019, 263, 398–408. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Bigeard, F.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Influence of emulsion and powder size on the stability of encapsulated D-limonene by spray drying. Innov. Food Sci. Emerg. Technol. 2005, 6, 107–114. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Cruz-Olivares, J.E.; Barragán-Huerta, B.; Dublán-García, O.; Román-Guerrero, A.; Pérez-Alonso, C. Microencapsulation by spray drying of lemon essential oil: Evaluation of mixtures of mesquite gum–nopal mucilage as new wall materials. J. Microencapsul. 2017, 34, 395–407. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Acuña-Avila, P.E.; Rodríguez-Huezo, M.E.; Román-Guerrero, A.; Varela-Guerrero, V.; Pérez-Alonso, C. Effect of chia mucilage addition on oxidation and release kinetics of lemon essential oil microencapsulated using mesquite gum—Chia mucilage mixtures. Int. Food Res. J. 2019, 116, 1010–1019. [Google Scholar] [CrossRef]
- Carvalho Gualberto, N.; Santos de Oliveira, C.; Pedreira Nogueira, J.; Silva de Jesus, M.; Santos Araujo, H.C.; Rajan, M.; Santos Leite Neta, M.T.; Narain, N. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extraction. Int. Food Res. J. 2021, 147, 110538. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Du, J.; Zhang, L.; Li, W. Properties of pectin extracted from fermented and steeped hawthorn wine pomace: A comparison. Carbohydr. Polym. 2018, 197, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.A.; Aparecida de Oliveira Silva, D.; Nunes, C.A.; Pereira, J. Experimental techniques for the extraction of taro mucilage with enhanced emulsifier properties using chemical characterization. Food Chem. 2020, 327, 127095. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Martínez, C.V.; Medina-Torres, L.; González-Laredo, R.F.; Calderas, F.; Sánchez-Olivares, G.; Herrera-Valencia, E.E.; Gallegos Infante, J.A.; Rocha-Guzman, N.E.; Rodríguez-Ramírez, J. Study of spray drying of the Aloe vera mucilage (Aloe vera barbadensis Miller) as a function of its rheological properties. LWT—Food Sci. Technol. 2014, 55, 426–435. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilusLa-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT—Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sanchez, J.A.; Beristain, C.I.; Jimenez Fernandez, M. Microencapsulation of Eugenia uniflora L. juice by spray drying using fructans with different degrees of polymerisation. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Homayouni-Rad, A.; Mortazavian, A.M.; Mashkani, M.G.; Hajipour, N.; Pourjafar, H. Effect of Alyssum homolocarpum mucilage and inulin microencapsulation on the survivability of Lactobacillus casei in simulated gastrointestinal and high temperature conditions. Biocatal. Agric. Biotechnol. 2021, 35, 102075. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Campo, C.; Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; Rios, A.O.; Flôres, S.H. Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispanica L.) as wall material: Characterization and stability evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Monge Neto, A.A.; Fonseca Tomazini, L.; Gouveia Mizuta, A.; Gomes Correa, R.C.; Scaramal Madrona, G.; Faria de Moraes, F.; Peralta, R.M. Direct microencapsulation of an annatto extract by precipitation of psyllium husk mucilage polysaccharides. Food Hydrocoll. 2021, 112, 106333. [Google Scholar] [CrossRef]


| Parameter | LGP | SD-AV | SD-OFI |
|---|---|---|---|
| TCC 1 | 190.9 ± 0.2 a | 42.6 ± 0.2 b | 31.4 ± 0.3 c |
| TEAC 2 | 32.2 ± 0.3 a | 26.8 ± 0.2 b | 23.2 ± 0.2 c |
| Total dietary fiber 3 | - | 22.8 ± 0.1 b | 32.1 ± 0.1 a |
| Color parameters | |||
| L* (luminosity) | 70.83 ± 0.02 b | 68.94 ± 0.01 b | 77.80 ± 0.02 a |
| a* | 13.07 ± 0.04 a | 7.48 ± 0.02 b | 5.89 ± 0.02 c |
| b* | 24.27 ± 0.01 b | 31.61 ± 0.03 a | 22.39 ± 0.01 b |
| Cab* (chroma) | 27.29 ± 0.02 b | 31.78 ± 0.03 a | 22.56 ± 0.02 b |
| hab* (hue) | 61.55 ± 0.03 b | 77.27 ± 0.01 a | 77.19 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers 2022, 14, 310. https://doi.org/10.3390/polym14020310
Otálora MC, Wilches-Torres A, Gómez Castaño JA. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers. 2022; 14(2):310. https://doi.org/10.3390/polym14020310
Chicago/Turabian StyleOtálora, María Carolina, Andrea Wilches-Torres, and Jovanny A. Gómez Castaño. 2022. "Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials" Polymers 14, no. 2: 310. https://doi.org/10.3390/polym14020310
APA StyleOtálora, M. C., Wilches-Torres, A., & Gómez Castaño, J. A. (2022). Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers, 14(2), 310. https://doi.org/10.3390/polym14020310






