Thermal and Rheological Properties of Gluten-Free, Starch-Based Model Systems Modified by Hydrocolloids
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of Model Systems
2.2. Water Content of Model System Ingredients
2.3. Starch Suspensions Preparation
2.4. Model Systems Preparation for DSC and Rheological Analysis
2.5. DSC of Starch and Model System Suspensions
2.6. Rheology of Starch and Model System Suspensions
2.7. The Size and Molecular Weight of Starch and Hydrocolloids
2.8. Data Analysis
3. Results and Discussion
3.1. Starch Gelatinization
3.2. Effects of Hydrocolloids on a Gelatinization Behavior of Starches
3.3. Effects of Hydrocolloids on Rheological Properties of Starch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houben, A.; Höchstötter, A.; Becker, T. Possibilities to Increase the Quality in Gluten-Free Bread Production: An Overview. Eur. Food Res. Technol. 2012, 235, 195–208. [Google Scholar]
- Capriles, V.D.; Arêas, J.A.G. Novel Approaches in Gluten-Free Breadmaking: Interface between Food Science, Nutrition, and Health. Compr. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Anton, A.A.; Artfield, S.D. Hydrocolloids in Gluten-Free Breads: A Review. Int. J. Food Sci. Nutr. 2008, 59, 11–23. [Google Scholar]
- Padalino, L.; Conte, A.; Nobile, M.A. Del Overview on the General Approaches to Improve Gluten-Free Pasta and Bread. Foods 2016, 5, 87. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Application of Hydrocolloids as Baking Improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar]
- Mir, S.A.; Shah, M.A.; Naik, H.R.; Zargar, I.A. Influence of Hydrocolloids on Dough Handling and Technological Properties of Gluten-Free Breads. Trends Food Sci. Technol. 2016, 51, 49–57. [Google Scholar]
- Mollakhalili Meybodi, N.; Mohammadifar, M.A.; Feizollahi, E. Gluten-Free Bread Quality: A Review of the Improving Factors. J. Food Qual. Hazards Control 2015, 2, 81–85. [Google Scholar]
- Šmídová, Z.; Rysová, J. Gluten-Free Bread and Bakery Products Technology. Foods 2022, 11, 480. [Google Scholar]
