Terephthalaldehyde–Phenolic Resins as a Solid-Phase Extraction System for the Recovery of Rare-Earth Elements
Abstract
:1. Introduction
2. Experimental
2.1. Material and Methods
2.2. General Procedure for Resole Preparation
2.3. Resin Characterization Techniques
2.4. Sorption Experiments
2.4.1. Resin Conversion to Na+ Form
2.4.2. Batch Extraction
2.4.3. Adsorption Kinetic
2.4.4. Adsorption Mechanism
3. Results and Discussion
3.1. Resin Synthesis and Characterization
3.2. Sorption Experiments
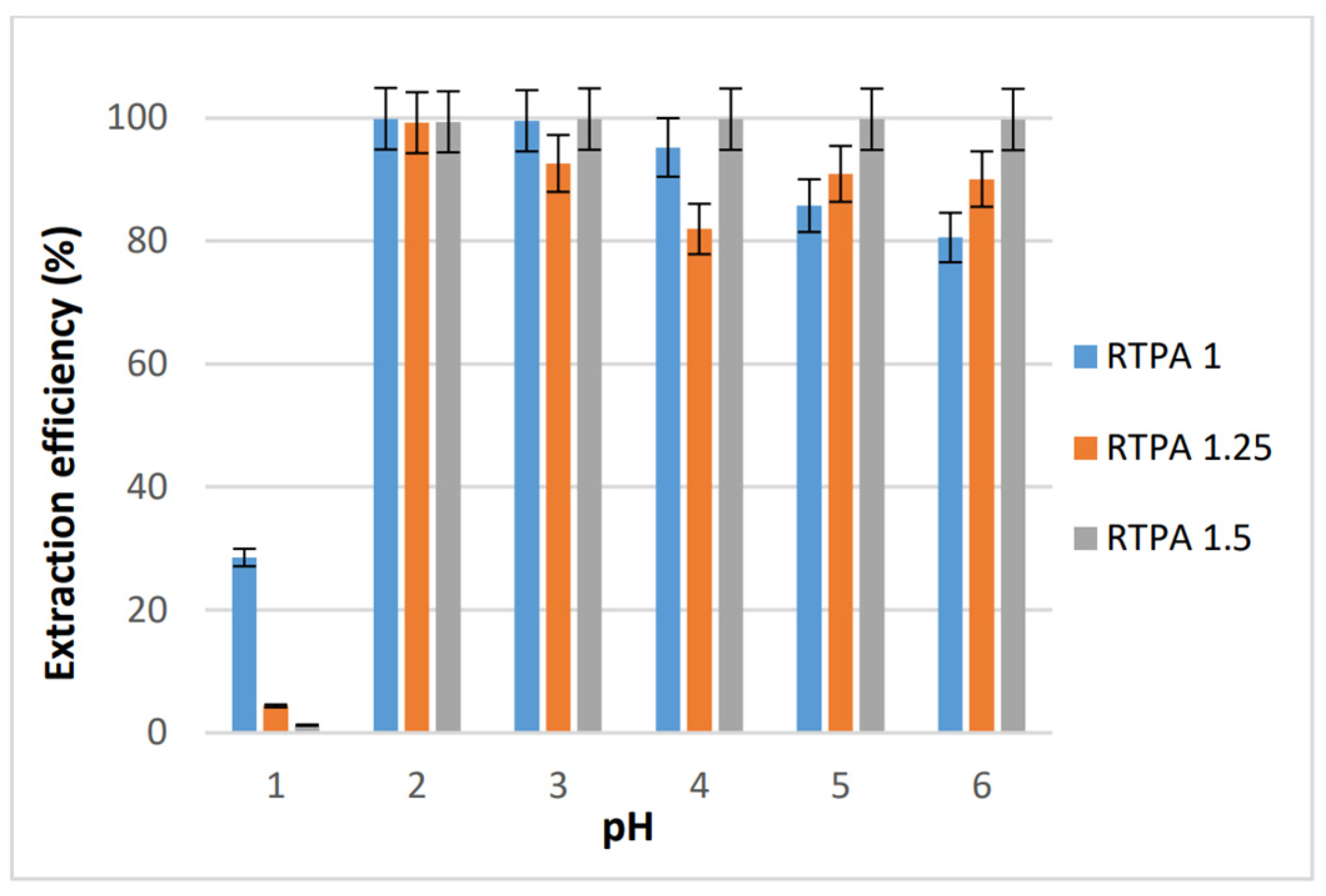
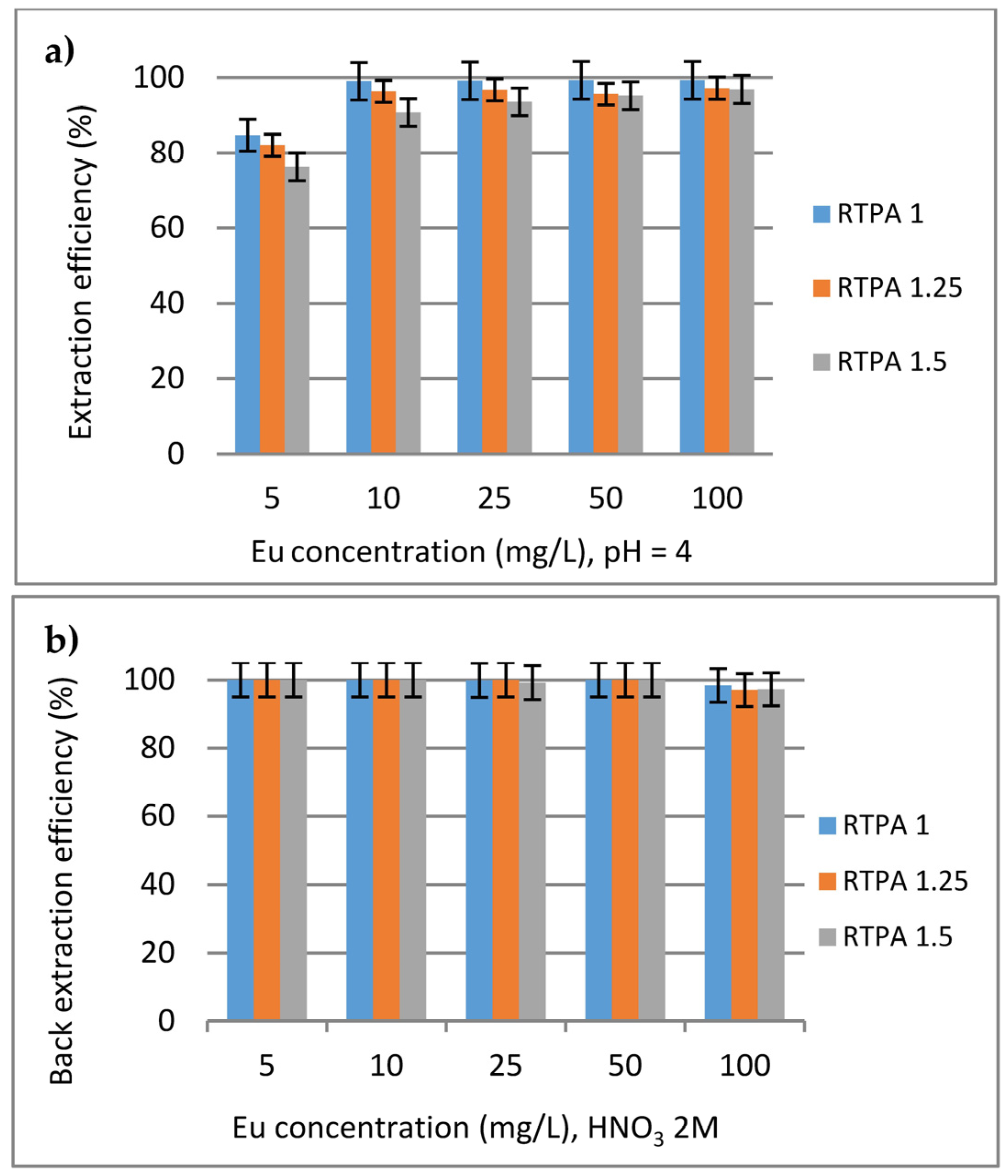
3.2.1. Extraction and Desorption Experiments
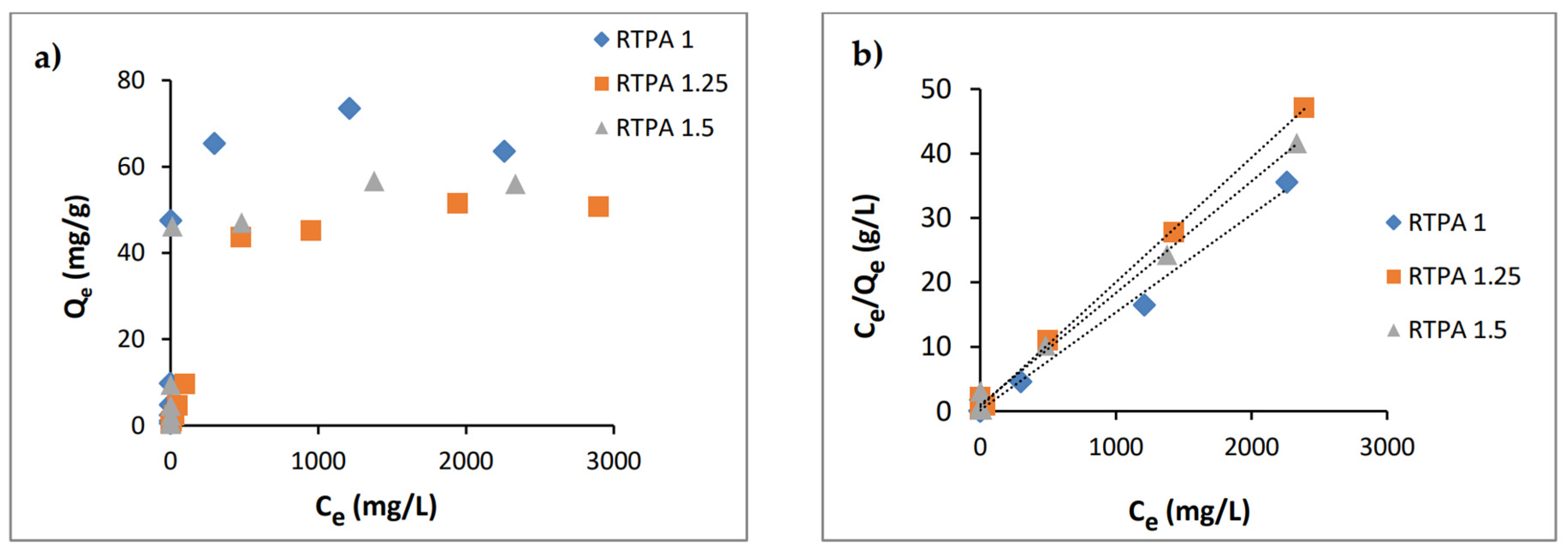
3.2.2. Adsorption Isotherms
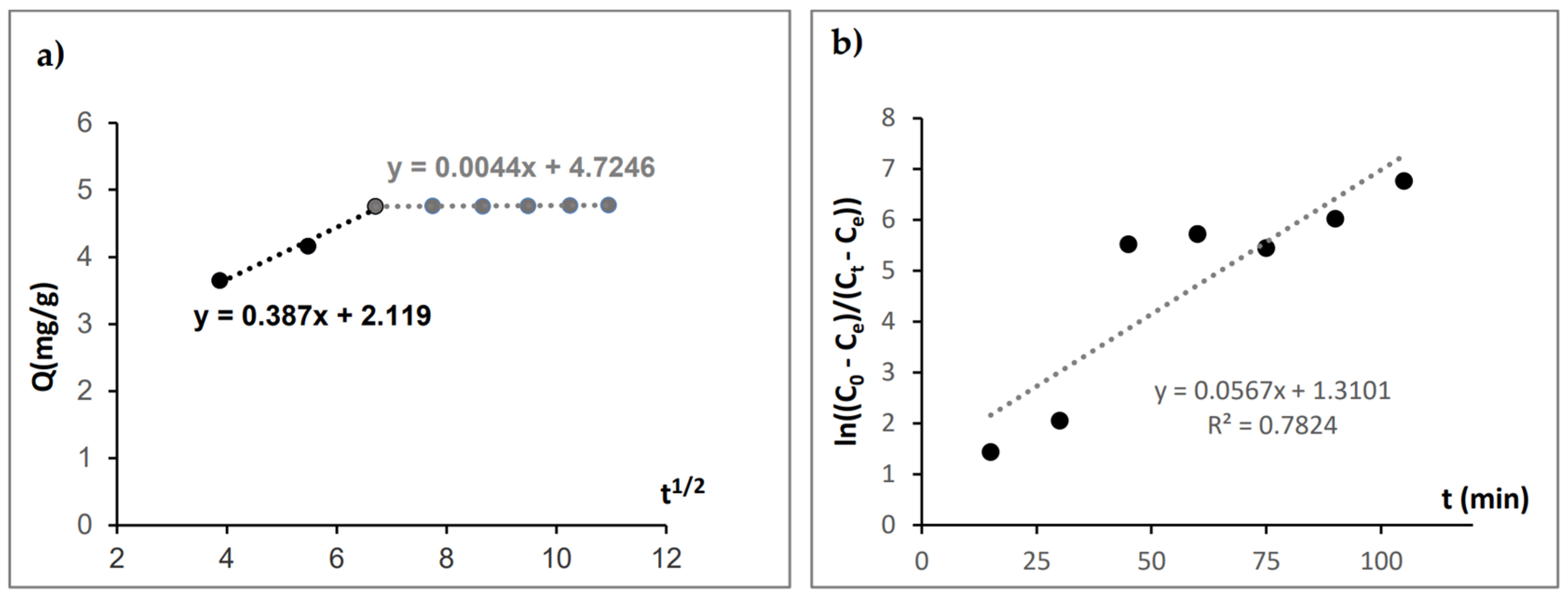
3.2.3. Adsorption Kinetic Studies
3.2.4. Adsorption Mechanism Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reisman, D.; Weber, R.; Mckernan, J.; Northeim, C. Rare Earth Elements: A Review of Production, Processing, Recycling and Associated Environmental Issues; United States Environmental Protection Agency: Washington, DC, USA, 2013.
