Influence of Starch on the Structure–Properties Relationship in Polyethylene Glycol/Polycaprolactone Diol Polyurethanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zwitterionic Starch
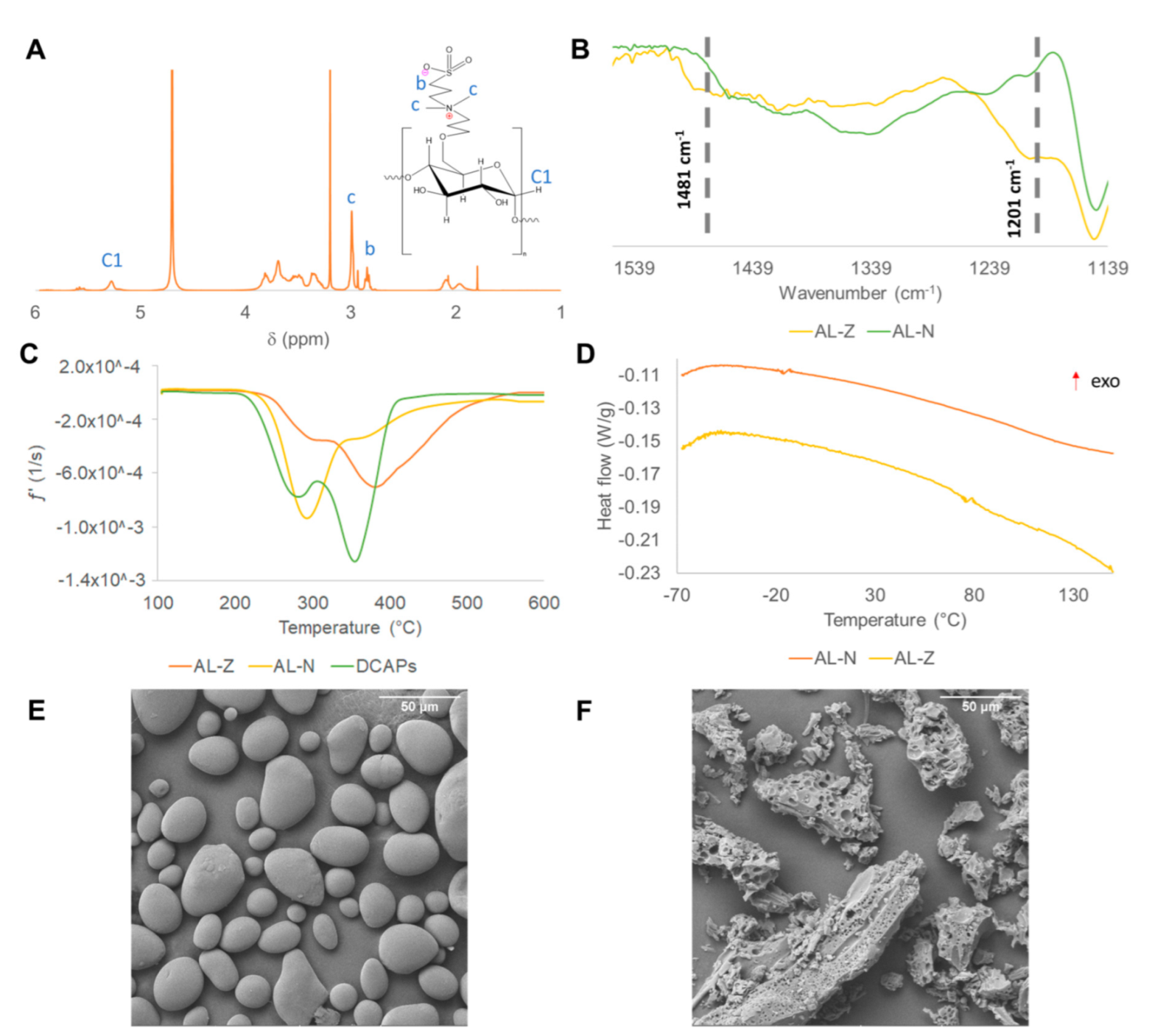
2.3. Chemical Structure, Thermal and Morphology Characterization of Zwitterionic Starch
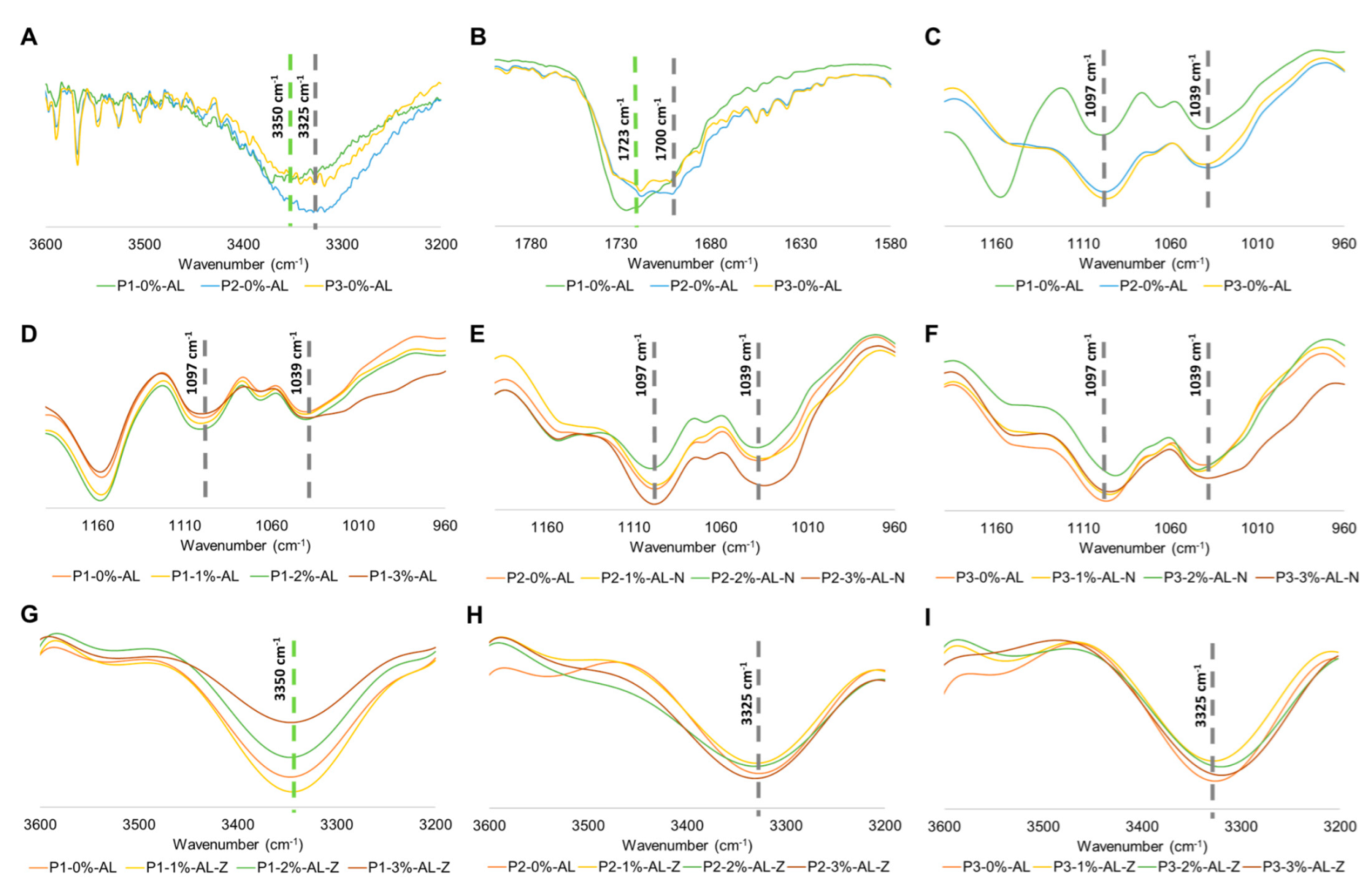
2.4. Synthesis of Polyurethane Composites
2.5. Physicochemical Characterization
2.6. Thermo-Mechanical Properties
2.7. Statistics Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Haponiuk, J.T.; Formela, K. PU Polymers, Their Composites, and Nanocomposites: State of the Art and New Challenges. Polyurethane Polymers: Composites and Nanocomposites. Polyurethane Polym. 2017, 1–20. [Google Scholar] [CrossRef]
- Zieber, L.; Or, S.; Ruvinov, E.; Cohen, S. Microfabrication of channel arrays promotes vessel-like network formation in cardiac cell construct and vascularization in vivo. Biofabrication 2014, 6, 24102. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Herráez, P.; Garbayo, E.; Simón-Yarza, T.; Formiga, F.; Prosper, F.; Blanco-Prieto, M. Adipose-derived stem cells combined with Neuregulin-1 delivery systems for heart tissue engineering. Eur. J. Pharm. Biopharm. 2013, 85, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Yang, H.; Wang, L.; Thomson, J.A.; Turng, L.S.; Guan, G. Surface modification of polytetrafluoroethylene (PTFE) with a heparin-immobilized extracellular matrix (ECM) coating for small-diameter vascular grafts applications. Mater. Sci. Eng. 2021, 128, 112301. [Google Scholar] [CrossRef]
- Mora-Cortes, L.F.; Rivas-Muñoz, A.N.; Neira-Velázquez, M.G.; Contreras-Esquivel, J.C.; Roger, P.; Mora-Cura, Y.N.; Soria-Arguello, G.; Bolaina-Lorenzo, E.D.; Reyna-Martínez, R.; Zugasti-Cruz, A.; et al. Biocompatible enhancement of poly (ethylene terephthalate) (PET) waste films by cold plasma aminolysis. J. Chem. Technol. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Kianpour, G.; Bagheri, R.; Pourjavadi, A.; Ghanbari, H. In situ synthesized TiO2-polyurethane nanocomposite for bypass graft application: In vitro endothelialization and degradation. Mater. Sci. Eng. 2020, 114, 111043. [Google Scholar] [CrossRef]
- Lee, T.H.; Yen, C.T.; Hsu, S.H. Preparation of Polyurethane-Graphene Nanocomposite and Evaluation of Neurovascular Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 597–609. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Emechebe, G.A.; Kim, D.-W.; Cho, H.-J.; Park, C.H.; Kim, C.S.; Jeong, I.S. Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review. Cardiovasc. Eng. Technol. 2020, 11, 495–521. [Google Scholar] [CrossRef]
- Gostev, A.A.; Karpenko, A.A.; Laktionov, P.P. Polyurethanes in cardiovascular prosthetics. Polym. Bull. 2018, 75, 4311–4325. [Google Scholar] [CrossRef]
