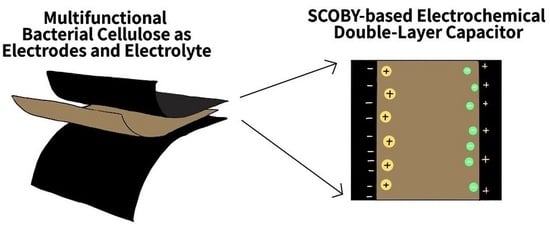
Multifunction Web-like Polymeric Network Bacterial Cellulose Derived from SCOBY as Both Electrodes and Electrolytes for Pliable and Low-Cost Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BC-NaBr-MMT (BXD) Electrolytes
2.3. Characterization of BC-NaBr-MMT Electrolytes (BXD)
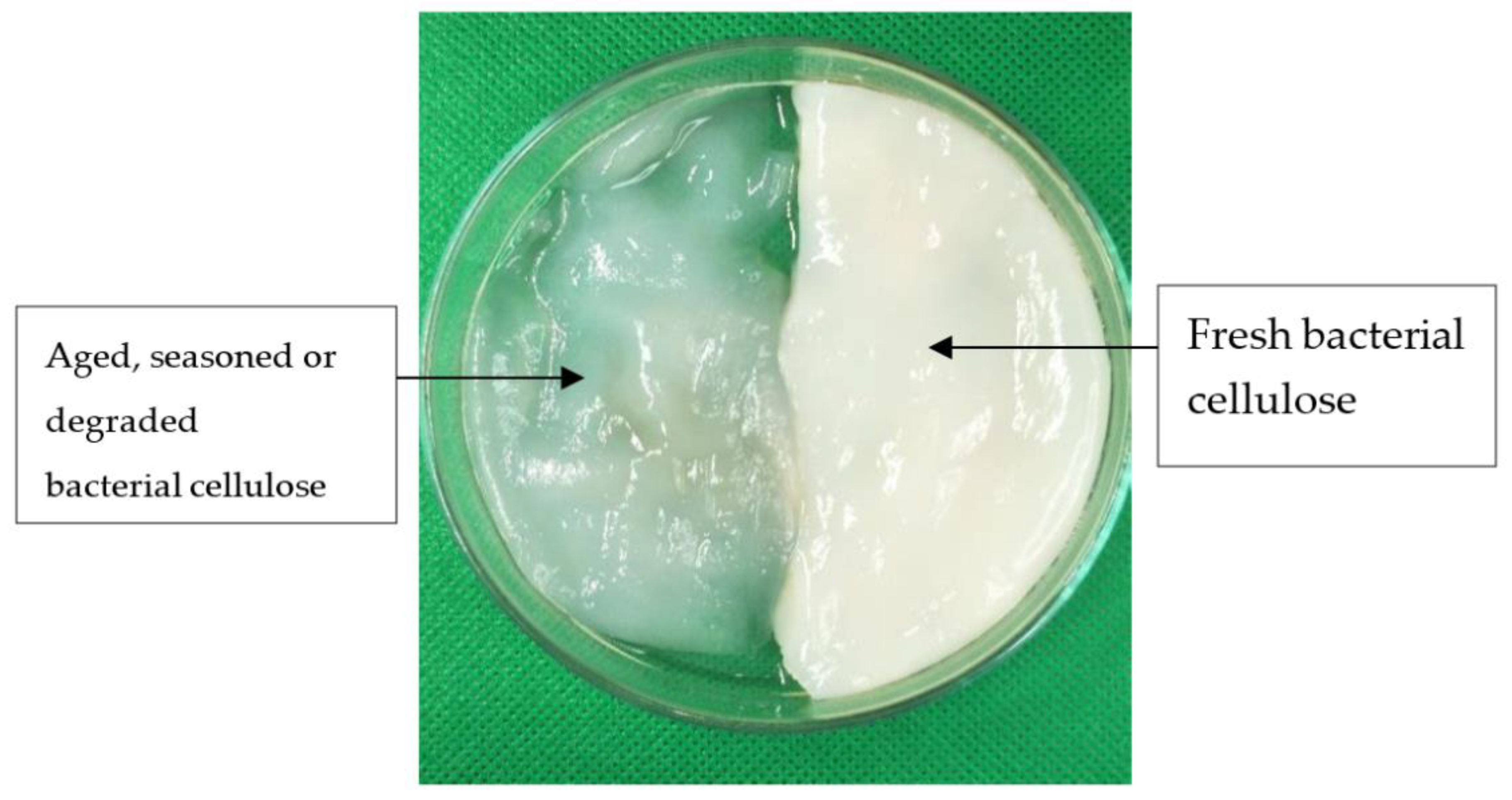
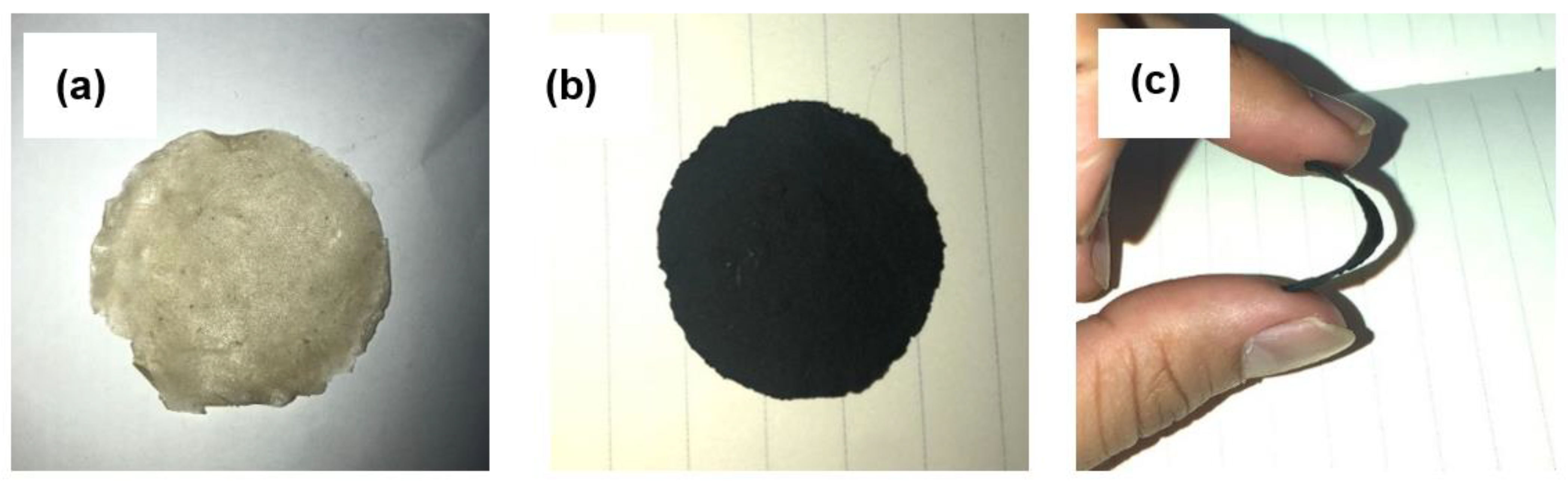
2.4. Preparation of BC-CNT Electrodes (BXC)
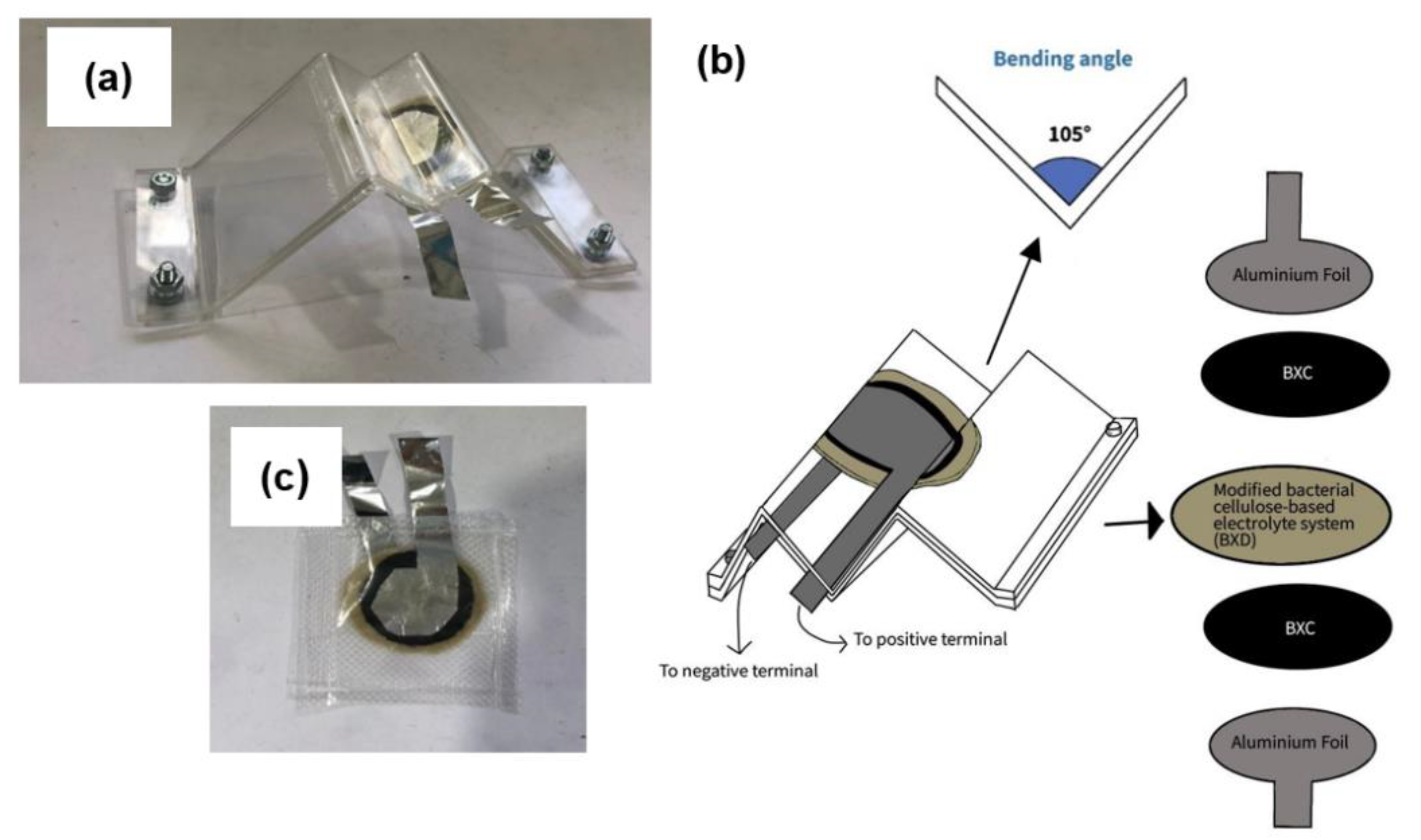
2.5. Construction of Full Bacterial Cellulose-Based EDLC
2.6. Characterization of Full Bacterial Cellulose-Based EDLC
3. Results
3.1. BC-NaBr-MMT (BXD) Electrolytes Study
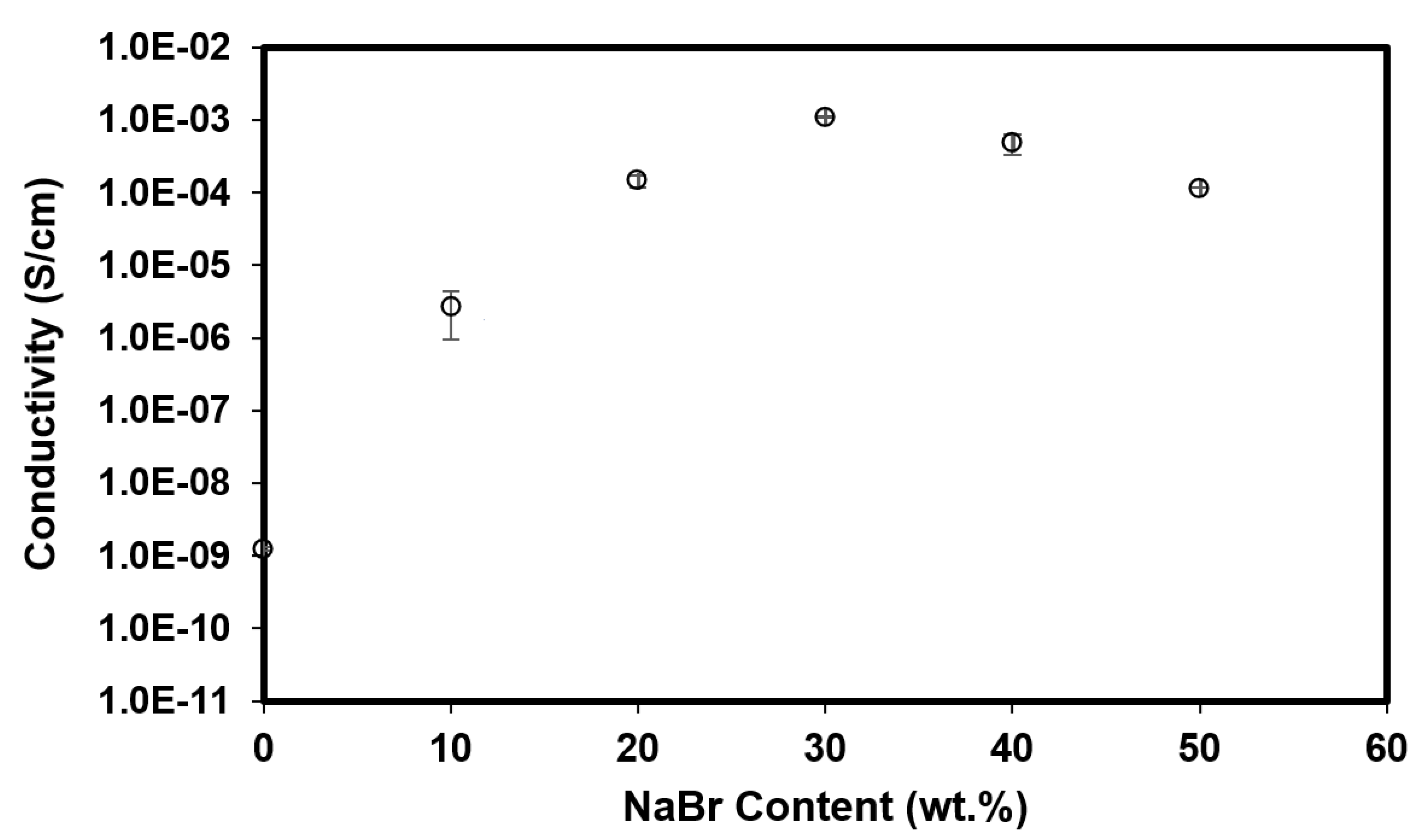
3.1.1. Ionic Conductivity Analysis of the Bacterial Cellulose Electrolytes
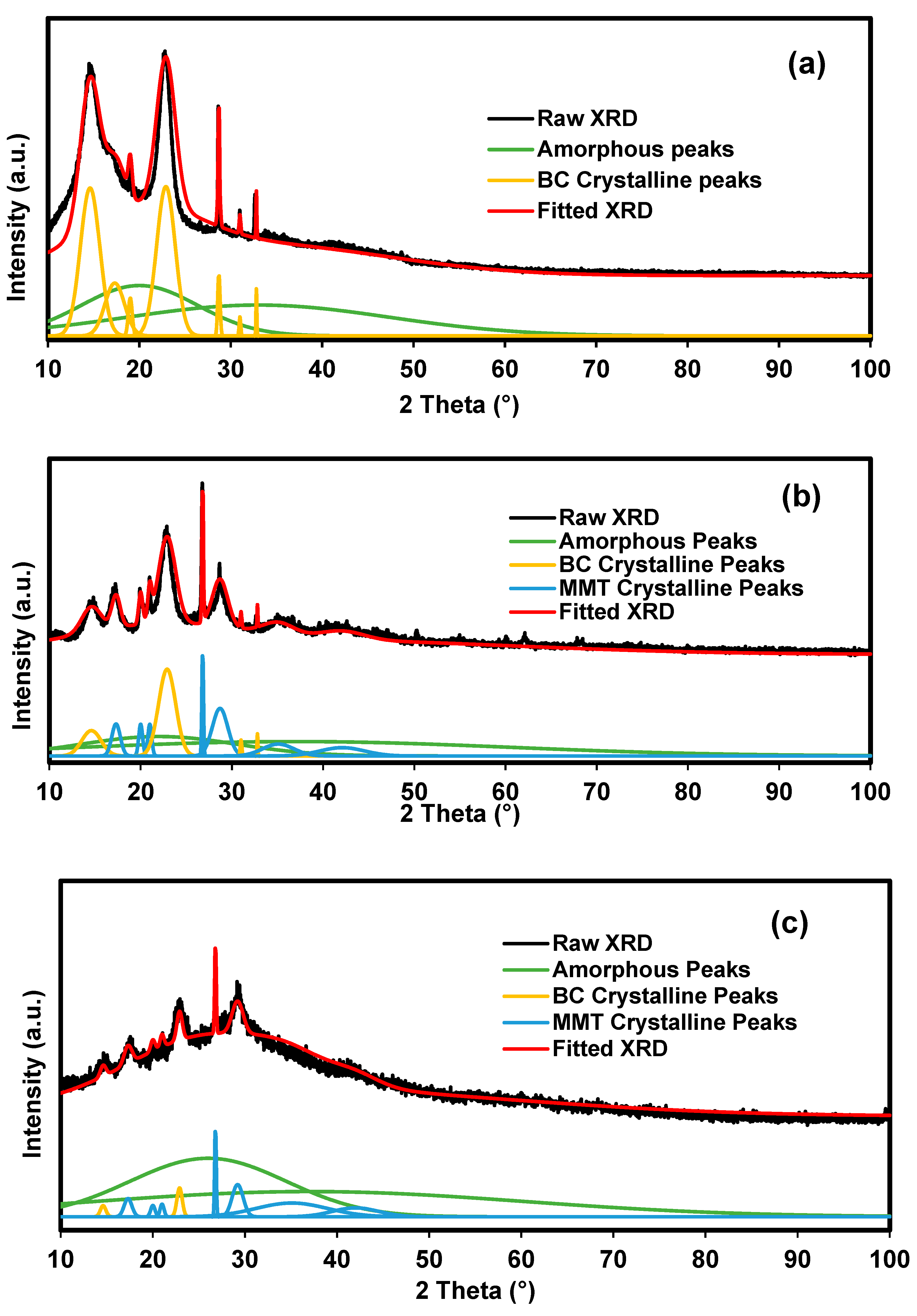
3.1.2. Crystallinity Analysis of the Bacterial Cellulose Electrolytes
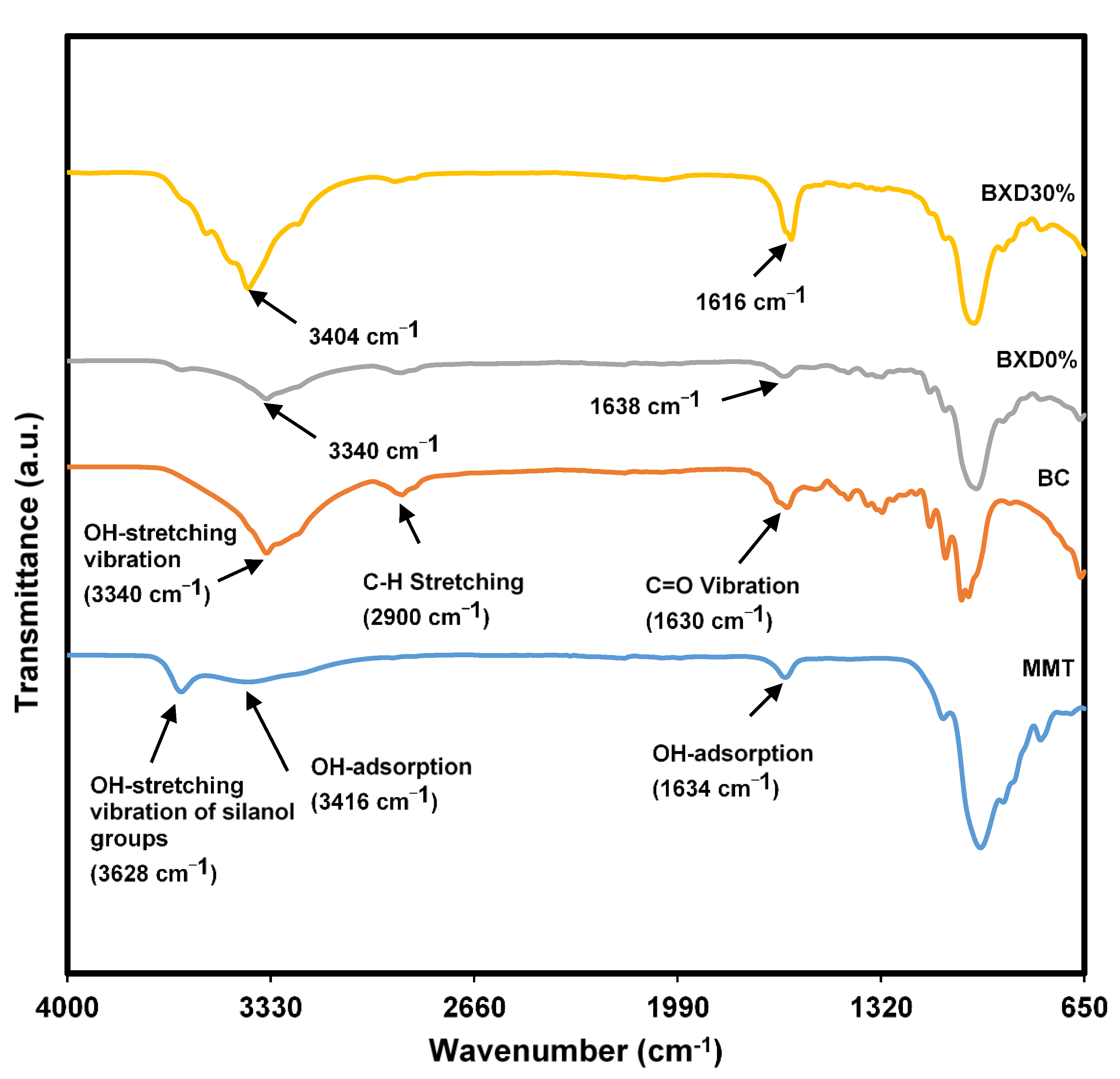
3.1.3. Complexation within the Bacterial Cellulose Electrolytes
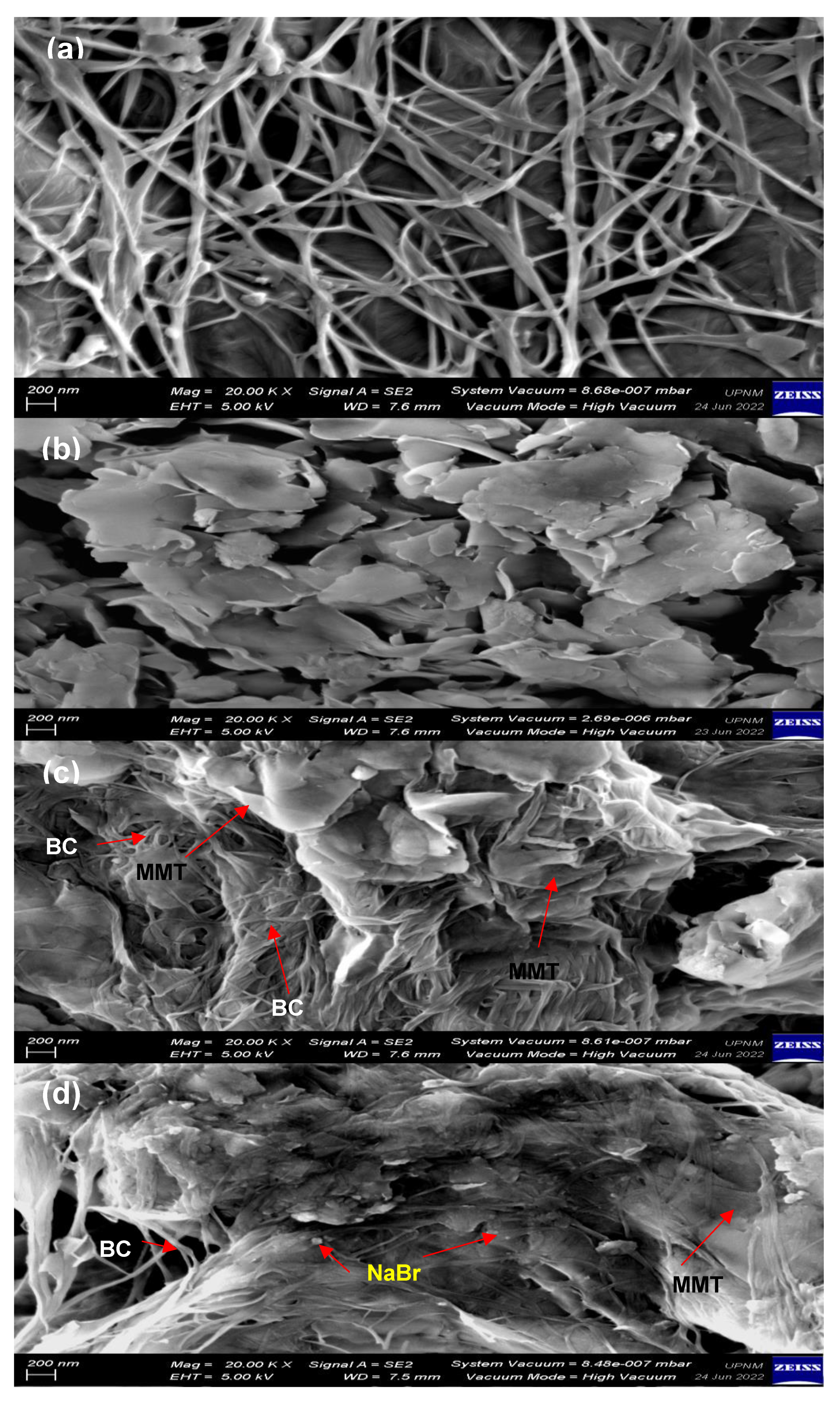
3.1.4. Surface Morphology of the Bacterial Cellulose Electrolytes
3.1.5. Contribution of Ions and Electrons in The Bacterial Cellulose Electrolytes
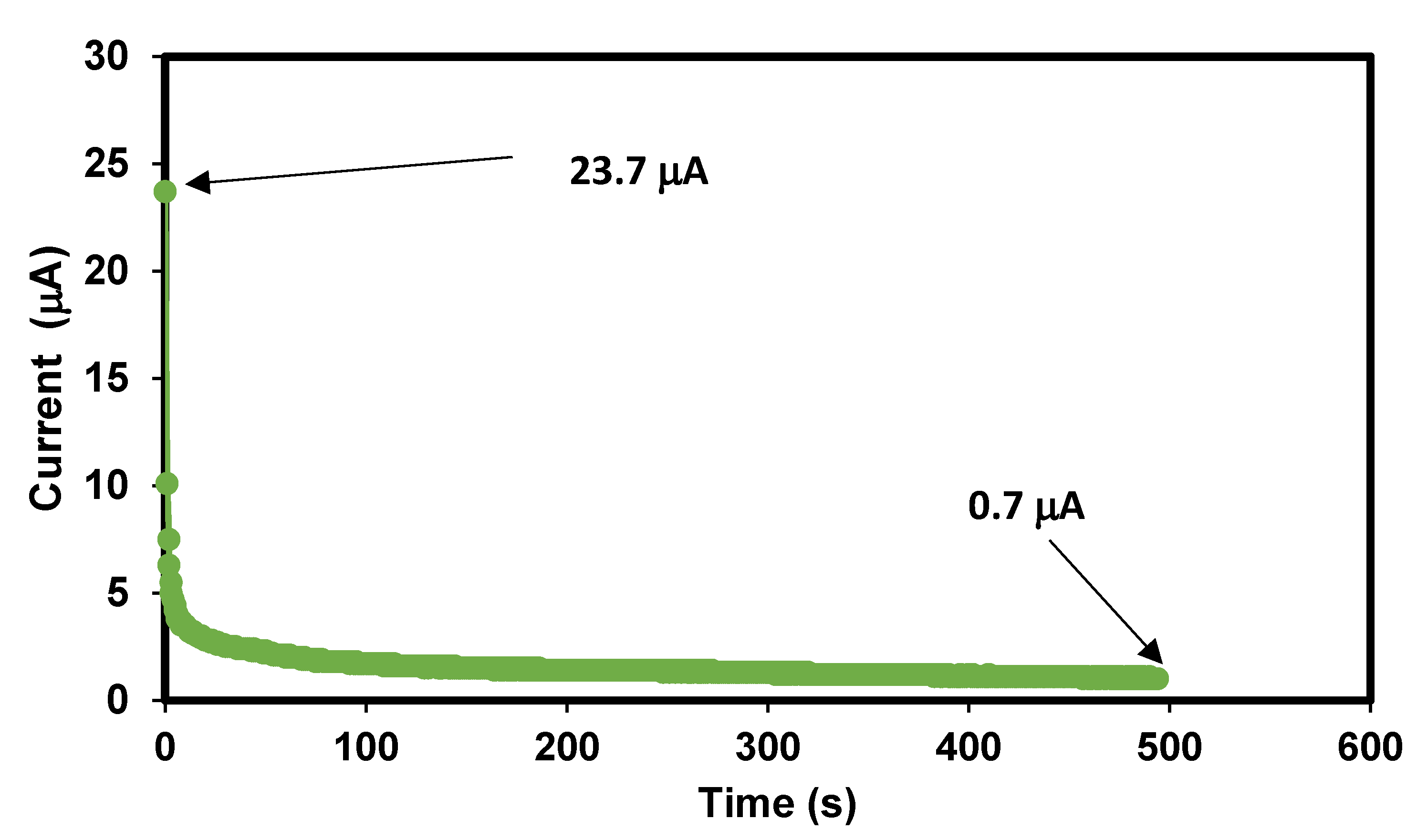
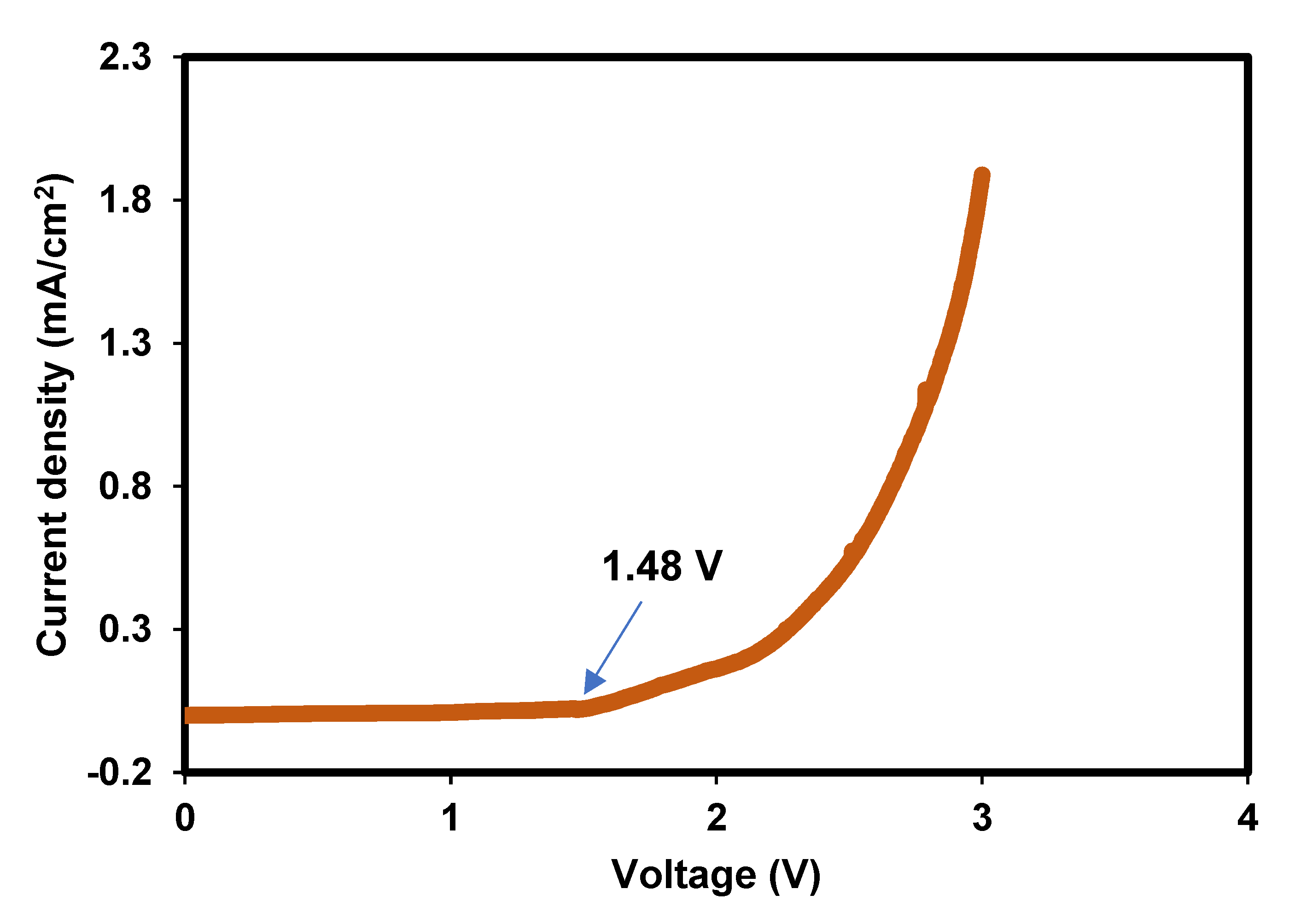
3.1.6. Potential Limit Test for the Bacterial Cellulose Electrolytes
3.2. Full Bacterial Cellulose-Based EDLC Study
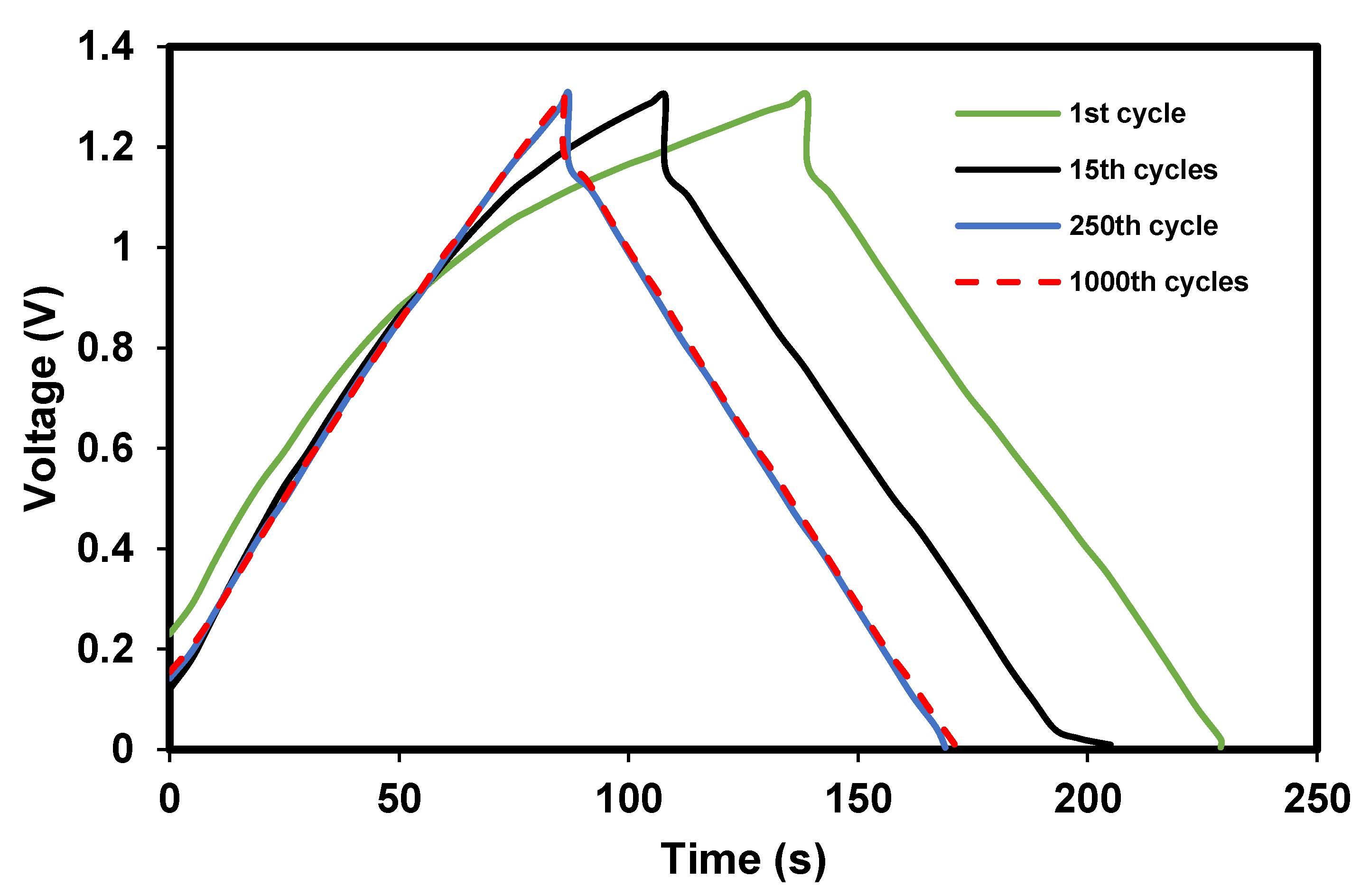
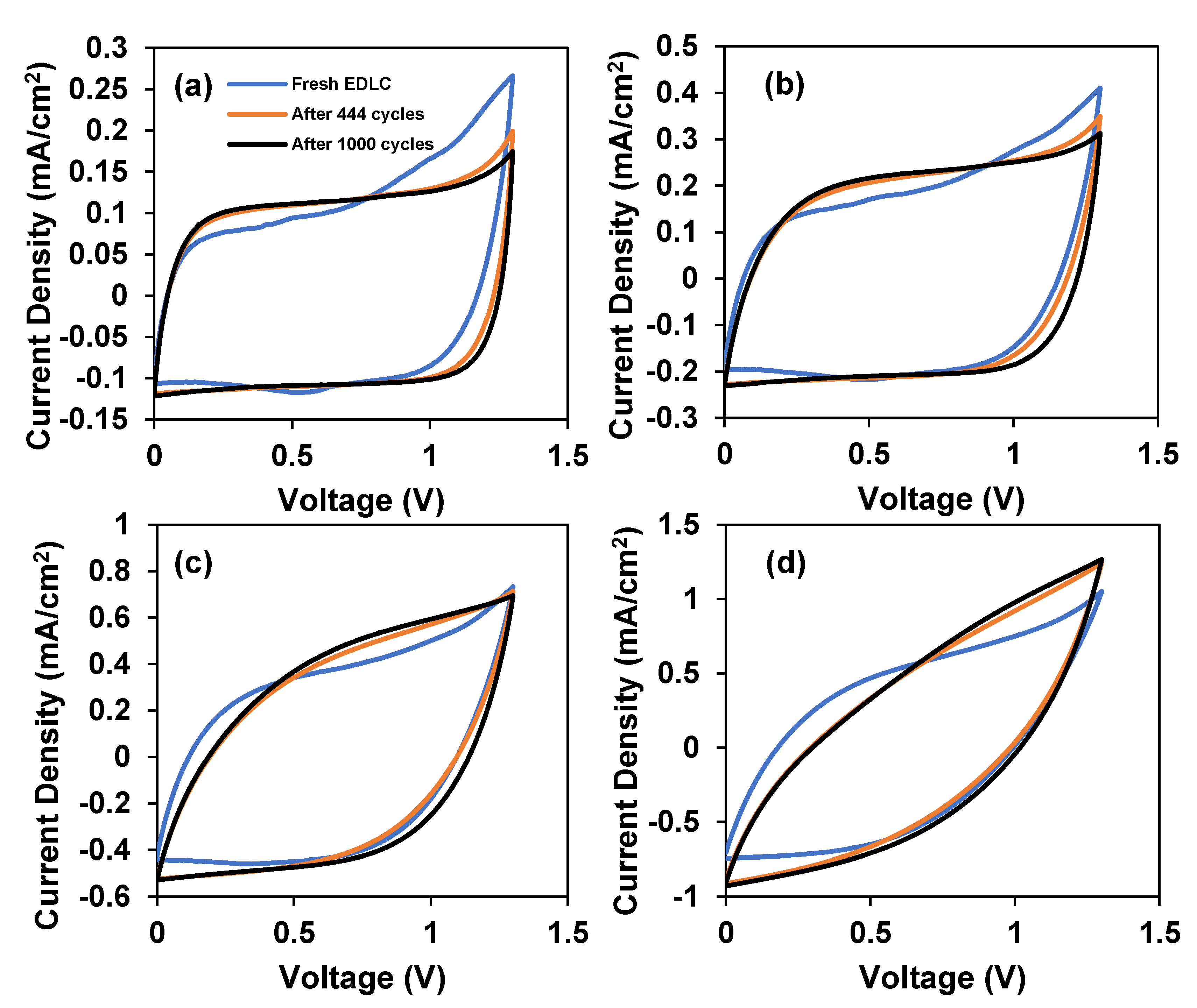
3.2.1. Important Storage Properties
3.2.2. Storing and Energy Storage Mechanism
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razali, N.M.M.; Sohaimi, R.M.; Othman, R.N.I.R.; Abdullah, N.; Demon, S.Z.N.; Jasmi, L.; Yunus, W.M.Z.W.; Ya′acob, W.M.W.; Salleh, E.M.; Norizan, M.N.; et al. Comparative Study on Extraction of Cellulose Fiber from Rice Straw Waste from Chemo-Mechanical and Pulping Method. Polymers 2022, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Araújo, S.; Gominho, J.; Evtuguin, D. Cellulose structural changes during mild torrefaction of eucalyptus wood. Polymers 2020, 12, 2831. