Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material
Abstract
:1. Introduction
1.1. The Additives
1.2. Recycling
1.3. Challenge
2. Materials and Methods
2.1. Materials and Reagents
2.2. PET’s Coating with Chitosan
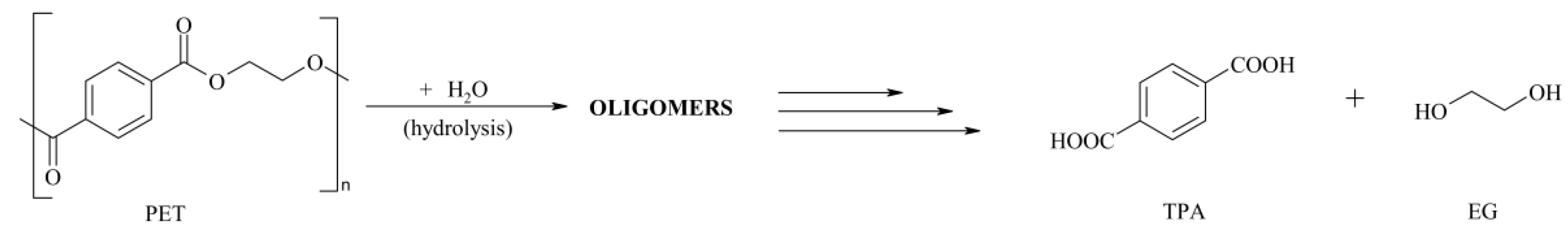
2.3. PET Hydrolysis
2.3.1. Neutral Hydrolysis
2.3.2. Alkaline Hydrolysis
2.4. Analytical Methods
2.5. Antimicrobial Testing
- -
- Preparation of microbiological media (TSB, NA, and PCA),
- -
- Production of buffer solutions (PBS) and cleavage of bacteria,
- -
- Incubation (at 37 °C and 25 °C),
- -
- Evaluation of antimicrobial effectiveness.
3. Results and Discussion
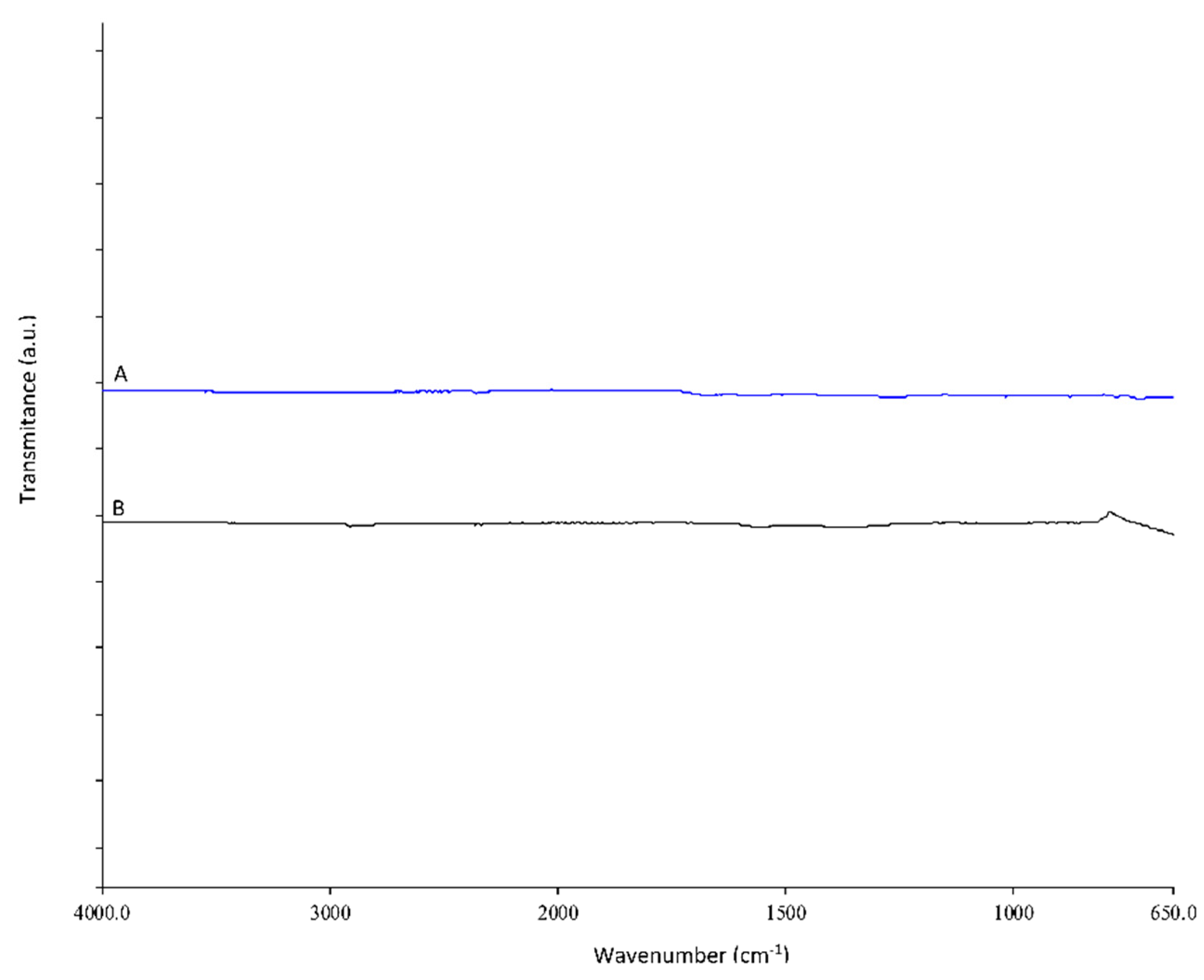