- Navas-Gómez, K.; Valero, M.F. Why polyurethanes have been used in the manufacture and design of cardiovascular devices: A systematic review. Materials 2020, 13, 3250. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Alquichire, S.; Morales-Gonzalez, M.; Diaz, L.E.; Valero, M.F. Surface response methodology-based mixture design to study the influence of polyol blend composition on polyurethanes’ properties. Molecules 2018, 23, 1942. [Google Scholar] [CrossRef] [Green Version]
- Adipurnama, I.; Yang, M.-C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2017, 5, 22–37. [Google Scholar] [CrossRef]
- Król, P.; Uram, Ł.; Król, B.; Pielichowska, K.; Sochacka-Piętal, M.; Walczak, M. Synthesis and property of polyurethane elastomer for biomedical applications based on nonaromatic isocyanates, polyesters, and ethylene glycol. Colloid Polym. Sci. 2020, 298, 1077–1093. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Niu, Y.; He, T.; Chen, K.C.; Xu, K. Alternating block polyurethanes based on PCL and PEG as potential nerve regeneration materials. J. Biomed. Mater. Res. Part A 2014, 102, 685–697. [Google Scholar] [CrossRef]
- Vadillo, J.; Larraza, I.; Calvo-Correas, T.; Gabilondo, N.; Derail, C.; Eceiza, A. Role of in situ added cellulose nanocrystals as rheological modulator of novel waterborne polyurethane urea for 3D-printing technology. Cellulose 2021, 28, 4729–4744. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Villani, M.; Consonni, R.; Canetti, M.; Bertoglio, F.; Iervese, S.; Bruni, G.; Visai, L.; Iannace, S.; Bertini, F. Polyurethane-based composites: Effects of antibacterial fillers on the physical-mechanical behavior of thermoplastic polyurethanes. Polymers 2020, 12, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arévalo, F.R.; Osorio, S.A.; Valcárcel, N.A.; Ibarra, J.C.; Valero, M.F. Characterization and in vitro biocompatibility of binary mixtures of chitosan and polyurethanes synthesized from chemically modified castor oil, as materials for medical use. Polym. Renew. Resour. 2018, 9, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Hormaiztegui, M.E.V.; Marin, D.; Gañán, P.; Stefani, P.M.; Mucci, V.; Aranguren, M.I. Nanocelluloses reinforced bio-waterborne polyurethane. Polymers 2021, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- Gaaz, T.S.; Sulong, A.B.; Ansari, M.N.M.; Kadhum, A.A.H.; Al-Amiery, A.A.; Nassir, M.H. Effect of starch loading on the thermo-mechanical and morphological properties of polyurethane composites. Materials 2017, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch Staerke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Carvalho, A.J.F. Starch: Major sources, properties and applications as thermoplastic materials. Monomers Polym. Compos. Renew. Resour. 2008, 15, 321–342. [Google Scholar]