- Goff, H.D.; Guo, Q. The Role of Hydrocolloids in the Development of Food Structure. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2020; Chapter 1; Volume 2020, pp. 3–28. ISBN 9781782629221. [Google Scholar]
- Kroll, J.E. Dickinson: An Introduction to Food Colloids. 207 Seiten, Zahlr. Abb. Oxford University Press, Oxford, New York, Tokyo 1992. Preis: 13,95 £. Food/Nahrung 1992, 36, 514. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar]
- Gao, Z.; Fang, Y.; Cao, Y.; Liao, H.; Nishinari, K.; Phillips, G.O. Hydrocolloid-Food Component Interactions. Food Hydrocoll. 2017, 68, 149–156. [Google Scholar] [CrossRef]
- Mahmoud, R.M.; Yousif, E.I.; Gadallah, M.G.E.; Alawneh, A.R. Formulations and Quality Characterization of Gluten-Free Egyptian Balady Flat Bread. Ann. Agric. Sci. 2013, 58, 19–25. [Google Scholar] [CrossRef][Green Version]
- Sabanis, D.; Tzia, C. Effect of Hydrocolloids on Selected Properties of Gluten-Free Dough and Bread. Food Sci. Technol. Int. 2011, 17, 279–291. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding Gluten-Free Dough for Reaching Breads with Physical Quality and Nutritional Balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar]
- Zoghi, A.; Mirmahdi, R.S.; Mohammadi, M. The Role of Hydrocolloids in the Development of Gluten-Free Cereal-Based Products for Coeliac Patients: A Review. Int. J. Food Sci. Technol. 2021, 56, 3138–3147. [Google Scholar]
- Zhao, F.; Li, Y.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Gu, Z.; Li, Z. Co-Supported Hydrocolloids Improve the Structure and Texture Quality of Gluten-Free Bread. LWT 2021, 152, 112248. [Google Scholar] [CrossRef]
- Padalino, L.; Mastromatteo, M.; Sepielli, G.; Del Nobile, M.A. Formulation Optimization of Gluten-Free Functional Spaghetti Based on Maize Flour and Oat Bran Enriched in β-Glucans. Materials 2011, 4, 2119–2135. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A Review: Interaction of Starch/Non-Starch Hydrocolloid Blending and the Recent Food Applications. Food Biosci. 2017, 19, 110–120. [Google Scholar]