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.A.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, B.R.; Wang, Q.; Gu, S.; Li, W.W.; Jin, J. High-strength internal cross-linking bacterial cellulose-network-based gel polymer electrolyte for dendrite-suppressing and high-rate lithium batteries. ACS Appl. Mater. Interfaces 2018, 10, 17809. [Google Scholar] [CrossRef]
- Teo, L.P.; Buraidah, M.H.; Arof, A.K. Development on solid polymer electrolytes for electrochemical devices. Molecules 2021, 26, 6499. [Google Scholar] [CrossRef] [PubMed]
- Betlej, I.; Zakaria, S.; Krajewski, K.J.; Poruszewski, B. Bacterial cellulose–properties and its potential application. Sains Malays 2021, 50, 493–505. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Liu, D.; Lu, S.; Li, F.; Gao, G. Bacterial cellulose composite solid polymer electrolyte with high tensile strength and lithium dendrite inhibition for long life battery. Energy Environ. Mater. 2021, 4, 434–443. [Google Scholar] [CrossRef]
- Yue, L.; Xie, Y.; Zheng, Y.; He, W.; Guo, S.; Sun, Y. Sulfonated bacterial cellulose/polyaniline composite membrane for use as gel polymer electrolyte. Compos. Sci. Technol. 2017, 145, 122–131. [Google Scholar] [CrossRef]
- Sabrina, Q.; Ratri, C.; Hardiansyah, A.; Lestariningsih, T.; Subhan, A.; Rifai, A. Preparation and characterization of nanofibrous cellulose as solid polymer electrolyte for lithium ion battery applications. RSC Adv. 2021, 11, 22929. [Google Scholar] [CrossRef]
- Luo, W.; Guo, N.; Wang, L.; Cao, Y.; Xu, M.; Jia, D. From powders to freestanding electrodes: Assembly active particles into bacterial cellulose for high performance supercapacitors. Electrochim. Acta 2021, 387, 138560. [Google Scholar] [CrossRef]