- Jin, M.M.; Shi, M.J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Zuber, M.; Kamal, S.; Aslam, N. Starch based polyurethanes: A critical review updating recent literature. Carbohydr. Polym. 2015, 134, 784–798. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, Z.; Li, C.; Wei, Z.; Jain, P.; Wu, K. Progress in biodegradable zwitterionic materials. Polym. Degrad. Stab. 2017, 139, 1–19. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Li, J.; Dong, D.; Zhang, Y.; Yao, F. Ionic starch-based hydrogels for the prevention of nonspecific protein adsorption. Carbohydr. Polym. 2015, 117, 384–391. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Yang, H.; Zhu, C.; Yang, J.; Yao, F. Preparation and characterization of protein resistant zwitterionic starches: The effect of substitution degrees. Starch Staerke 2015, 67, 920–929. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Ma, C.; Zhang, Y. Thermal behavior of sweet potato starch by non-isothermal thermogravimetric analysis. Materials 2019, 12, 699. [Google Scholar] [CrossRef] [Green Version]
- Arévalo, F.; Uscategui, Y.L.; Diaz, L.; Cobo, M.; Valero, M.F. Effect of the incorporation of chitosan on the physico-chemical, mechanical properties and biological activity on a mixture of polycaprolactone and polyurethanes obtained from castor oil. J. Biomater. Appl. 2016, 31, 708–720. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Morales-Gonzalez, M.; Navas-Gómez, K.; Diaz, L.E.; Gómez-Tejedor, J.A.; Serrano, M.A.; Valero, M.F. Influence of polyol/crosslinker blend composition on phase separation and thermo-mechanical properties of polyurethane thin films. Polymers 2020, 12, 666. [Google Scholar] [CrossRef] [Green Version]
- Morales-Gonzalez, M.; Arévalo-Alquichire, S.; Diaz, L.E.; Sans, J.Á.; Vilariño-Feltrer, G.; Gómez-Tejedor, J.A.; Valero, M.F. Hydrolytic stability and biocompatibility on smooth muscle cells of polyethylene glycol-polycaprolactone-based polyurethanes. J. Mater. Res. 2020, 35, 3276–3285. [Google Scholar] [CrossRef]
- Valero, M.F.; Díaz, L.E. Polyurethane networks from pentaerythritol-modified castor oil and lysine polyisocyanates: Synthesis, mechanical, and thermal properties and in vitro degradation. Quim. Nova 2014, 37, 1441–1445. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Arévalo-Alquichire, S.J.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Díaz, L.E.; Valero, M.F. Polyurethane-based bioadhesive synthesized from polyols derived from castor oil (Ricinus communis) and low concentration of chitosan. J. Mater. Res. 2017, 32, 3699–3711. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Z.; Liang, L.; Lan, K.; Zheng, Q.; Wang, Y.; Guo, Y.; Zhu, K.; Mehmood, R.; Wang, B. A facile heparin/carboxymethyl chitosan coating mediated by polydopamine on implants for hemocompatibility and antibacterial properties. Appl. Surf. Sci. 2020, 528, 146539. [Google Scholar] [CrossRef]