- Blengini, G.A.; EL Latunussa, C.; Eynard, U.; Torres de Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the Review of the List of Critical Raw Materials; European Commission: Brussels, Belgium, 2020; ISBN 978-92-76-21049-8. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths: A Critical Review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Li, X.Z.; Sun, Y.P. Progress in Solid-Liquid Extraction Resin for Separation of Rare Earth Elements. J. Rare Earths 2005, 23, 581–592. [Google Scholar]
- Rychkov, V.; Kirillov, E.; Kirillov, S.; Bunkov, G.; Botalov, M.; Semenishchev, V.; Smyshlyaev, D.; Malyshev, A.; Taukin, A.; Akcil, A. Rare Earth Element Preconcentration from Various Primary and Secondary Sources by Polymeric Ion Exchange Resins. Sep. Purif. Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Page, M.J.; Soldenhoff, K.; Ogden, M.D. Comparative Study of the Application of Chelating Resins for Rare Earth Recovery. Hydrometallurgy 2017, 169, 275–281. [Google Scholar] [CrossRef]
- Gupta, N.K.; Choudhary, B.C.; Gupta, A.; Achary, S.N.; Sengupta, A. Graphene-Based Adsorbents for the Separation of f-Metals from Waste Solutions: A Review. J. Mol. Liq. 2019, 289, 111121. [Google Scholar] [CrossRef]
- Parrish, J.R. New Chelating Resins. Chem. Ind. 1955, 14, 386–387. [Google Scholar]
- Pennington, L.; Williams, M. Chelating Ion Exchange Resins. Ind. Eng. Chem. 1959, 51, 759–762. [Google Scholar] [CrossRef]
- Tarase, M.V.; Zade, A.B.; Gurnule, W.B. Resin I: Synthesis, Characterization, and Ion-Exchange Properties of Terpolymer Resins Derived from 2,4-Dihydroxypropiophenone, Biuret, and Formaldehyde. J. Appl. Polym. Sci. 2008, 108, 738–746. [Google Scholar] [CrossRef]
- Azarudeen, R.S.; Ahamed, M.A.R.; Burkanudeen, A.R. Chelating Terpolymer Resin: Synthesis, Characterization and Its Ion-Exchange Properties. Desalination 2011, 268, 90–96. [Google Scholar] [CrossRef]
- Azarudeen, R.S.; Subha, R.; Jeyakumar, D.; Burkanudeen, A.R. Batch Separation Studies for the Removal of Heavy Metal Ions Using a Chelating Terpolymer: Synthesis, Characterization and Isotherm Models. Sep. Purif. Technol. 2013, 116, 366–377. [Google Scholar] [CrossRef]