3.1. PET Coating and Its Active Concept Monitoring
3.2. Antimicrobial Testing
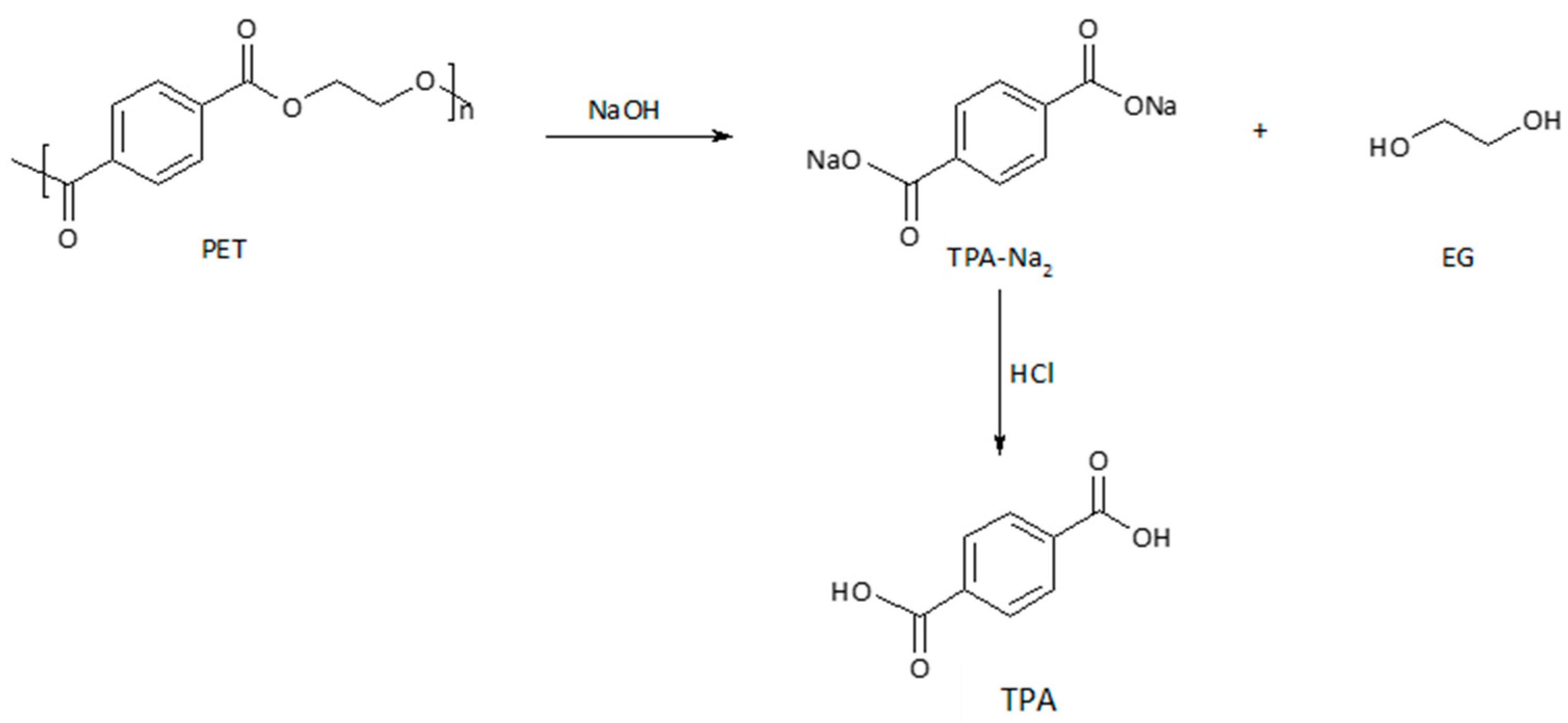
3.3. Chemical Recycling—Isolation of Secondary Raw Material TPA
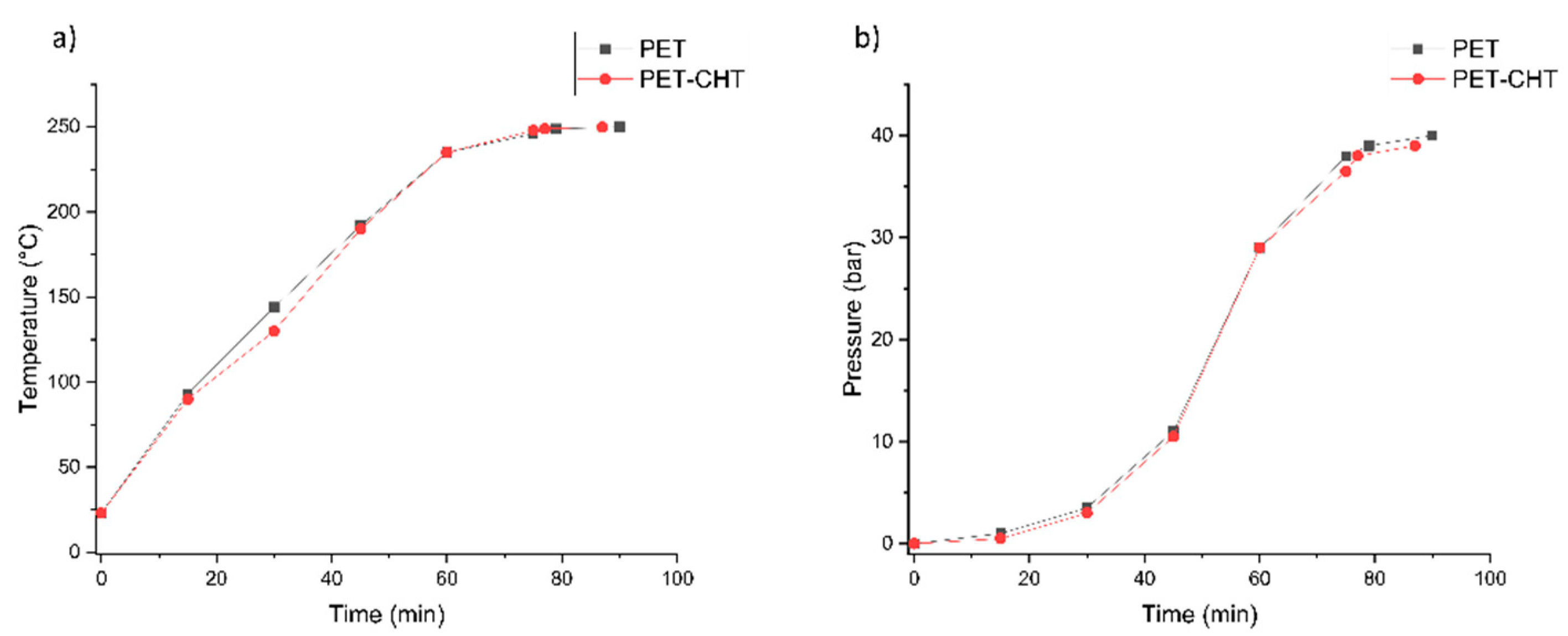
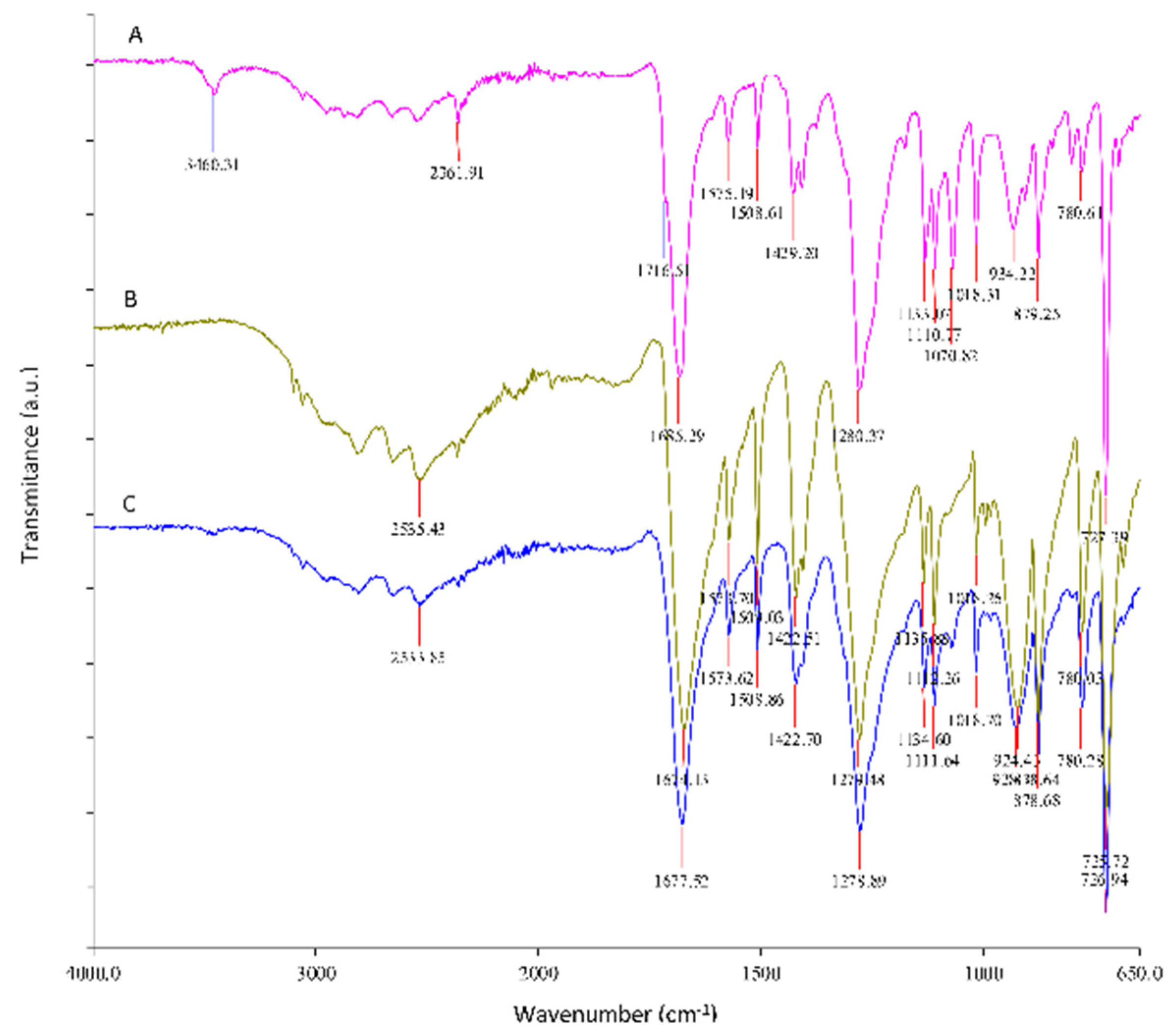
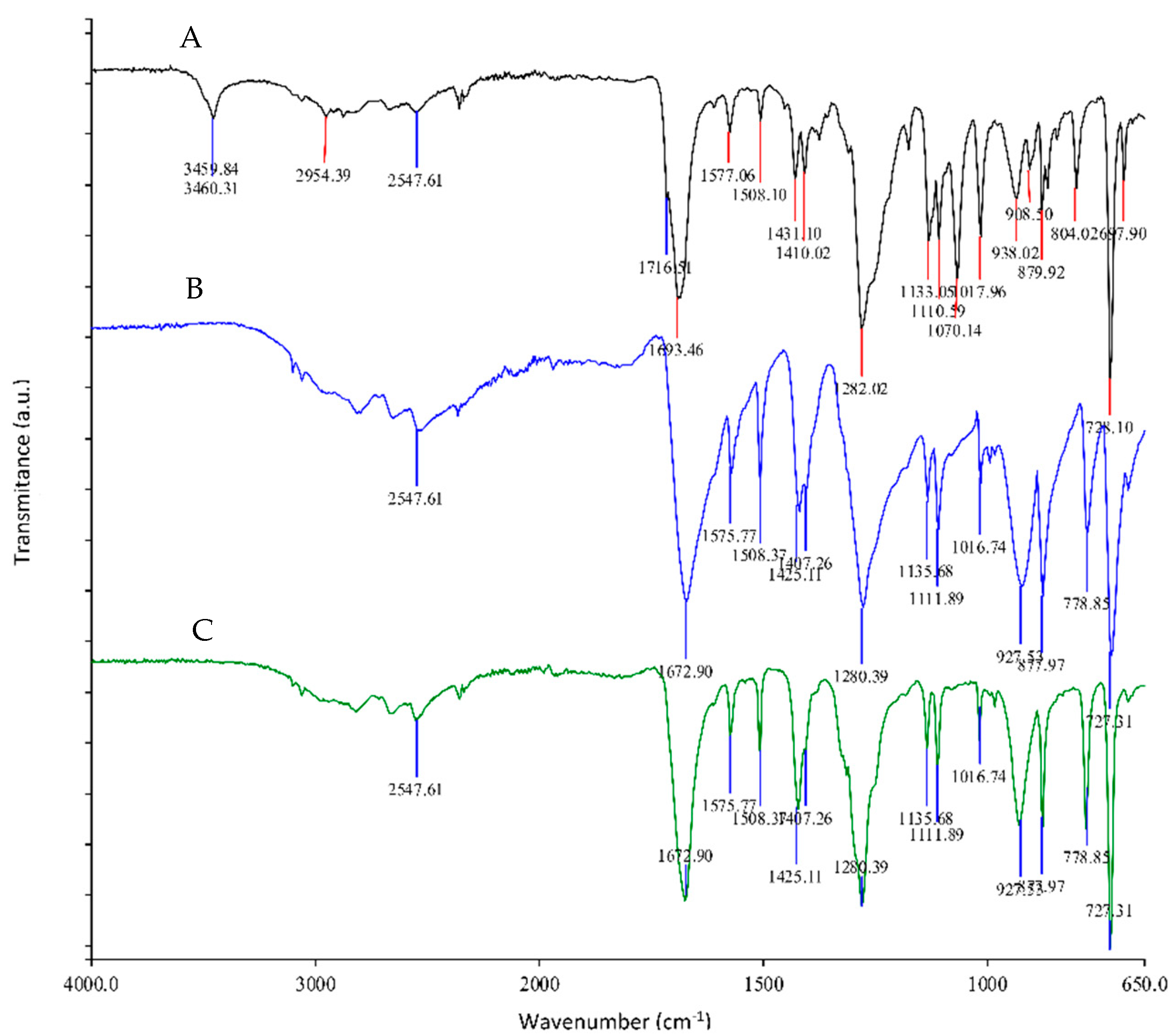
3.3.1. Neutral Hydrolysis Condition
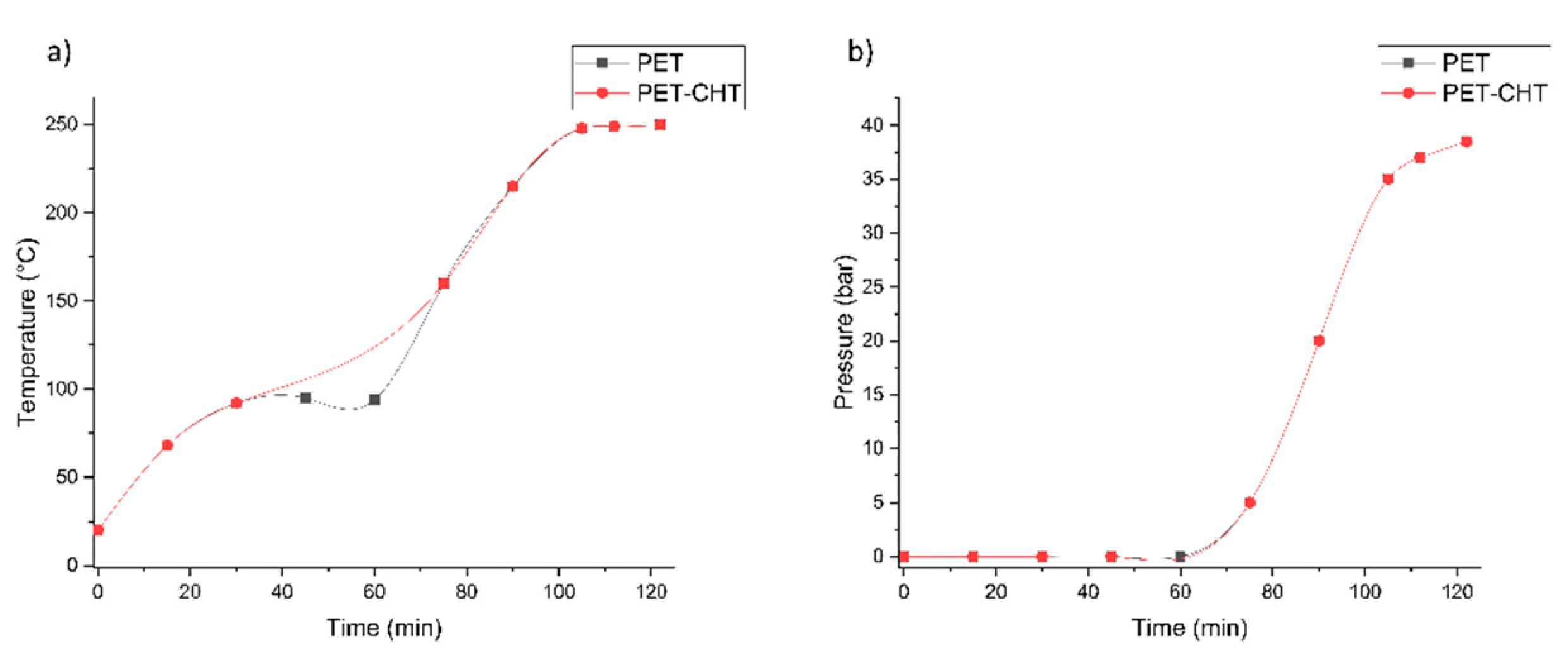
3.3.2. Alkaline Hydrolysis Conditions
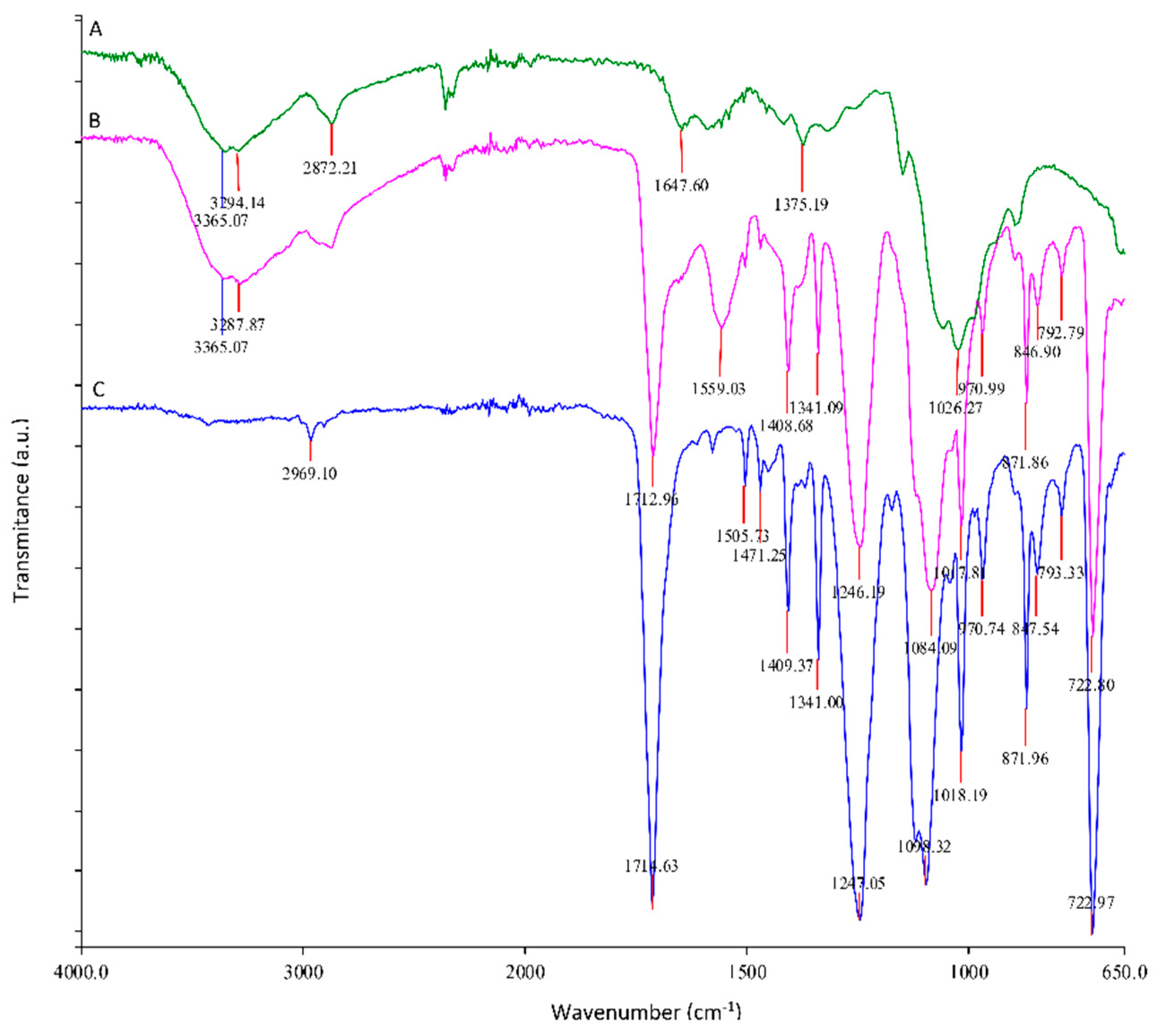
3.4. Characterization of Impurities
4. Comparison of the Classical TPA Synthesis Route with the Chemical Recycling of PET Food Packaging Waste and Isolation of TPA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, P.D.; Santos, T.R.; Duarte, A.C. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures; European Parliamentary Research Service (EPRS): Rue Wiertz, Belgium, 2020. [Google Scholar] [CrossRef]
- Heidbreder, L.M.; Bablok, I.; Drews, S.; Menzel, C. Tackling the plastic problem: A review on perceptions, behaviors, and interventions. Sci. Total Environ. 2019, 668, 1077–1093. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Ishigaki, T.; Sugano, W.; Nakanishi, A.; Tateda, M.; Ike, M.; Fujita, M. The degradability of biodegradable plastics in aerobic and anaerobic waste landfill model reactors. Chemosphere 2004, 54, 225–233. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S. Plastic Films in Food Packaging: Materials, Technology and Applications; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kochhar, A. Active Packaging in Food Industry: A Review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 plasma treatment on the deposition of chitosan onto polyethylene terephthalate (PET) surfaces. Int. J. Adhes. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Trends in Chitosan as a Primary Biopolymer for Functional Films and Coatings Manufacture for Food and Natural Products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Potrč, S.; Sterniša, M.; Smole Možina, S.; Knez Hrnčič, M.; Fras Zemljič, L. Bioactive Characterization of Packaging Foils Coated by Chitosan and Polyphenol Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 2610. [Google Scholar] [CrossRef]
- Souza, V.G.; Pires, J.R.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical Characterization of Packaging Foils Coated by Chitosan and Polyphenols Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Tkavc, T.; Vesel, A.; Šauperl, O. Chitosan coatings onto polyethylene terephthalate for the development of potential active packaging material. Appl. Surf. Sci. 2013, 265, 697–703. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Delva, L.; Karen; Kets, V.; Kuzmanović, M.; Demets, R.; Hubo, S.; Mys, N.; De Meester, S.; Ragaert, K. An Introductory Review Mechanical