- Zao, H.; Li, K.-C.; Wu, W.; Li, Q.; Jiang, Y.; Cheng, B.-X.; Huang, C.-X.; Li, H.-N. Microstructure and viscoelastic behavior of waterborne polyurethane/cellulose nanofiber nanocomposite. J. Ind. Eng. Chem. 2022, 110, 150–157. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- PigłoPigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and thermodynamics of thermal degradation of different starches and estimation the OH group and H2O content on the surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, J.; Rubio, L.; Albores-Velasco, M. Thermal degradation of poly (sulfobetaines). J. Appl. Polym. Sci. 1999, 73, 1409–1414. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Li, L.; Ye, C.; Lin, X.; Qiu, T. Novel multi–SO3H functionalized ionic liquids as highly efficient catalyst for synthesis of biodiesel. Green Energy Environ. 2021, 6, 271–282. [Google Scholar] [CrossRef]
- Dereszewska, A.; Olayo, R.; Cardoso, J. Synthesis and Thermal Degradation of Poly (n-butylisocyanate) Modified by 1,3-Propanesultone. J. Appl. Polym. Sci. 2003, 90, 3594–3601. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Zewen, Y.; Liu, Y.; Meng, X.; Zhang, W.; Zhang, H.; Yang, G.; Shaojie, W. High-efficiency emulsification anionic surfactant for enhancing heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128654. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, B.; Niu, B.; Ji, X.; He, Y.; Shi, M. Understanding the Structure, Thermal, Pasting, and Rheological Properties of Potato and Pea Starches Affected by Annealing Using Plasma-Activated Water. Front. Nutr. 2022, 9, 842662. [Google Scholar] [CrossRef]
- Fonseca-Santanilla, E.B.; Betancourt-López, L.L. Physicochemical and structural characterization of starches from Andean roots and tubers grown in Colombia. Food Sci. Technol. Int. 2022, 28, 144–156. [Google Scholar] [CrossRef]
- Govindaraju, I.; Sunder, M.; Chakraborty, I.; Mumbrekar, K.D.; Sankar Mal, S.; Mazumder, N. Investigation of physico-chemical properties of native and gamma irradiated starches. Mater. Today Proc. 2022, 55, 12–16. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Fontes, R.L.S.; Fontes-Sant’Ana, G.C.; Calado, V.; López, E.O.; Rocha-Leão, M.H.M. Extraction, Modification, and Chemical, Thermal and Morphological Characterization of Starch From the Agro-Industrial Residue of Mango (Mangifera indica L.) var. Ubá. Starch Staerke 2019, 71, 1800023. [Google Scholar] [CrossRef] [Green Version]
- Wongsamut, C.; Suwanpreedee, R.; Manuspiya, H. Thermoplastic polyurethane-based polycarbonate diol hot melt adhesives: The effect of hard-soft segment ratio on adhesion properties. Int. J. Adhes. Adhes. 2020, 102, 102677. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Benes, H.; Donato, R.K.; Datta, J. The role of hydrogen bonding on tuning hard-soft segments in bio-based thermoplastic poly(ether-urethane)s. J. Clean. Prod. 2020, 274, 122678. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.; Li, K.; Zhang, Z. Hydrogen bonds and FTIR peaks of polyether polyurethane-urea. Key Eng. Mater. 2019, 815, 151–156. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [Green Version]