- Hager, A.S.; Arendt, E.K. Influence of Hydroxypropylmethylcellulose (HPMC), Xanthan Gum and Their Combination on Loaf Specific Volume, Crumb Hardness and Crumb Grain Characteristics of Gluten-Free Breads Based on Rice, Maize, Teff and Buckwheat. Food Hydrocoll. 2013, 32, 195–203. [Google Scholar] [CrossRef]
- Belorio, M.; Gómez, M. Effect of Hydration on Gluten-Free Breads Made with Hydroxypropyl Methylcellulose in Comparison with Psyllium and Xanthan Gum. Foods 2020, 9, 1548. [Google Scholar] [CrossRef]
- Crockett, R.; Ie, P.; Vodovotz, Y. How Do Xanthan and Hydroxypropyl Methylcellulose Individually Affect the Physicochemical Properties in a Model Gluten-Free Dough? J. Food Sci. 2011, 76, E274–E282. [Google Scholar] [CrossRef]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The Role of Hydrocolloids in Gluten-Free Bread and Pasta; Rheology, Characteristics, Staling and Glycemic Index. Foods 2021, 10, 3121. [Google Scholar]
- Rosell, C.M.; Yokoyama, W.; Shoemaker, C. Rheology of Different Hydrocolloids-Rice Starch Blends. Effect of Successive Heating-Cooling Cycles. Carbohydr. Polym. 2011, 84, 373–382. [Google Scholar] [CrossRef]
- Sivaramakrishnan, H.P.; Senge, B.; Chattopadhyay, P.K. Rheological Properties of Rice Dough for Making Rice Bread. J. Food Eng. 2004, 62, 37–45. [Google Scholar] [CrossRef]
- Morreale, F.; Garzón, R.; Rosell, C.M. Understanding the Role of Hydrocolloids Viscosity and Hydration in Developing Gluten-Free Bread. A Study with Hydroxypropylmethylcellulose. Food Hydrocoll. 2018, 77, 629–635. [Google Scholar] [CrossRef]
- Horvat, M.; Pannuri, A.; Romeo, T.; Dogsa, I.; Stopar, D. Viscoelastic Response of Escherichia Coli Biofilms to Genetically Altered Expression of Extracellular Matrix Components. Soft Matter 2019, 15, 5042–5051. [Google Scholar] [CrossRef]
- Benigar, E.; Zupančič Valant, A.; Dogsa, I.; Sretenovic, S.; Stopar, D.; Jamnik, A.; Tomšič, M. Structure and Dynamics of a Model Polymer Mixture Mimicking a Levan-Based Bacterial Biofilm of Bacillus Subtilis. Langmuir 2016, 32, 8182–8194. [Google Scholar] [CrossRef]
- Stojković, B.; Sretenovic, S.; Dogsa, I.; Poberaj, I.; Stopar, D. Viscoelastic Properties of Levan-DNA Mixtures Important in Microbial Biofilm Formation as Determined by Micro- and Macrorheology. Biophys. J. 2015, 108, 758–765. [Google Scholar] [CrossRef]