Recycling of Polymers for Dummies. Capture, Plastics Res 2019, 1–25. Available online: https://www.researchgate.net/publication/333390524 (accessed on 30 October 2021).
- Navarro, R.; Ferrándiz, S.; López, J.; Seguí, V.J. The influence of polyethylene in the mechanical recycling of polyethylene terephtalate. J. Mater. Process. Technol. 2008, 195, 110–116. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- EC 2023/2006; Commission Regulation (EC) No 282/2008 of 27 March 2008 on Recycled Plastic Materials and Articles Intended to Come into Contact with Foods and Amending Regulation (EC) No 2023/2006 (Text with EEA Relevance). European Commission: Brussels, Belgium, 2008.
- EC 1935/2004; Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. European Parliament, C.o.t.E.U.: Strasbourg, France, 2004.
- Muncke, J.; Backhaus, T.; Geueke, B.; Maffini, M.V.; Martin, O.V.; Myers, J.P.; Soto, A.M.; Trasande, L.; Trier, X.; Scheringer, M. Scientific Challenges in the Risk Assessment of Food Contact Materials. Environ. Health Perspect. 2017, 125, 095001. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Department of Health and Human Services. Food Drugs 2019, 21. [Google Scholar]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- 2019/904; Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. European Parliament, C.o.t.E.U.: Strasbourg, France, 2019.