- Malay, O.; Oguz, O.; Kosak, C.; Yilgor, E.; Yilgor, I.; Menceloglu, Y.Z. Polyurethaneurea-silica nanocomposites: Preparation and investigation of the structure-property behavior. Polymer 2013, 54, 5310–5320. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Hsu, S.L. An analysis of phase separation kinetics of model polyurethanes. Macromolecules 1989, 22, 1100–1105. [Google Scholar] [CrossRef]
- Vakili, H.; Mohseni, M.; Makki, H.; Yahyaei, H.; Ghanbari, H.; González, A.; Irusta, L. Synthesis of segmented polyurethanes containing different oligo segments: Experimental and computational approach. Prog. Org. Coat. 2021, 150, 105965. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Niu, Y.; Ye, J.; Huang, S.; Liu, C.; Xu, K. Synthesis and wound healing of alternating block polyurethanes based on poly (lactic acid) (PLA) and poly (ethylene glycol) (PEG). J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.A.; Gnanasekaran, D.; Reddy, B.S.R. Synthesis of different soft segmented polyurethane membranes: Structural characterization and antimicrobial activity studies. J. Polym. Mater. 2014, 31, 305–316. [Google Scholar]
- Kumar, S.; Tewatia, P.; Samota, S.; Rattan, G.; Kaushik, A. Ameliorating properties of castor oil based polyurethane hybrid nanocomposites via synergistic addition of graphene and cellulose nanofibers. J. Ind. Eng. Chem. 2022, 109, 492–509. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Chan, H.-M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, G. Effects of soft segment characteristics on the properties of biodegradable amphiphilic waterborne polyurethane prepared by a green process. J. Mater. Sci. 2020, 55, 3139–3156. [Google Scholar] [CrossRef]
- Polo Fonseca, L.; Bergamo Trinca, R.; Isabel Felisberti, M. Thermo-responsive polyurethane hydrogels based on poly (ethylene glycol) and poly (caprolactone): Physico-chemical and mechanical properties. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Kendaganna Swamy, B.K. Structure-property relationship of starch-filled chain-extended polyurethanes. J. Appl. Polym. Sci. 2003, 90, 2945–2954. [Google Scholar] [CrossRef]
- Omidi-Ghallemohamadi, M.; Jafari, P.; Behniafar, H. Polyurethane elastomer–silica hybrid films based on oxytetramethylene soft segments: Thermal and thermo-mechanical investigations. J. Polym. Res. 2021, 28, 3. [Google Scholar] [CrossRef]
- Weng, F.; Zhang, P.; Koranteng, E.; Zhang, Y.; Wu, Q.; Zeng, G. Effects of shell powder size and content on the properties of polycaprolactone composites. J. Appl. Polym. Sci. 2021, 138, 51264. [Google Scholar] [CrossRef]