- Bhatt, R.R.; Shah, B.A. Sorption Studies of Heavy Metal Ions by Salicylic Acid–Formaldehyde–Catechol Terpolymeric Resin: Isotherm, Kinetic and Thermodynamics. Arab. J. Chem. 2015, 8, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Arrambide Cruz, C.; Marie, S.; Arrachart, G.; Pellet-Rostaing, S. Selective Extraction and Separation of Germanium by Catechol Based Resins. Sep. Purif. Technol. 2018, 193, 214–219. [Google Scholar] [CrossRef]
- Ebraheem, K.A.K.; Mubarak, M.S.; Yassien, Z.J.; Khalili, F. Chelation Properties of Poly(8–Hydroxyquinoline 5,7–Diylmethylene) Crosslinked with Bisphenol—A Toward Lanthanum(III), Cerium(III), Neodimium(III), Samarium(III), and Gadolinium(III) Ions. Sep. Sci. Technol. 2000, 35, 2115–2125. [Google Scholar] [CrossRef]
- Draye, M.; Czerwinski, K.; Favre-Réguillon, A.; Foos, J.; Guy, A.; Lemaire, M. Selective Separation of Lanthanides with Phenolic Resins: Extraction Behavior and Thermal Stability. Sep. Sci. Technol. 2000, 35, 1117–1132. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Ahmad, A.; Khalili, F.I.; Mubarak, M.S. Chelation Properties of Some Phenolic-Formaldehyde Polymers Toward Some Trivalent Lanthanide Ions. Solvent Extr. Ion Exch. 2004, 22, 721–735. [Google Scholar] [CrossRef]
- Ameta, R.; Patel, V.; Joshi, J. Polychelates of Phenolic Ion-Exchange Resin: Synthesis and Characterization. Iran. Polym. J. 2007, 16, 615–625. [Google Scholar]
- Arrambide, C.; Arrachart, G.; Berthalon, S.; Wehbie, M.; Pellet-Rostaing, S. Extraction and Recovery of Rare Earths by Chelating Phenolic Copolymers Bearing Diglycolamic Acid or Diglycolamide Moieties. React. Funct. Polym. 2019, 142, 147–158. [Google Scholar] [CrossRef]
- Favre-Réguillon, A.; Dunjic, B.; Lemaire, M.; Chomel, R. Synthesis and Evaluation of Resorcinol-Based Ion-Exchange Resins for the Selective Removal of Cesium. Solvent Extr. Ion Exch. 2001, 19, 181–191. [Google Scholar] [CrossRef]