- Carmona, J.A.; Ramírez, P.; Calero, N.; Muñoz, J. Large Amplitude Oscillatory Shear of Xanthan Gum Solutions. Effect of Sodium Chloride (NaCl) Concentration. J. Food Eng. 2014, 126, 165–172. [Google Scholar] [CrossRef]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The Rheology of Gelatin Hydrogels Modified by κ-Carrageenan. LWT 2015, 63, 612–619. [Google Scholar] [CrossRef]
- Ward, F.M.; Andon, S.A. Hydrocolloids as Film Formers, Adhesives, and Gelling Agents for Bakery and Cereal Products. Cereal Foods World 2002, 47, 52–55. [Google Scholar]
- Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch Characteristics Linked to Gluten-Free Products. Foods 2017, 6, 29. [Google Scholar]
- Miyazaki, M.; Van Hung, P.; Maeda, T.; Morita, N. Recent Advances in Application of Modified Starches for Breadmaking. Trends Food Sci. Technol. 2006, 17, 591–599. [Google Scholar]
- Hug-Iten, S.; Escher, F.; Conde-Petit, B. Structural Properties of Starch in Bread and Bread Model Systems: Influence of an Antistaling α-Amylase. Cereal Chem. 2001, 78, 421–428. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Carvalho, A.J.F. Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342. ISBN 9780080453163. [Google Scholar]
- Wang, S.; Copeland, L. Molecular Disassembly of Starch Granules during Gelatinization and Its Effect on Starch Digestibility: A Review. Food Funct. 2013, 4, 1564–1580. [Google Scholar]
- Kusunose, C.; Fujii, T.; Matsumoto, H. Role of Starch Granules in Controlling Expansion of Dough during Baking. Cereal Chem. 1999, 76, 920–924. [Google Scholar] [CrossRef]
- Borwankar, R.P. Food Texture and Rheology: A Tutorial Review. J. Food Eng. 1992, 16, 1–16. [Google Scholar] [CrossRef]
- Milas, M.; Rinaudo, M.; Tinland, B. The Viscosity Dependence on Concentration, Molecular Weight and Shear Rate of Xanthan Solutions. Polym. Bull. 1985, 14, 157–164. [Google Scholar] [CrossRef]