- Vrsaljko, D.; Blagojević, S.L.; Leskovac, M.; Kovačević, V. Effect of calcium carbonate particle size and surface pretreatment on polyurethane composite Part I: Interface and mechanical properties. Mater. Res. Innov. 2008, 12, 40–46. [Google Scholar] [CrossRef]
- Bharadwaj-Somaskandan, S.; Krishnamurthi, B.; Sergeeva, T.; Shutov, F. Macro- and Microfillers as Reinforcing Agents for Polyurethane Elastomers. J. Elastomers Plast. 2003, 35, 325–334. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asensio, M.; Costa, V.; Nohales, A.; Bianchi, O.; Gómez, C.M. Tunable structure and properties of segmented thermoplastic polyurethanes as a function offlexible segment. Polymers 2019, 11, 1910. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wang, H.; Pan, H.; Yan, T.; Zong, C. The effect of mixed soft segment on the microstructure of thermoplastic polyurethane. J. Appl. Polym. Sci. 2021, 138, 51346. [Google Scholar] [CrossRef]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Amirkiai, A.; Panahi-Sarmad, M.; Sadeghi, G.M.M.; Arjmand, M.; Abrisham, M.; Dehghan, P.; Nazockdast, H. Microstructural design for enhanced mechanical and shape memory performance of polyurethane nanocomposites: Role of hybrid nanofillers of montmorillonite and halloysite nanotube. Appl. Clay Sci. 2020, 198, 105816. [Google Scholar] [CrossRef]
- Jayanarayanan, K.; Rasana, N.; Mishra, R.K. Dynamic Mechanical Thermal Analysis of Polymer Nanocomposites. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3, pp. 123–157. [Google Scholar]
- Loh, X.J.; Colin Sng, K.B.; Li, J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly (ε-caprolactone), poly (ethylene glycol) and poly (propylene glycol). Biomaterials 2008, 29, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Jalili Marand, M.; Rezaei, M.; Babaie, A.; Lotfi, R. Synthesis, characterization, crystallinity, mechanical properties, and shape memory behavior of polyurethane/hydroxyapatite nanocomposites. J. Intell. Mater. Syst. Struct. 2020, 31, 1662–1675. [Google Scholar] [CrossRef]


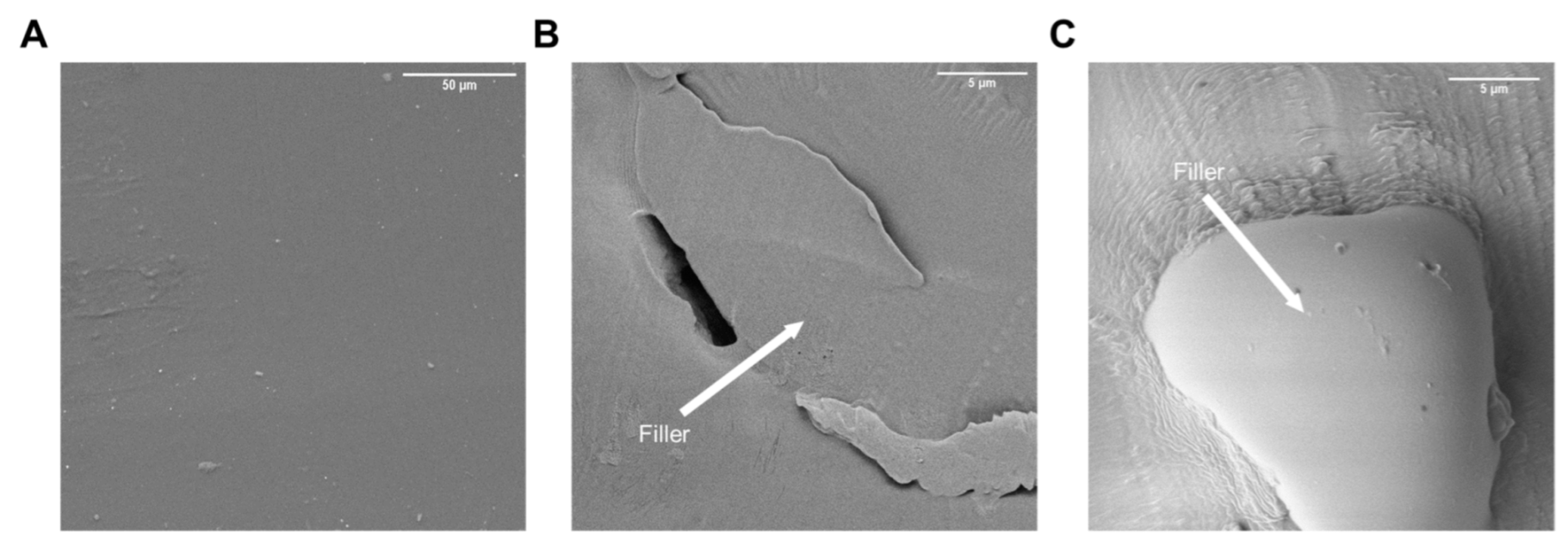

| PEG | PCL | PE | Starch | Sample * |
|---|---|---|---|---|
| 5% | 90% | 5% | 0% | P1-0%-AL |
| 1% | P1-1%-AL-(N ó Z) | |||
| 2% | P1-2%-AL-(N ó Z) | |||
| 3% | P1-3%-AL-(N ó Z) | |||
| 45% | 45% | 10% | 0% | P2-0%-AL |
| 1% | P2-1%-AL-(N ó Z) | |||
| 2% | P2-2%-AL-(N ó Z) | |||
| 3% | P2-3%-AL-(N ó Z) | |||
| 46.25% | 46.25% | 7.5% | 0% | P3-0%-AL |
| 1% | P3-1%-AL-(N ó Z) | |||
| 2% | P3-2%-AL-(N ó Z) | |||
| 3% | P3-3%-AL-(N ó Z) |
| PU | Starch | Concentration | Contact Angle (°) ** | Area Under Curve (%Wt·h) ** | Tmax (°C) ** | Tensile Strenght (σ) (MPa) ** | Module (E) (MPa) ** |
|---|---|---|---|---|---|---|---|
| P1 | AL-0% | 103.7 ± 6.6 a | 1.60 ± 0.93 a | 420.29 ± 12.61 a | 2.4 ± 0.28 a | 1.99 ± 0.53 abcd | |