- Banerjee, D.; Rao, M.; Wattal, P. Separation and Recovery of Cs from High Active Waste Simulant Using Resorcinol Formaldehyde Polycondensate Resin: Batch and Column Studies. Sep. Sci. Technol. 2013, 48, 133–139. [Google Scholar] [CrossRef]
- Arrachart, G.; Kenaan, A.; Gracia, S.; Turgis, R.; Dubois, V.; Pellet-Rostaing, S. Design and Evaluation of Chelating Resins through EDTA- and DTPA-Modified Ligands. Sep. Sci. Technol. 2015, 50, 1882–1889. [Google Scholar] [CrossRef]
- Palamarchuk, M.; Egorin, A.; Tokar, E.; Tutov, M.; Marinin, D.; Avramenko, V. Decontamination of Spent Ion-Exchangers Contaminated with Cesium Radionuclides Using Resorcinol-Formaldehyde Resins. J. Hazard. Mater. 2017, 321, 326–334. [Google Scholar] [CrossRef]
- Foyer, G.; Chanfi, B.H.; Boutevin, B.; Caillol, S.; David, G. New Method for the Synthesis of Formaldehyde-Free Phenolic Resins from Lignin-Based Aldehyde Precursors. Eur. Polym. J. 2016, 74, 296–309. [Google Scholar] [CrossRef]
- Foyer, G.; Chanfi, B.-H.; Virieux, D.; David, G.; Caillol, S. Aromatic Dialdehyde Precursors from Lignin Derivatives for the Synthesis of Formaldehyde-Free and High Char Yield Phenolic Resins. Eur. Polym. J. 2016, 77, 65–74. [Google Scholar] [CrossRef]
- Granado, L.; Tavernier, R.; Foyer, G.; David, G.; Caillol, S. Comparative Curing Kinetics Study of High Char Yield Formaldehyde- and Terephthalaldehyde-Phenolic Thermosets. Thermochim. Acta 2018, 667, 42–49. [Google Scholar] [CrossRef]
- Granado, L.; Tavernier, R.; Foyer, G.; David, G.; Caillol, S. Catalysis for Highly Thermostable Phenol-Terephthalaldehyde Polymer Networks. Chem. Eng. J. 2020, 379, 122237. [Google Scholar] [CrossRef]
- Durairaj, R.B. Resorcinol Based Resins and Applications. In Resorcinol, Chemistry, Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2005; pp. 178–261. ISBN 978-3-540-28090-3. [Google Scholar]