- Brunchi, C.E.; Morariu, S.; Bercea, M. Intrinsic Viscosity and Conformational Parameters of Xanthan in Aqueous Solutions: Salt Addition Effect. Colloids Surf. B Biointerfaces 2014, 122, 512–519. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of Heat-Moisture Treatment and Annealing in Starches: A Review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Schirmer, M.; Jekle, M.; Becker, T. Starch Gelatinization and Its Complexity for Analysis. Starch-Stärke 2015, 67, 30–41. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wang, L.J.; Zou, H.; Li, D.; Adhikari, B. Studies on the Starch-Water Interactions between Partially Gelatinized Corn Starch and Water during Gelatinization. Carbohydr. Polym. 2014, 101, 727–732. [Google Scholar] [CrossRef]
- Donovan, J.W. Phase Transitions of the Starch–Water System. Biopolymers 1979, 18, 263–275. [Google Scholar] [CrossRef]
- Evans, I.D.; Haisman, D.R. The Effect of Solutes on the Gelatinization Temperature Range of Potato Starch. Starch-Stärke 1982, 34, 224–231. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Non-Equilibrium Melting of Native Granular Starch: Part I. Temperature Location of the Glass Transition Associated with Gelatinization of A-Type Cereal Starches. Carbohydr. Polym. 1988, 8, 183–208. [Google Scholar] [CrossRef]
- Waigh, T.A.; Gidley, M.J.; Komanshek, B.U.; Donald, A.M. The Phase Transformations in Starch during Gelatinisation: A Liquid Crystalline Approach. Carbohydr. Res. 2000, 328, 165–176. [Google Scholar] [CrossRef]
- Tananuwong, K.; Reid, D.S. DSC and NMR Relaxation Studies of Starch-Water Interactions during Gelatinization. Carbohydr. Polym. 2004, 58, 345–358. [Google Scholar] [CrossRef]
- Justel, F.J.; Villca, G.; Jimenez, Y.P. Measurement and Modelling of Water Activity, Density, Sound Velocity, Refractive Index and Viscosity of the Na2MoO4 + Poly(Ethylene Glycol) + H2O System in the Temperature Range from 313.15 to 333.15 K. Fluid Phase Equilib. 2020, 518, 112628. [Google Scholar] [CrossRef]
- Spies, R.D.; Hoseney, R.C. Effect of Sugars on Starch Gelatinization. Cereal Chem. 1982, 59, 128–131. [Google Scholar]