| AL-N | 1% | 104.6 ± 4.7 a | 0.96 ± 0.72 a | 407.72 ± 11.16 a | 3.67 ± 0.18 b | 2.13 ± 0.08 abcd | |
| 2% | 100.5 ±5.2 a | 2.52 ± 1.21 a | 415.78 ± 13.52 a | 2.34 ± 0.77 a | 2.25 ± 0.65 acd | ||
| 3% | 104.4 ± 1.8 a | 2.34 ± 0.52 a | 406.67 ± 5.52 a | 2.24 ± 0.27 a | 2.55 ± 0.23 a | ||
| AL-Z | 1% | 101.0 ± 1.3 a | 2.12 ± 1.43 a | 424.89 ± 8.10 a | 1.28 ± 0.14 c | 1.20 ± 0.024 bcd | |
| 2% | 102.9 ± 1.5 a | 3.58 ± 0.44 a | 428.61 ± 2.04 a | 1.19 ± 0.039 c | 1.27 ± 0.12 cd | ||
| 3% | 110.3 ± 3.4 a | 3.68 ± 1.10 a | 429.22 ± 2.53 a | 1.15 ± 0.17 c | 1.46 ± 0.014 d | ||
| p-values | |||||||
| Starch | 0.524 | 0.071 | 0.004 * | <0.001 * | <0.001 * | ||
| Concentration | 0.181 | 0.030 * | 0.704 | 0.002 * | 0.300 | ||
| Starch: Concentration | 0.332 | 0.620 | 0.248 | <0.001 * | 0.070 | ||
| P2 | AL-0% | 99.5 ± 6.5 a | 23.86 ± 6.76 a | 395.89 ± 5.59 a | 4.76 ± 0.16 a | 3.21 ± 0.96 a | |
| AL-N | 1% | 99.5 ± 7.7 a | 24.17 ± 5.24 a | 398.83 ± 3.75 a | 4.08 ± 0.62 abc | 3.27 ± 0.72 a | |
| 2% | 102.8 ± 3.6 a | 22.12 ± 2.79 a | 400.89 ± 2.59 a | 4.48 ± 0.90 a | 3.65 ± 0.81 a | ||
| 3% | 104.3 ± 1.4 a | 20.34 ± 2.28 a | 405.22 ± 3.14 a | 4.12 ± 0.13 abc | 4.05 ± 0.47 a | ||
| AL-Z | 1% | 83.4 ± 8.4 abc | 30.52 ± 2.19 a | 400.78 ± 8.68 a | 3.82 ± 0.47 abc | 3.20 ± 0.28 a | |
| 2% | 57.9 ± 15.8 bc | 29.38 ± 4.19 a | 403.78 ± 2.45 a | 2.87 ± 0.34 bc | 3.05 ± 0.60 a | ||
| 3% | 68.5 ± 17.9 c | 30.90 ± 1.88 a | 402.83 ± 13.10 a | 2.59 ± 0.93 c | 3.04 ± 1.2 a | ||
| p-values | |||||||
| Starch | <0.001 * | 0.004 * | 0.823 | 0.002 * | 0.220 | ||
| Concentration | 0.028 * | 0.615 | 0.200 | 0.003 * | 0.888 | ||
| Starch: Concentration | 0.006 * | 0.257 | 0.902 | 0.042 * | 0.677 | ||
| P3 | AL-0% | 80.5 ± 17.6 ab | 32.28 ± 1.98 a | 401.11 ± 4.94 a | 3.13 ± 0.19 a | 1.52 ± 0.19 a | |
| AL-N | 1% | 81.3 ± 11.8 ab | 36.57 ± 3.18 a | 406.44 ± 2.38 a | 2.87 ± 0.96 a | 1.37 ± 0.24 a | |
| 2% | 77.1 ± 3.7 ab | 35.67 ± 2.45 a | 409.61 ± 3.67 a | 2.48 ± 0.35 acd | 1.32 ± 0.06 a | ||
| 3% | 84.5 ± 5.5 a | 31.85 ± 0.94 a | 412.11 ± 2.43 a | 1.78 ± 0.29 bcde | 1.12 ± 0.19 a | ||
| AL-Z | 1% | 57.5 ± 16.1 ab | 34.39 ± 2.48 a | 412.56 ± 2.59 a | 1.54 ± 0.29 cde | 1.51 ± 0.31 a | |
| 2% | 43.1 ± 24.2 ab | 29.67 ± 5.64 a | 412.78 ± 0.09 a | 1.63 ± 0.37 de | 1.39 ± 0.19 a | ||
| 3% | 39.4 ± 14.8 b | 29.30 ± 10.86 a | 411.99 ± 9.78 a | 1.20 ± 0.30 e | 1.17 ± 0.21 a | ||
| p-values | |||||||
| Starch | <0.001 * | 0.187 | 0.249 | <0.001 * | 0.448 | ||
| Concentration | 0.128 | 0.378 | 0.003 * | <0.001 * | 0.034 * | ||
| Starch: Concentration | 0.108 | 0.749 | 0.625 | 0.102 | 0.946 | ||
| PU | Starch | Concentration | Max E’ (MPa) ** | Tg from DMA (°C) ** | Tg from DSC (°C) | DC (%) |
|---|---|---|---|---|---|---|
| P1 | AL-0% | 3004 ± 1508 a | −32.89 ± 0.74 a | −46.7 | 73.6% | |