- Liu, C.; Hung, C.-W.; Cheng, I.-C.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Dielectric Barrier Discharge Plasma Jet (DBDjet) Processed Reduced Graphene Oxide/Polypyrrole/Chitosan Nanocomposite Supercapacitors. Polymers 2021, 13, 3585. [Google Scholar] [CrossRef] [PubMed]
- Hamsan, M.H.; Nofal, M.M.; Aziz, S.B.; Brza, M.A.; Dannoun, E.M.A.; Murad, A.R.; Kadir, M.F.Z.; Muzakir, S.K. Plasticized polymer blend electrolyte based on chitosan for energy storage application: Structural, circuit modeling, morphological and electrochemical properties. Polymers 2021, 13, 1233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded graphite-based materials for supercapacitors: A review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef]
- Lee, D.; Shin, Y.; Park, J. Recent Progress in Polysaccharide Aerogels: Their Synthesis, Application, and Future Outlook. Polymers 2021, 13, 1347. [Google Scholar]
- Tran, C.; Hoffmann, A.; Kuehne, A.J.C. High Throughput Centrifugal Electrospinning of Polyacrylonitrile Nanofibers for Carbon Fiber Nonwovens. Polymers 2021, 13, 1313. [Google Scholar]
- Kolosov, D.A.; Glukhova, O.E. A new composite material on the base of carbon nanotubes and boron clusters b12 as the base for high-performance supercapacitor electrodes. C 2021, 7, 26. [Google Scholar] [CrossRef]
- Gun’ko, V.M. Polymer adsorbents vs. functionalized oxides and carbons: Particulate morphology and textural and surface characteristics. Polymers 2021, 13, 1249. [Google Scholar] [CrossRef]
- Queirós, G.; Rey-Raap, N.; Pereira, C.; Pereira, M.F.R. CNT-based materials as electrodes for flexible supercapacitors. U Porto J. Eng. 2021, 7, 151–162. [Google Scholar] [CrossRef]