- Granado, L.; Tavernier, R.; Henry, S.; Auke, R.O.; Foyer, G.; David, G.; Caillol, S. Toward Sustainable Phenolic Thermosets with High Thermal Performances. ACS Sustain. Chem. Eng. 2019, 7, 7209–7217. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of Thermodynamic Parameters from Langmuir Isotherm Constant-Revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Yuh-Shan, H. Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; McKay, G. Sorption Kinetics for the Removal of Copper and Zinc from Effluents Using Bone Char. Sep. Purif. Technol. 2000, 19, 55–64. [Google Scholar] [CrossRef]
- Trieu, Q.A.; Pellet-Rostaing, S.; Arrachart, G.; Traore, Y.; Kimbel, S.; Daniele, S. Interfacial Study of Surface-Modified ZrO2 Nanoparticles with Thioctic Acid for the Selective Recovery of Palladium and Gold from Electronic Industrial Wastewater. Sep. Purif. Technol. 2020, 237, 116353. [Google Scholar] [CrossRef]
- Das, A.; Chandrakumar, K.R.S.; Paul, B.; Chopade, S.M.; Majumdar, S.; Singh, A.K.; Kain, V. Enhanced Adsorption and Separation of Zirconium and Hafnium under Mild Conditions by Phosphoric Acid Based Ligand Functionalized Silica Gels: Insights from Experimental and Theoretical Investigations. Sep. Purif. Technol. 2020, 239, 116518. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of Sorption Kinetics: The Pseudo-Second Order Equation and the Sorbate Intraparticle Diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, Kinetics, Mechanism, and Process Design for the Sorption of Methylene Blue onto Rice Husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Morris, C.J. Advance in Water Pollution Research: Removal of Biological Resistant Pollutions from Wastewater by Adsorption. In Proceedings of the 1st International Conference on Water Pollution Research, London, UK, 27–28 September 1962. [Google Scholar]
- Obradovic, B. Adsorption Kinetics Modeling. Hem. Ind. 2020, 74, 65–70. [Google Scholar] [CrossRef]
- Spahn, H.; Schlünder, E.U. The Scale-up of Activated Carbon Columns for Water Purification, Based on Results from Batch Tests—I: Theoretical and Experimental Determination of Adsorption Rates of Single Organic Solutes in Batch Tests. Chem. Eng. Sci. 1975, 30, 529–537. [Google Scholar] [CrossRef]