- Singh, J.; Singh, N. Studies on the Morphological and Rheological Properties of Granular Cold Water Soluble Corn and Potato Starches. Food Hydrocoll. 2003, 17, 63–72. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Belz, M.C.E.; Heitmann, M.; Zannini, E.; Arendt, E.K. Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads. Foods 2016, 5, 30. [Google Scholar] [CrossRef]
- Bahaji, A.; Li, J.; Sánchez-López, Á.M.; Baroja-Fernández, E.; Muñoz, F.J.; Ovecka, M.; Almagro, G.; Montero, M.; Ezquer, I.; Etxeberria, E.; et al. Starch Biosynthesis, Its Regulation and Biotechnological Approaches to Improve Crop Yields. Biotechnol. Adv. 2014, 32, 87–106. [Google Scholar]
- Allan, M.C.; Rajwa, B.; Mauer, L.J. Effects of Sugars and Sugar Alcohols on the Gelatinization Temperature of Wheat Starch. Food Hydrocoll. 2018, 84, 593–607. [Google Scholar] [CrossRef]
- Brown, S.A.; French, D. Specific Adsorption of Starch Oligosaccharides in the Gel Phase of Starch Granules. Carbohydr. Res. 1977, 59, 203–212. [Google Scholar] [CrossRef]
- Bemiller, J.N. Pasting, Paste, and Gel Properties of Starch-Hydrocolloid Combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar]
- Zhang, Y.; Gu, Z.; Zhu, L.; Hong, Y. Comparative Study on the Interaction between Native Corn Starch and Different Hydrocolloids during Gelatinization. Int. J. Biol. Macromol. 2018, 116, 136–143. [Google Scholar] [CrossRef]
- Wilderjans, E.; Pareyt, B.; Goesaert, H.; Brijs, K.; Delcour, J.A. The Role of Gluten in a Pound Cake System: A Model Approach Based on Gluten-Starch Blends. Food Chem. 2008, 110, 909–915. [Google Scholar] [CrossRef]
- Bent, A.J. Functional Additives for Bakery Foods. Trends Food Sci. Technol. 1991, 2, 210. [Google Scholar] [CrossRef]
- Song, K.W.; Kim, Y.S.; Chang, G.S. Rheology of Concentrated Xanthan Gum Solutions: Steady Shear Flow Behavior. Fibers Polym. 2006, 7, 129–138. [Google Scholar] [CrossRef]
- Mezger, T.G. European Coatings Tech Files the Rheology Handboo; Vincentz Network: Hanover, Germany, 2014; ISBN 978-3-86630-890-9. [Google Scholar]
- Tadros, T.F. Rheology of Dispersions: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2010; ISBN 9783527320035. [Google Scholar]