- Kato, M.; Hiraoka, K.; Seki, S. Investigation of the ionic conduction mechanism of polyether/Li7La3Zr2O12 composite solid electrolytes by electrochemical impedance spectroscopy. J. Electrochem. Soc. 2020, 167, 070559. [Google Scholar] [CrossRef]
- Johari, S.N.; Tajuddin, N.A.; Hanibah, H.; Deraman, S.K. A review: Ionic conductivity of solid polymer electrolyte based polyethylene oxide. Int. J. Electrochem. Sci. 2021, 16, 211049. [Google Scholar] [CrossRef]
- Olopade, B.K.; Oranusi, S.; Nwinyi, O.C.; Njobeh, P.B.; Lawal, I.A. Characterization of nanoformulations from montmorillonite clay for the decontamination of zearalenonein cereals using x-ray diffraction technique. J. Phys. Conf. 2019, 1299, 012107. [Google Scholar] [CrossRef]
- Sun, H.; Peng, T.; Liu, B.; Xian, H. Effects of montmorillonite on phase transition and size of TiO2 nanoparticles in TiO2/montmorillonite nanocomposites. Appl. Clay Sci. 2015, 11, 440–446. [Google Scholar] [CrossRef]
- Mao, R.; Meng, N.; Tu, W.; Peijs, T. Toughening mechanisms in cellulose nanopaper: The contribution of amorphous regions. Cellulose 2017, 24, 4627–4639. [Google Scholar] [CrossRef]
- Wasim, M.; Khan, M.R.; Mushtaq, M.; Naeem, A.; Han, M.; Wei, Q. Surface modification of bacterial cellulose by copper and zinc oxide sputter coating for UV-resistance/antistatic/antibacterial characteristics. Coatings 2020, 10, 364. [Google Scholar] [CrossRef] [Green Version]
- Makarov, I.S.; Shambilova, G.K.; Vinogradov, M.I.; Zatonskih, P.V.; Gromovykh, T.I.; Lutsenko, S.V.; Arkharova, N.A.; Ku-lichikhin, V.G. Films of bacterial cellulose prepared from solutions in n-methylmorpholine-n-oxide: Structure and proper-ties. Processes 2020, 8, 171. [Google Scholar] [CrossRef]
- Praveena, S.D.; Ravindrachary, V.; Bhajantri, I.; Harisha, R.F.; Guruswamy, A.; Hegde, B. Inhibition and quenching effect on positr onium formation in metal salt doped polymer blend. In Proceedings of the AIP Conference Proceedings. Solid State Physics Symposium 2017, Mumbai, India, 26–30 October 2017. [Google Scholar]