| AL-N | 1% | 3042 ± 697 a | −32.65 ± 0.39 a | −47.7 | 63.7% | |
| 2% | 2703 ± 1357 a | −33.02 ± 0.42 a | −46.8 | 63.2% | ||
| 3% | 3041 ± 729 a | −33.61 ± 0.80 a | −47.7 | 68.9% | ||
| AL-Z | 1% | 3297 ± 127 a | −33.75 ± 1.75 a | −50.2 | 68.9% | |
| 2% | 3328 ± 388 a | −34.83 ± 0.82 a | −49.7 | 54.1% | ||
| 3% | 3512 ± 647 a | −35.80 ± 2.05 a | −49.2 | 72.3% | ||
| p-values | ||||||
| Starch | 0.334 | 0.045 * | - | - | ||
| Concentration | 0.890 | 0.052 | - | - | ||
| Starch: Concentration | 0.922 | 0.682 | - | - | ||
| P2 | AL−0% | 4054 ± 274 a | −24.94 ± 1.77 a | −42.7 | 64.2% | |
| AL-N | 1% | 4708 ± 702 a | −28.90 ± 1.41 a | −41.9 | 69.2% | |
| 2% | 4513 ± 617 a | −24.28 ± 1.42 a | −40.3 | 59.3% | ||
| 3% | 4668 ± 455 a | −24.28 ± 1.26 a | −39.8 | 61.8% | ||
| AL-Z | 1% | 9588 ± 2903 bcd | −38.69 ± 4.43 bcd | −48.4 | 71.1% | |
| 2% | 8537 ± 3620 cd | −40.23 ± 2.83 cd | −44.2 | 72.4% | ||
| 3% | 6447 ± 451 d | −41.36 ± 5.86 d | −40.3 | 66.8% | ||
| p-values | ||||||
| Starch | <0.001 * | <0.001 * | - | - | ||
| Concentration | 0.012 * | 0.012 * | - | - | ||
| Starch: Concentration | 0.013 * | 0.014 * | - | - | ||
| P3 | AL-0% | 4913 ± 328a | −35.16 ± 0.92a | −48.0 | 71.3% | |
| AL-N | 1% | 6791 ± 2084 ab | −41.01 ± 1.61 ab | −48.1 | 60.6% | |
| 2% | 5744 ± 40 ab | −41.48 ± 1.65 ab | −48.1 | 69.0% | ||
| 3% | 6045 ± 1936 ab | −38.00 ± 1.75 a | −48.2 | 70.5% | ||
| AL-Z | 1% | 7981 ± 2676 ab | −40.66 ± 1.88 ab | −47.8 | 69.4% | |
| 2% | 8401 ± 1882 ab | −40.86 ± 5.03 ab | −48.3 | 72.1% | ||
| 3% | 11,694 ± 3883 b | −45.36 ± 0.46 b | −48.2 | 66.7% | ||
| p-values | ||||||
| Starch | 0.021 * | 0.092 | - | - | ||
| Concentration | 0.037 * | <0.001 * | - | - | ||
| Starch: Concentration | 0.215 | 0.043 * | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cespedes, J.F.; Arévalo-Alquichire, S.; Diaz, L.E.; Valero, M.F. Influence of Starch on the Structure–Properties Relationship in Polyethylene Glycol/Polycaprolactone Diol Polyurethanes. Polymers 2022, 14, 3184. https://doi.org/10.3390/polym14153184
Cespedes JF, Arévalo-Alquichire S, Diaz LE, Valero MF. Influence of Starch on the Structure–Properties Relationship in Polyethylene Glycol/Polycaprolactone Diol Polyurethanes. Polymers. 2022; 14(15):3184. https://doi.org/10.3390/polym14153184
Chicago/Turabian StyleCespedes, Jhoan F., Said Arévalo-Alquichire, Luis E. Diaz, and Manuel F. Valero. 2022. "Influence of Starch on the Structure–Properties Relationship in Polyethylene Glycol/Polycaprolactone Diol Polyurethanes" Polymers 14, no. 15: 3184. https://doi.org/10.3390/polym14153184
APA StyleCespedes, J. F., Arévalo-Alquichire, S., Diaz, L. E., & Valero, M. F. (2022). Influence of Starch on the Structure–Properties Relationship in Polyethylene Glycol/Polycaprolactone Diol Polyurethanes. Polymers, 14(15), 3184. https://doi.org/10.3390/polym14153184