- Montes, L.; Rosell, C.M.; Moreira, R. Rheological Properties of Corn Starch Gels with the Addition of Hydroxypropyl Methylcellulose of Different Viscosities. Front. Nutr. 2022, 9, 436. [Google Scholar] [CrossRef]
- Crawford, N.C.; Popp, L.B.; Johns, K.E.; Caire, L.M.; Peterson, B.N.; Liberatore, M.W. Shear Thickening of Corn Starch Suspensions: Does Concentration Matter? J. Colloid Interface Sci. 2013, 396, 83–89. [Google Scholar] [CrossRef]



| Sample Name * | m (Xanthan Gum)/g | m (HPMC)/g | m (Gluten)/g | m (Potato Starch)/g | m (Corn Starch)/g | m (Water)/g |
|---|---|---|---|---|---|---|
| S1 | / | / | / | 1.50 | / | 1.50 |
| S2 | 0.12 | 0.24 | / | 1.14 | / | 1.50 |
| S3 | / | / | / | / | 1.50 | 1.50 |
| S4 | 0.12 | 0.24 | / | / | 1.14 | 1.50 |
| S5 | / | / | / | 1.28 | 0.23 | 1.50 |
| S6 | 0.12 | 0.24 | / | 0.97 | 0.17 | 1.50 |
| S7 | / | 0.24 | / | 1.07 | 0.19 | 1.50 |
| S8 | 0.12 | / | / | 1.17 | 0.21 | 1.50 |
| S9 | / | / | 0.36 | 0.97 | 0.17 | 1.50 |
| S10 | / | 0.13 | / | / | / | 1.50 |
| S11 | 0.13 | / | / | / | / | 1.50 |
| S12 | / | / | 0.13 | / | / | 1.50 |
| 20% Corn Starch | 30% Corn Starch | 40% Corn Starch | 50% Corn Starch | 60% Corn Starch | |
|---|---|---|---|---|---|
| ΔHtr (J/g starch) | 22.3 ± 1.8 | 15.9 ± 1.3 | 14.9 ± 1.2 | 12.6 ± 1.0 | 10.4 ± 1.0 |
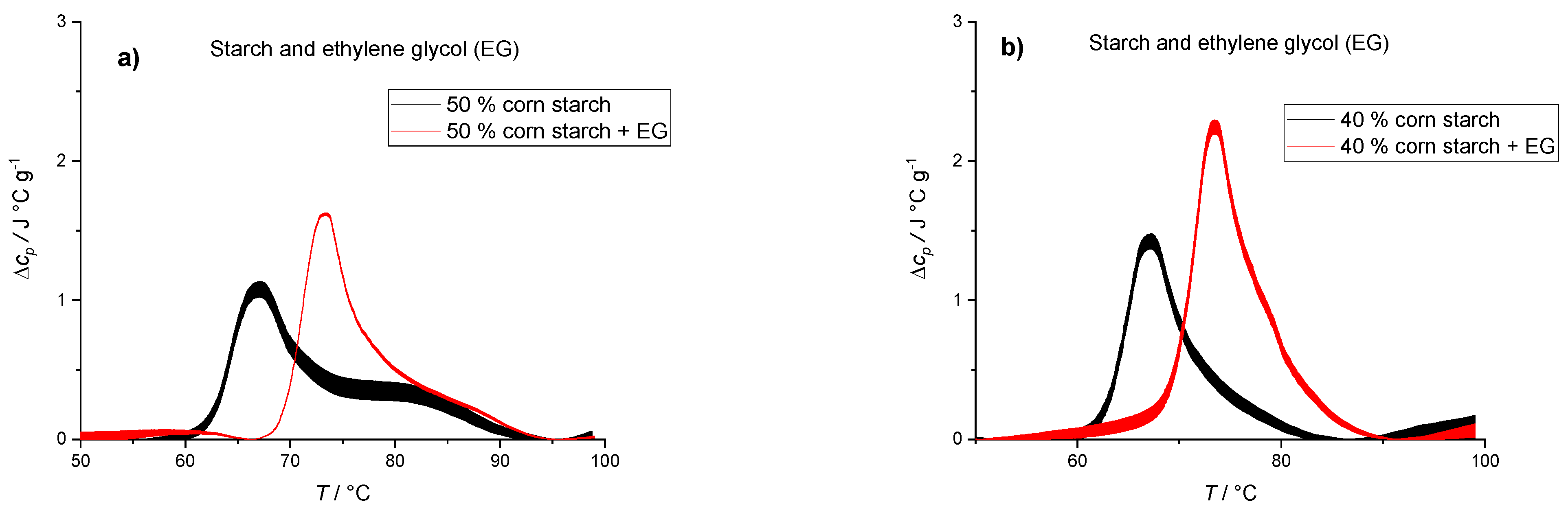
| 50% Corn Starch | 50% Corn Starch + EG | 40% Corn Starch | 40% Corn Starch + EG | |
|---|---|---|---|---|
| ΔHtr (J/g starch) | 12.6 ± 1.0 | 14.2 ± 0.6 | 14.9 ± 1.2 | 18.6 ± 1.5 |
| Ttr (°C) | 68.0 ± 0.5 | 73.0 ± 0.5 | 68.0 ± 0.5 | 74.0 ± 0.5 |
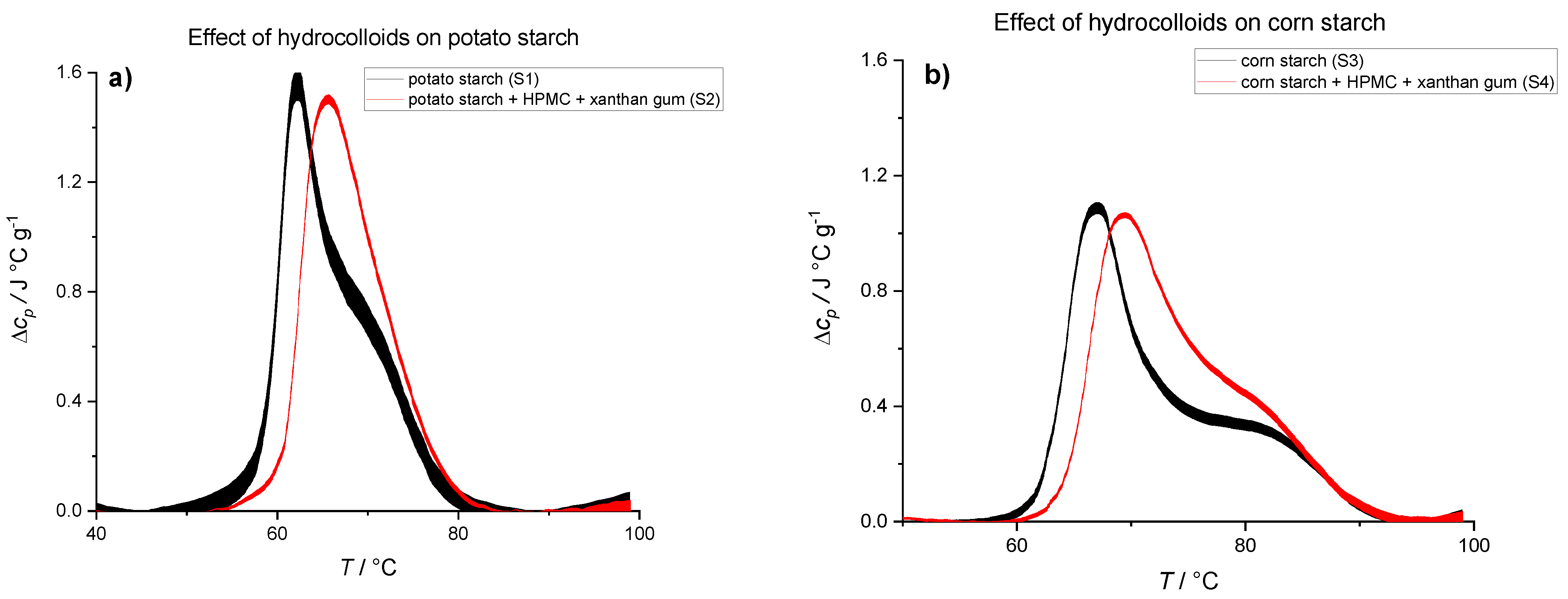
| Potato Starch (S1) | Potato Starch + HC Mixture (S2) | Corn Starch (S3) | Corn Starch + HC Mixture (S4) | |
|---|---|---|---|---|
| ΔHtr (J/g starch) | 15.7 ± 1.9 | 15.8 ± 0.5 | 12.8 ± 0.8 | 13.8 ± 1.0 |
| Ttr (°C) | 62.3 ± 0.5 | 66.1 ± 0.5 | 67.4 ± 0.5 | 70.2 ± 0.5 |
| Starch Mix (Potato:Corn = 85:15) (S5) | Starch Mix + HC Mixture (S6) | Starch Mix + % HPMC (S7) | Starch Mix + 4% Xanthan (S8) | Starch Mix + Gluten (S9) | |
|---|---|---|---|---|---|