- Pambudi, A.B.; Priyangga, A.; Hartanto, D.; Atmaja, L. Intramolecular hydrogen bond and vibrational spectroscopic study of cellulose oligosaccharide using density functional theory. In Proceedings of the 4th International Seminar on Chemistry, Surabaya, Indonesia, 7–8 October 2020. [Google Scholar]
- Akpomie, K.G.; Dawodu, F.A. Acid-modified montmorillonite for sorption of heavy metals from automobile effluent. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Danková, Z.; Mockovčiaková, A.; Dolinska, S. Influence of ultrasound irradiation on cadmium cations adsorption by montmorillonite. Desalination Water Treat. 2014, 52, 5462–5469. [Google Scholar] [CrossRef]
- Ceyhun, B.; Tülin, K.; Erhan, P. Production and characterization of biodegradable bacterial cellulose membranes. Int. J. Eng. Sci. 2009, 3, 19–22. [Google Scholar]
- Potivara, K.; Phisalaphong, M. Development and characterization of bacterial cellulose reinforced with natural rubber. Materials 2019, 12, 2323. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.A.; Kamarudin, N.S.; Esa, F.; Kalil, M.S.; Kamarudin, S.K. Bacterial Cellulose as a Potential Hard Gelatin Capsule. J. Kejuruter. SI 2019, 1, 151–156. [Google Scholar]
- Jia, Y.; Wang, X.; Huo, M.; Zhai, X.; Li, F.; Zhong, C. Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater. Nanotechnol. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ul-Islama, M.; Khan, T.; Parka, J.K. Nanoreinforced bacterial cellulose–montmorillonite composites for biomedical applications. Carbohydr. Polym. 2012, 89, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.L.; Sun, D.P.; Hu, L.Y.; Li, Y.W.; Yang, J.Z. EVect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J. Ind. Microbiol. Biotechnol. 2007, 34, 483–489. [Google Scholar] [CrossRef]