- Bartolome, L.; Imran, M.; Cho, B.; Al-Masry, W.; Kim, D. Recent Developments in the Chemical Recycling of PET. Mater. Recycl.-Trends Perspect. 2012, 406, 576–596. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Mishra, R.K.; Thomas, S. 3—Materials Recovery, Direct Reuse and Incineration of PET Bottles. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 37–60. [Google Scholar] [CrossRef]
- Teixeira, W.; Steiner, C.; Martins, G.; Arruda, M. Charcoal (Biochar) as Soil Conditioner to Enhance Fertilizers and Water Use Efficiency in Agriculture in Acid Tropical Soils in the Central Amazon Basin; CIEC: Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Valh, J.V.; Vončina, B.; Lobnik, A.; Zemljič, L.F.; Škodič, L.; Vajnhandl, S. Conversion of polyethylene terephthalate to high-quality terephthalic acid by hydrothermal hydrolysis: The study of process parameters. Text. Res. J. 2020, 90, 1446–1461. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Gardini, F.; Lanciotti, R. Contribution of Two Different Packaging Material to Microbial Contamination of Peaches: Implications in Their Microbiological Quality. Front. Microbiol. 2016, 7, 938. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Rios, R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar] [CrossRef]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of poly(ethylene terephthalate)—A review focusing on chemical methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Fadzil, N.A.M.; Rahim, M.H.A.; Maniam, G.P. A brief review of para-xylene oxidation to terephthalic acid as a model of primary C–H bond activation. Chin. J. Catal. 2014, 35, 1641–1652. [Google Scholar] [CrossRef]
- Bohnet, M. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- James, G.S. Chemical Process and Design Handbook; McGraw-Hill Education: New York, NY, USA, 2002. [Google Scholar]
- Kuznetsova, N.I.; Bal’zhinimaev, B.S.; Bhattacharyya, A.; Walenga, J.T. An Improved Synthetic Approach for Obtaining High-Quality Terephthalic Acid: Eliminating the Need for Purification. ChemistrySelect 2017, 2, 11815–11820. [Google Scholar] [CrossRef]
- Wombat. Resyntex. Available online: https://cordis.europa.eu/project/id/641942 (accessed on 17 October 2021).
- Polymer Comply Europe. Demeto. Available online: https://www.demeto.eu/ (accessed on 12 January 2022).
- Mishra, S.; Goje, A.S. Kinetic and thermodynamic study of methanolysis of poly(ethylene terephthalate) waste powder. Polym. Int. 2003, 52, 337–342. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Chatziavgoustis, A.P.; Achilias, D.S. Poly (ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. 2002, 21, 250–259. [Google Scholar] [CrossRef]



| SAMPLES | Microbial Reduction (%) | ||
|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Escherichia coli ATCC 25922 | Candida glabrata EC 8706 | |
| PET foil | 0 ± 0 | 17 ± 1 | 0 ± 0 |
| PET–CHT | 76 ± 2 | 91 ± 2 | 42 ± 2 |
| Experiment # | m/(g) |
|---|---|
| 1 | 8.6 |
| 2 | 8.7 |
| 3 | 8.9 |
| Experiment # | m/(g) |
|---|---|
| 1 | 9.2 |
| 2 | 9.0 |
| 3 | 9.2 |
| Experiment # | m/(g) |
|---|---|
| 1 | 10.2 |
| 2 | 9.9 |
| 3 | 10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volmajer Valh, J.; Stopar, D.; Selaya Berodia, I.; Erjavec, A.; Šauperl, O.; Fras Zemljič, L. Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material. Polymers 2022, 14, 3244. https://doi.org/10.3390/polym14163244
Volmajer Valh J, Stopar D, Selaya Berodia I, Erjavec A, Šauperl O, Fras Zemljič L. Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material. Polymers. 2022; 14(16):3244. https://doi.org/10.3390/polym14163244
Chicago/Turabian StyleVolmajer Valh, Julija, Dimitrije Stopar, Ignacio Selaya Berodia, Alen Erjavec, Olivera Šauperl, and Lidija Fras Zemljič. 2022. "Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material" Polymers 14, no. 16: 3244. https://doi.org/10.3390/polym14163244
APA StyleVolmajer Valh, J., Stopar, D., Selaya Berodia, I., Erjavec, A., Šauperl, O., & Fras Zemljič, L. (2022). Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material. Polymers, 14(16), 3244. https://doi.org/10.3390/polym14163244