| ΔHtr (J/g of starch) | 14.7 ± 1.0 | 15.4 ± 1.0 | 18.2 ± 1.5 | 15.0 ± 1.2 | 16.5 ± 1.3 |
| Ttr (°C) | 62.5 ± 0.5 | 68.4 ± 0.5 | 64.9 ± 0.5 | 64.8 ± 0.5 | 64.6 ± 0.5 |
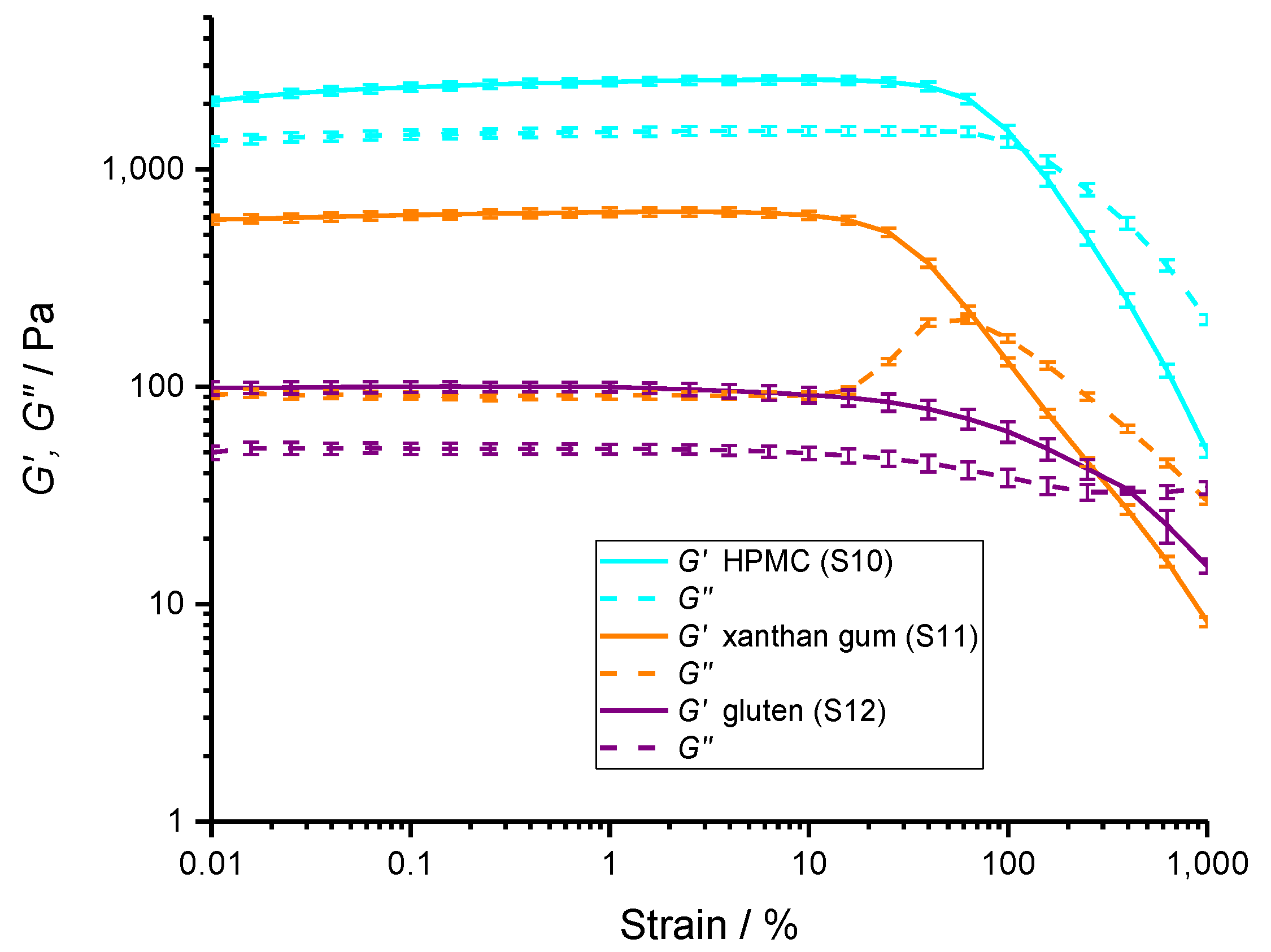
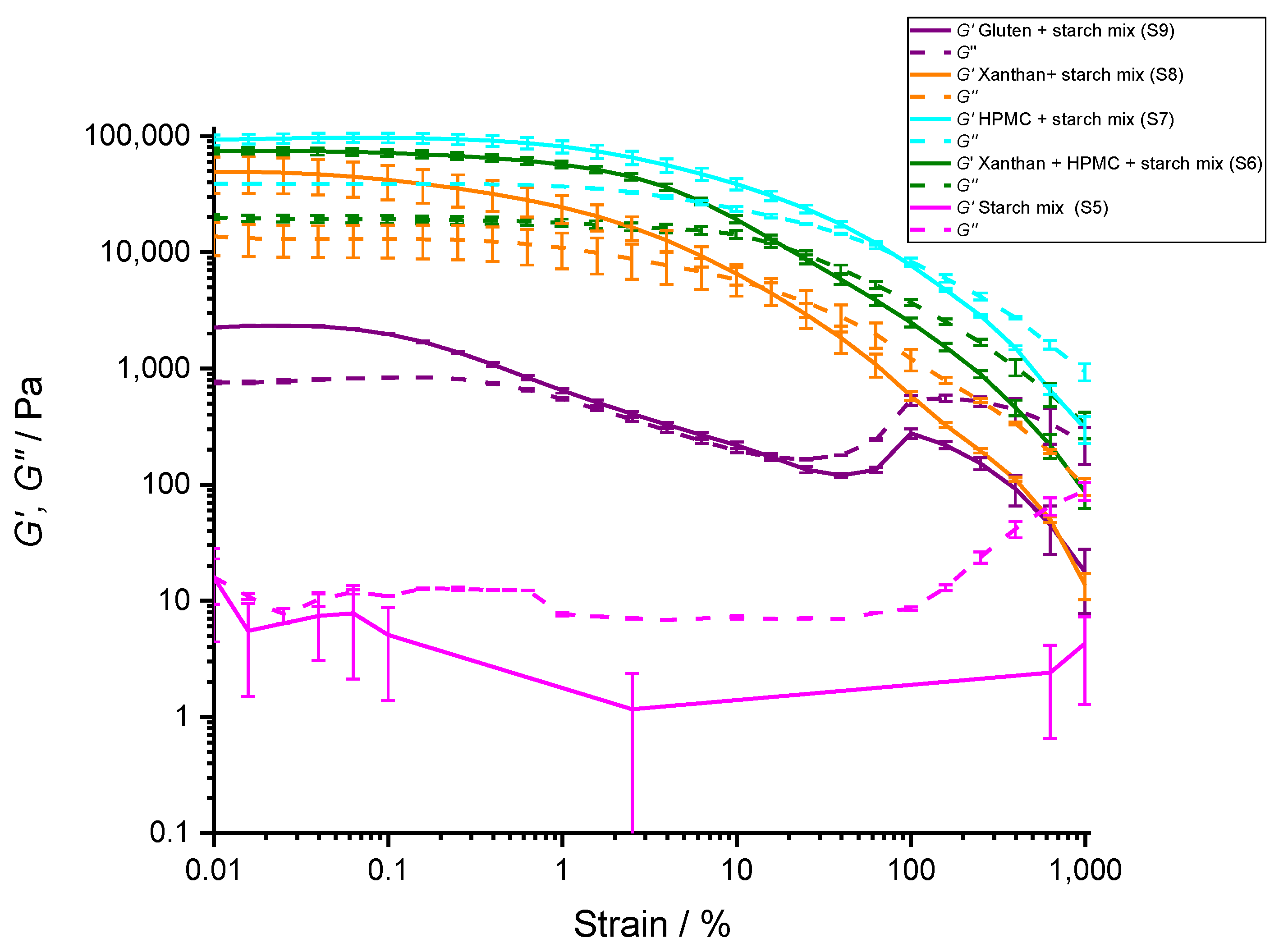
| G′ (KPa) (in LVE) | Loss Factor, Tan δ (in LVE) | LVE Critical Strain, γc at G′ (%) | Strain at Flow Transition Point (%) | Cohesive Energy Density, Ec (J m−3) | |
|---|---|---|---|---|---|
| 8% HPMC (S10) | 2.4 ± 0.1 | 0.61 ± 0.01 | 63 ± 12 | 130 ± 30 | 500 ± 100 |
| 8% Xanthan gum (S11) | 0.61 ± 0.03 | 0.15 ± 0.01 | 25± 5 | 80 ± 20 | 19 ± 6 |
| 8% gluten (S12) | 0.10 ± 0.01 | 0.52 ± 0.01 | 24 ± 14 | 500 ± 100 | 3 ± 2 |
| 8% HPMC + 42% starch mixture (S7) | 97 ± 9 | 0.40 ± 0.04 | 1.0 ± 0.1 | 80 ± 20 | 5 ± 1 |
| 4% Xanthan gum + 46% starch mixture (S8) | 50 ± 20 | 0.31 ± 0.01 | 0.16 ± 0.03 | 20 ± 5 | 0.06 ± 0.03 |
| 12% gluten + 38% starch mixture (S9) | 2.30 ± 0.05 | 0.42 ± 0.01 | 0.10 ± 0.03 | 20 ± 5 | 0.0011 ± 0.0005 |
| 8% HPMC + 4% Xanthan gum + 38% starch mixture (S6) | 74 ± 6 | 0.27 ± 0.01 | 0.39 ± 0.07 | 20 ± 5 | 0.6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megusar, P.; Stopar, D.; Poklar Ulrih, N.; Dogsa, I.; Prislan, I. Thermal and Rheological Properties of Gluten-Free, Starch-Based Model Systems Modified by Hydrocolloids. Polymers 2022, 14, 3242. https://doi.org/10.3390/polym14163242
Megusar P, Stopar D, Poklar Ulrih N, Dogsa I, Prislan I. Thermal and Rheological Properties of Gluten-Free, Starch-Based Model Systems Modified by Hydrocolloids. Polymers. 2022; 14(16):3242. https://doi.org/10.3390/polym14163242
Chicago/Turabian StyleMegusar, Polona, David Stopar, Natasa Poklar Ulrih, Iztok Dogsa, and Iztok Prislan. 2022. "Thermal and Rheological Properties of Gluten-Free, Starch-Based Model Systems Modified by Hydrocolloids" Polymers 14, no. 16: 3242. https://doi.org/10.3390/polym14163242
APA StyleMegusar, P., Stopar, D., Poklar Ulrih, N., Dogsa, I., & Prislan, I. (2022). Thermal and Rheological Properties of Gluten-Free, Starch-Based Model Systems Modified by Hydrocolloids. Polymers, 14(16), 3242. https://doi.org/10.3390/polym14163242