- Shetty, S.K.; Hegde, S.; Ravindrachary, V.; Sanjeev, G.; Bhajantri, R.F. Dielectric relaxations and ion transport study of NaCMC:NaNO3 solid polymer electrolyte films. Ionics 2021, 27, 2509–2525. [Google Scholar] [CrossRef]
- Bhargav, P.B.; Mohan, V.M.; Sharma, A.K.; Rao, V.V.R.N. Structural and electrical studies of sodium iodide doped poly (vinyl alcohol) polymer electrolyte films for their application in electrochemical cells. Ionics 2007, 13, 173–178. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Tenhaeff, W.E. Determining the absolute anodic stability threshold of polymer electrolytes: A capacity-based electrochemical method. Chem. Mater. 2021, 33, 1927–1934. [Google Scholar] [CrossRef]
- Arof, A.K.; Kufian, M.Z.; Shukur, M.F.; Aziz, M.F.; Abdelrahman, A.E.; Majid, S.R. Electrical double layer capacitor using poly(methyl methacrylate)-C4BO8Li gel polymer electrolyte and carbonaceous material from shells of mata kucing (Dimocarpus longan) fruit. Electrochim. Acta 2012, 74, 39–45. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Majid, S.R.; Arof, A.K. On complexation between methyl cellulose and ammonium nitrate. Mater. Res. Innov. 2009, 13, 239–242. [Google Scholar] [CrossRef]
- Pandey, G.P.; Yogesh, K.; Hashmi, S.A. Ionic liquid incorporated PEO based polymer electrolyte for electrical double layer capacitors: A comparative study with lithium and magnesium systems. Solid State Ionics 2011, 190, 93–98. [Google Scholar] [CrossRef]
- Lim, C.S.; Teoh, K.H.; Liew, C.W.; Ramesh, S. Electric double layer capacitor based on activated carbon electrode and biodegradable composite polymer electrolyte. Ionics 2014, 20, 251–258. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Hamsan, M.H.; Brza, M.A.; Abdullah, R.M.; Kadir, M.F.Z. Structural, impedance, and EDLC characteristics of proton conducting chitosan-based polymer blend electrolytes with high electrochemical stability. Molecules 2019, 24, 3508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, S.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of ohmic-drop on electrochemical performance of EDLC fabricated from PVA: Dextran: NH4I based polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 3734–3745. [Google Scholar] [CrossRef]
- Aziz, S.B.; Dannoun, E.M.A.; Hamsan, M.H.; Abdulwahid, R.T.; Mishra, K.; Nofal, M.M. Improving EDLC Device Performance Constructed from Plasticized Magnesium Ion Conducting Chitosan Based Polymer Electrolytes via Metal Complex Dispersion. Membranes 2021, 11, 289. [Google Scholar] [CrossRef]
- Rafidi, N.; Bashir, S.; Hina, M.; Gunalan, S.; Ramesh, S.; Ramesh, K. Renewable and soft dynamic supercapacitors based on poly (acrylamide) hydrogel electrolytes and porous carbon electrodes. Polym. Bull. 2022. accepted. [Google Scholar] [CrossRef]
- Markoulidis, F.; Dawe, A.; Lekakou, C. Electrochemical double-layer capacitors with lithium-ion electrolyte and electrode coatings with PEDOT:PSS binder. J. Appl. Electrochem. 2021, 51, 373–385. [Google Scholar] [CrossRef]


| Sample | χc (%) |
|---|---|
| BC | 33.5 |
| BXD0% | 27.3 |
| BXD30% | 10.9 |
| Electrolyte | Electrodes | Cs (F/g) | Cycles | Reference |
|---|---|---|---|---|
| PMMA-LiBOB | Carbon | 0.52 | 50 | [40] |
| Methylcellulose-NH4NO3 | Activated Carbon | 1.67 | - | [41] |
| PEO-Mg(Tf)2 + EMITf | MWCNT-AB-PVdF-HFP | 2.6–3.0 | - | [42] |
| PVA–LiClO4 | Activated carbon | 3.0 | 200 | [43] |
| Chitosan-PEO-NH4SCN | Carbon | 3.8 | - | [44] |
| PVA-Dextran-NH4I | Activated carbon-AB-PVdF | 4.2 | 100 | [45] |
| BC-MMT-NaBr | BC-MWCNT | 6.7 | 1000 | This work |
| Specific Capacitance (Ccyc) | ||||
|---|---|---|---|---|
| 10 mV/s | 20 mV/s | 50 mV/s | 100 mV/s | |