- Jackson, W.M.; Conley, R.T. High Temperature Oxidative Degradation of Phenol–Formaldehyde Polycondensates. J. Appl. Polym. Sci. 1964, 8, 2163–2193. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The Pyrolysis Mechanism of Phenol Formaldehyde Resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Belaid, K.; Kacha, S. Étude cinétique et thermodynamique de l’adsorption d’un colorant basique sur la sciure de bois. Rev. Sci. L’Eau/J. Water Sci. 2011, 24, 131–144. [Google Scholar] [CrossRef] [Green Version]
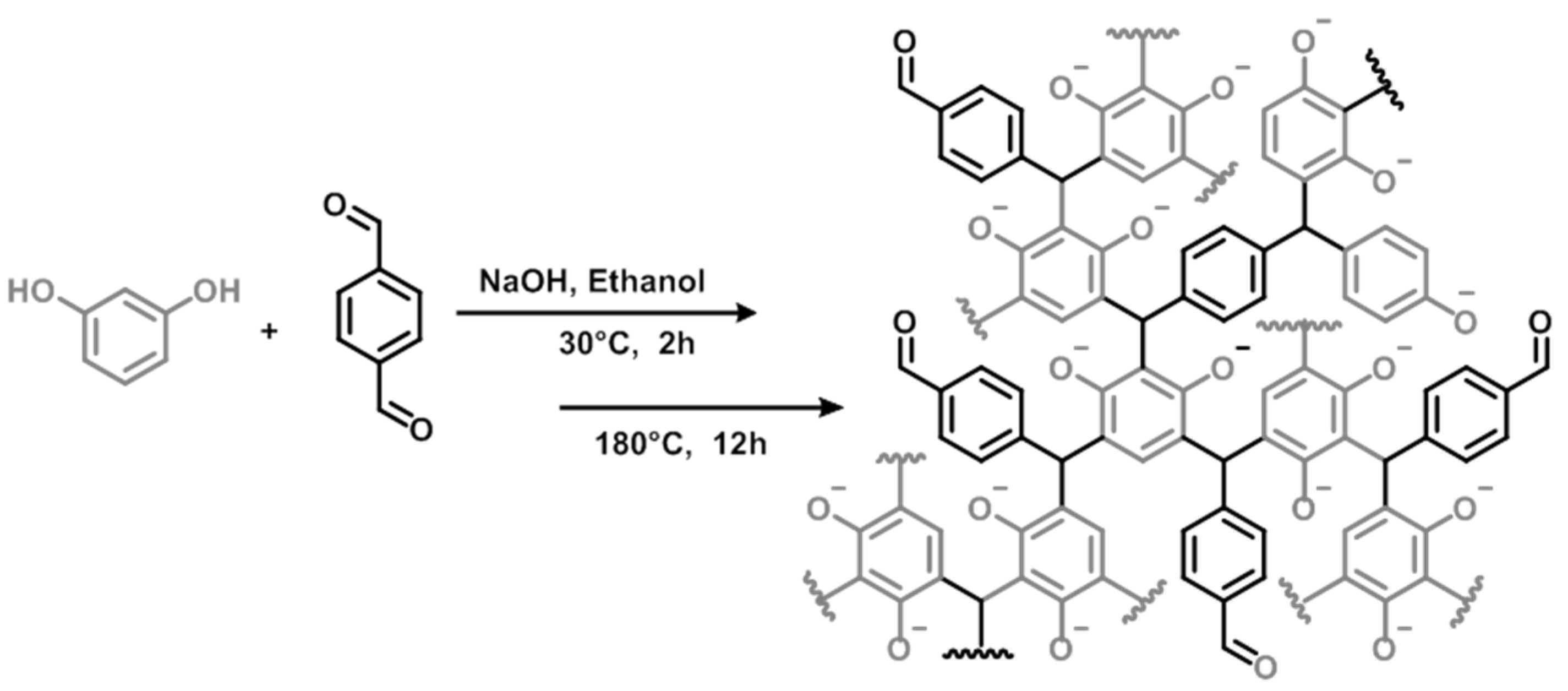
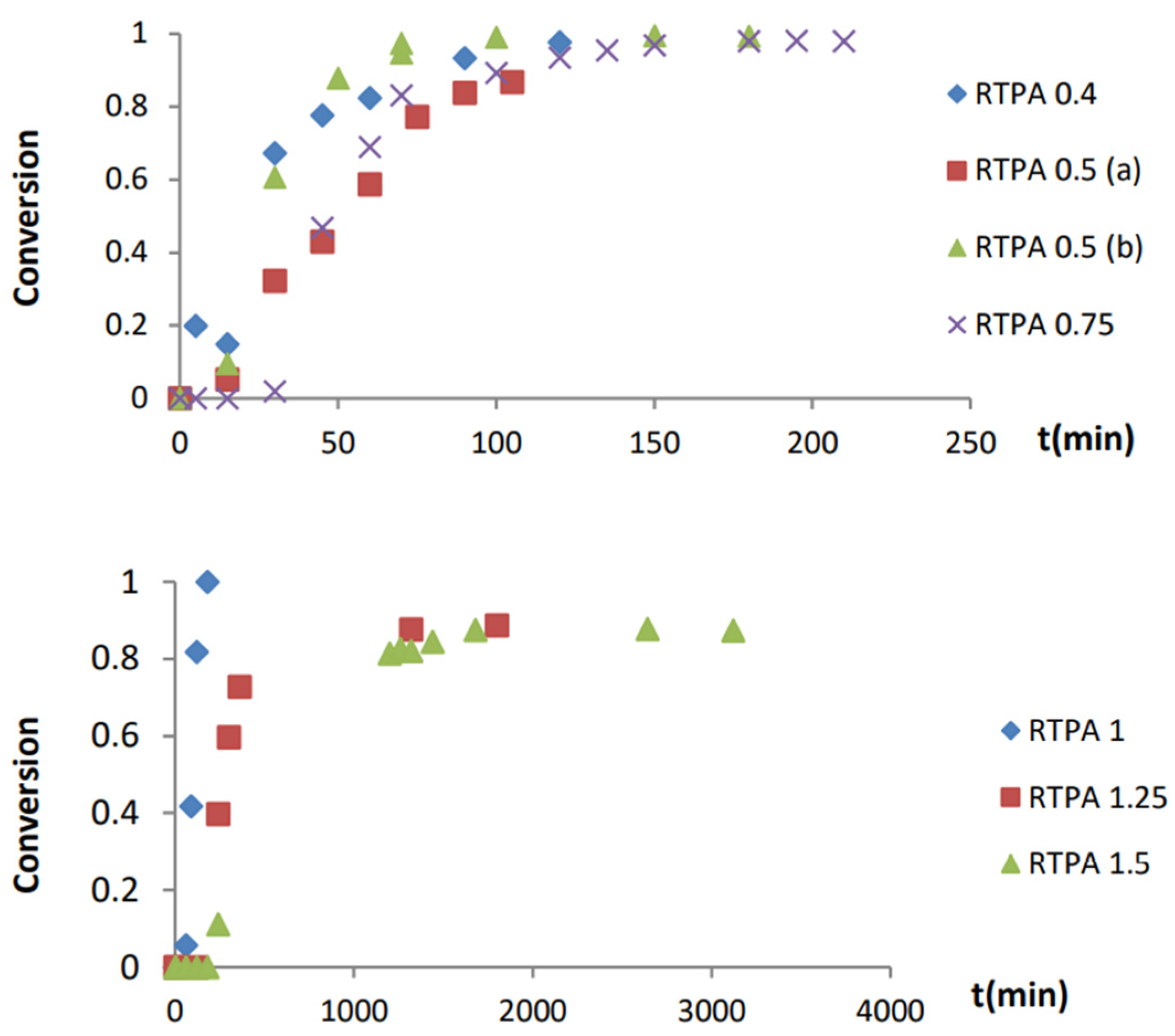
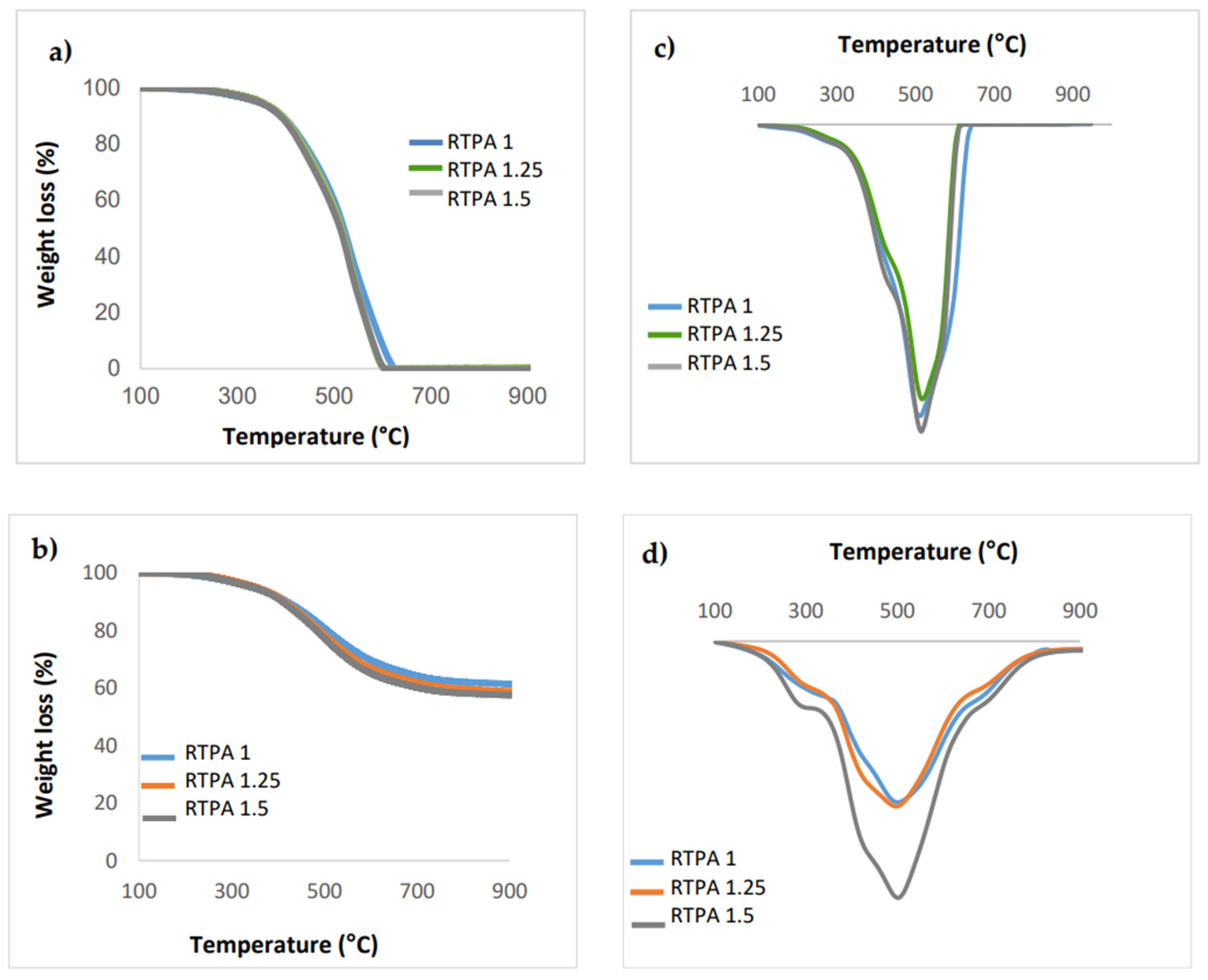
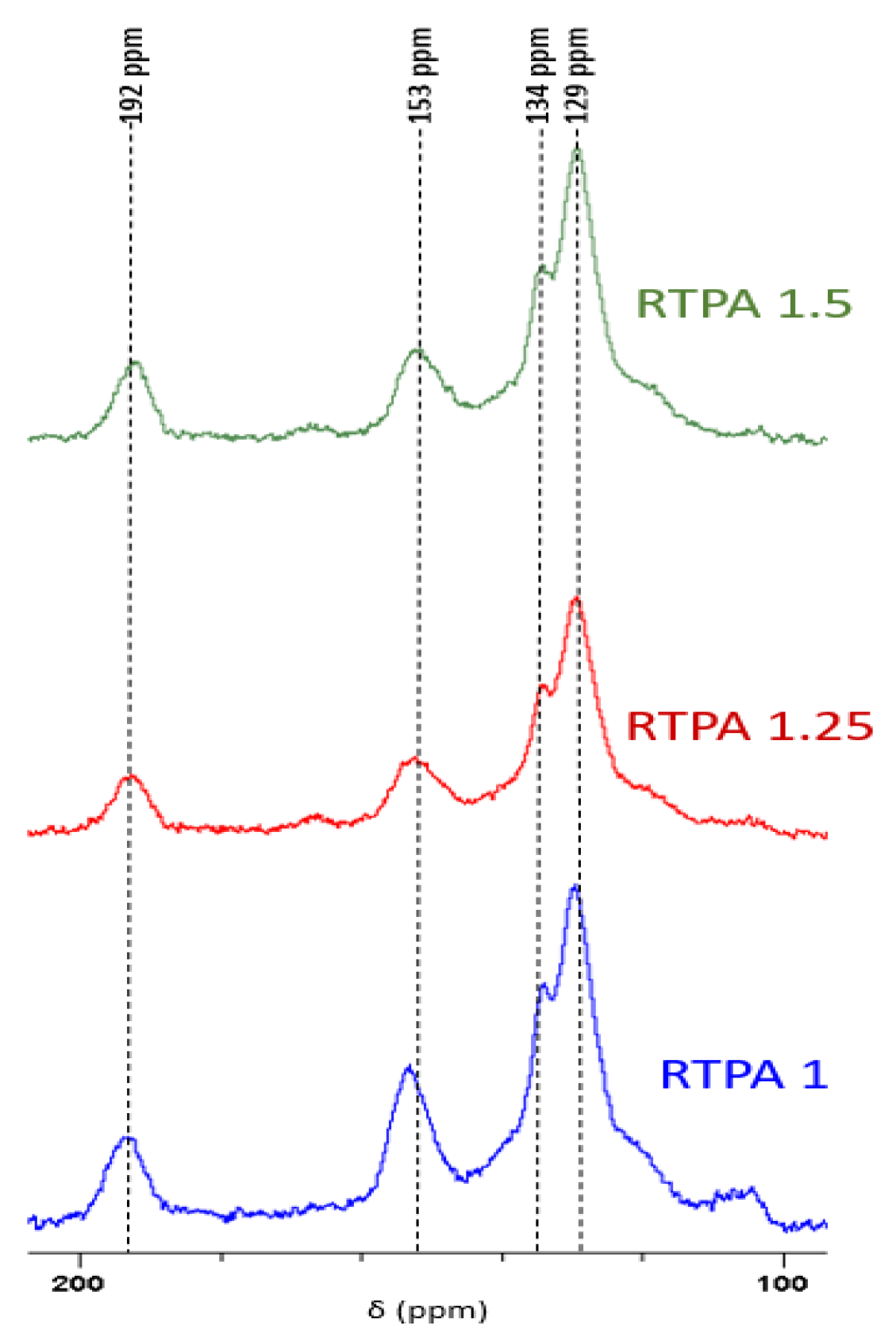

| Resin | Molar Ratio Resorcinol/TPA | T (°C) | Conversion (95%) |
|---|---|---|---|
| RTPA 0.4 | 1:0.4 | 30 | 2 h |
| RTPA 0.5 (a) | 1:0.5 | 30 | 2 h |
| RTPA 0.5 (b) | 1:0.5 | 40 | ≥1 h |
| RTPA 0.75 | 1:0.75 | 40 | 2 h |
| RTPA 1 | 1:1 | 40 | 5 h |
| RTPA 1.25 | 1:1.25 | 40 | ≤10 h |
| RTPA 1.5 | 1:1.5 | 40 | ≤50 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oye Auke, R.; Arrachart, G.; Tavernier, R.; David, G.; Pellet-Rostaing, S. Terephthalaldehyde–Phenolic Resins as a Solid-Phase Extraction System for the Recovery of Rare-Earth Elements. Polymers 2022, 14, 311. https://doi.org/10.3390/polym14020311
Oye Auke R, Arrachart G, Tavernier R, David G, Pellet-Rostaing S. Terephthalaldehyde–Phenolic Resins as a Solid-Phase Extraction System for the Recovery of Rare-Earth Elements. Polymers. 2022; 14(2):311. https://doi.org/10.3390/polym14020311
Chicago/Turabian StyleOye Auke, Ruth, Guilhem Arrachart, Romain Tavernier, Ghislain David, and Stéphane Pellet-Rostaing. 2022. "Terephthalaldehyde–Phenolic Resins as a Solid-Phase Extraction System for the Recovery of Rare-Earth Elements" Polymers 14, no. 2: 311. https://doi.org/10.3390/polym14020311
APA StyleOye Auke, R., Arrachart, G., Tavernier, R., David, G., & Pellet-Rostaing, S. (2022). Terephthalaldehyde–Phenolic Resins as a Solid-Phase Extraction System for the Recovery of Rare-Earth Elements. Polymers, 14(2), 311. https://doi.org/10.3390/polym14020311