| Fresh EDLC | 2.67 | 2.31 | 1.68 | 1.07 |
| After 444 cycles | 2.76 | 2.43 | 1.72 | 1.05 |
| After 1000 cycles | 2.78 | 2.75 | 1.86 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamsan, M.H.; Halim, N.A.; Demon, S.Z.N.; Sa’aya, N.S.N.; Kadir, M.F.Z.; Abidin, Z.H.Z.; Poad, N.A.; Kasim, N.F.A.; Razali, N.A.M.; Aziz, S.B.; et al. Multifunction Web-like Polymeric Network Bacterial Cellulose Derived from SCOBY as Both Electrodes and Electrolytes for Pliable and Low-Cost Supercapacitor. Polymers 2022, 14, 3196. https://doi.org/10.3390/polym14153196
Hamsan MH, Halim NA, Demon SZN, Sa’aya NSN, Kadir MFZ, Abidin ZHZ, Poad NA, Kasim NFA, Razali NAM, Aziz SB, et al. Multifunction Web-like Polymeric Network Bacterial Cellulose Derived from SCOBY as Both Electrodes and Electrolytes for Pliable and Low-Cost Supercapacitor. Polymers. 2022; 14(15):3196. https://doi.org/10.3390/polym14153196
Chicago/Turabian StyleHamsan, Muhamad Hafiz, Norhana Abdul Halim, Siti Zulaikha Ngah Demon, Nurul Syahirah Nasuha Sa’aya, Mohd Fakhrul Zamani Kadir, Zul Hazrin Zainal Abidin, Nursaadah Ahmad Poad, Nurul Farhana Abu Kasim, Nur Amira Mamat Razali, Shujahadeen B. Aziz, and et al. 2022. "Multifunction Web-like Polymeric Network Bacterial Cellulose Derived from SCOBY as Both Electrodes and Electrolytes for Pliable and Low-Cost Supercapacitor" Polymers 14, no. 15: 3196. https://doi.org/10.3390/polym14153196
APA StyleHamsan, M. H., Halim, N. A., Demon, S. Z. N., Sa’aya, N. S. N., Kadir, M. F. Z., Abidin, Z. H. Z., Poad, N. A., Kasim, N. F. A., Razali, N. A. M., Aziz, S. B., Ahmad, K. A., Miskon, A., & Nor, N. M. (2022). Multifunction Web-like Polymeric Network Bacterial Cellulose Derived from SCOBY as Both Electrodes and Electrolytes for Pliable and Low-Cost Supercapacitor. Polymers, 14(15), 3196. https://doi.org/10.3390/polym14153196