Improvement in Thermal Stability of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Blending with Native Cassava Starch
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PLLA/Starch and PLLA-PEG-PLLA/Starch Composites
2.3. Characterization of PLLA/Starch and PLLA-PEG-PLLA/Starch Composites
3. Results and Discussion
3.1. Thermal Transition Properties
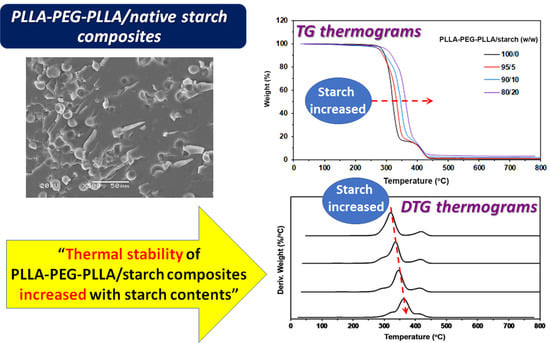
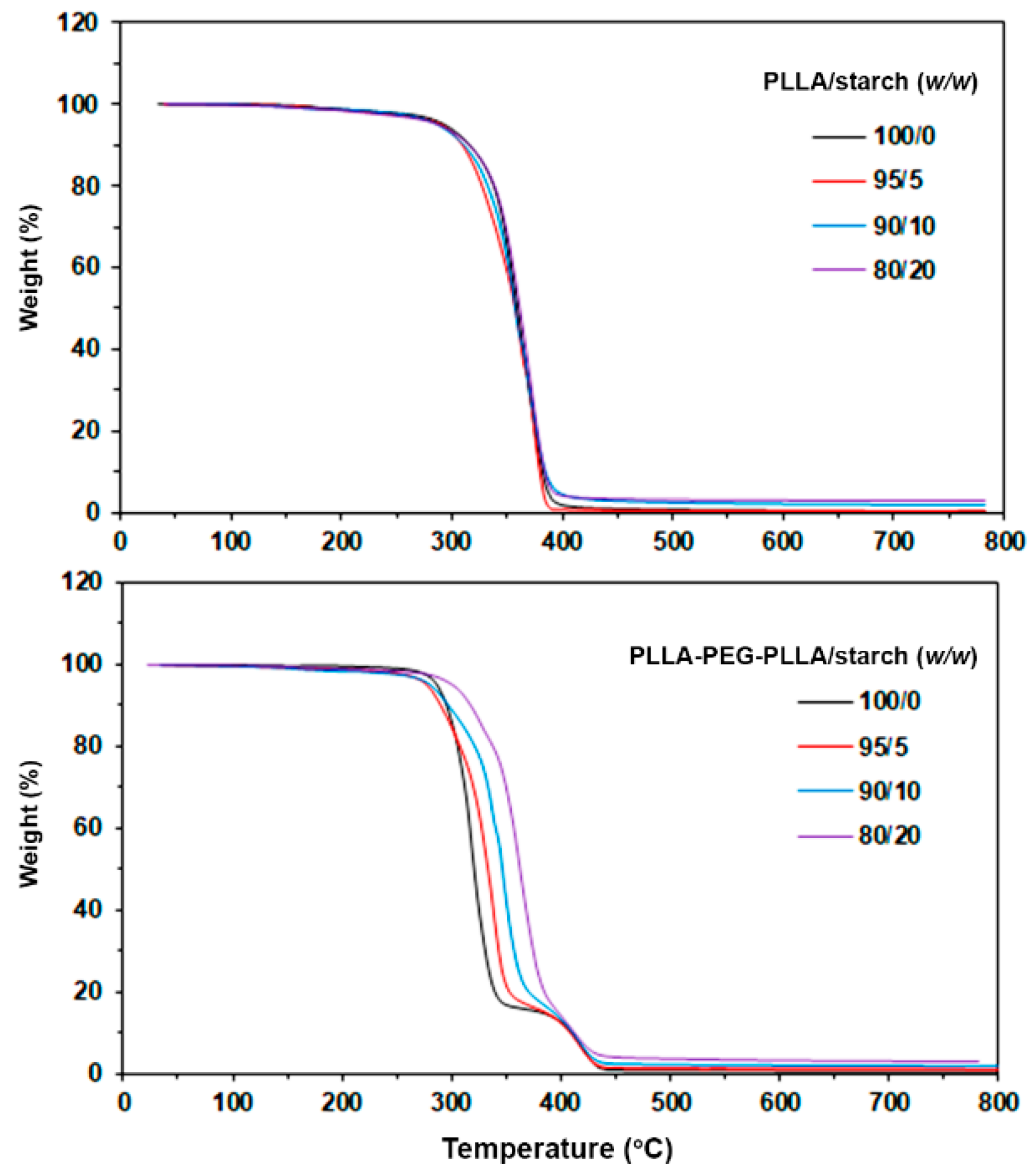
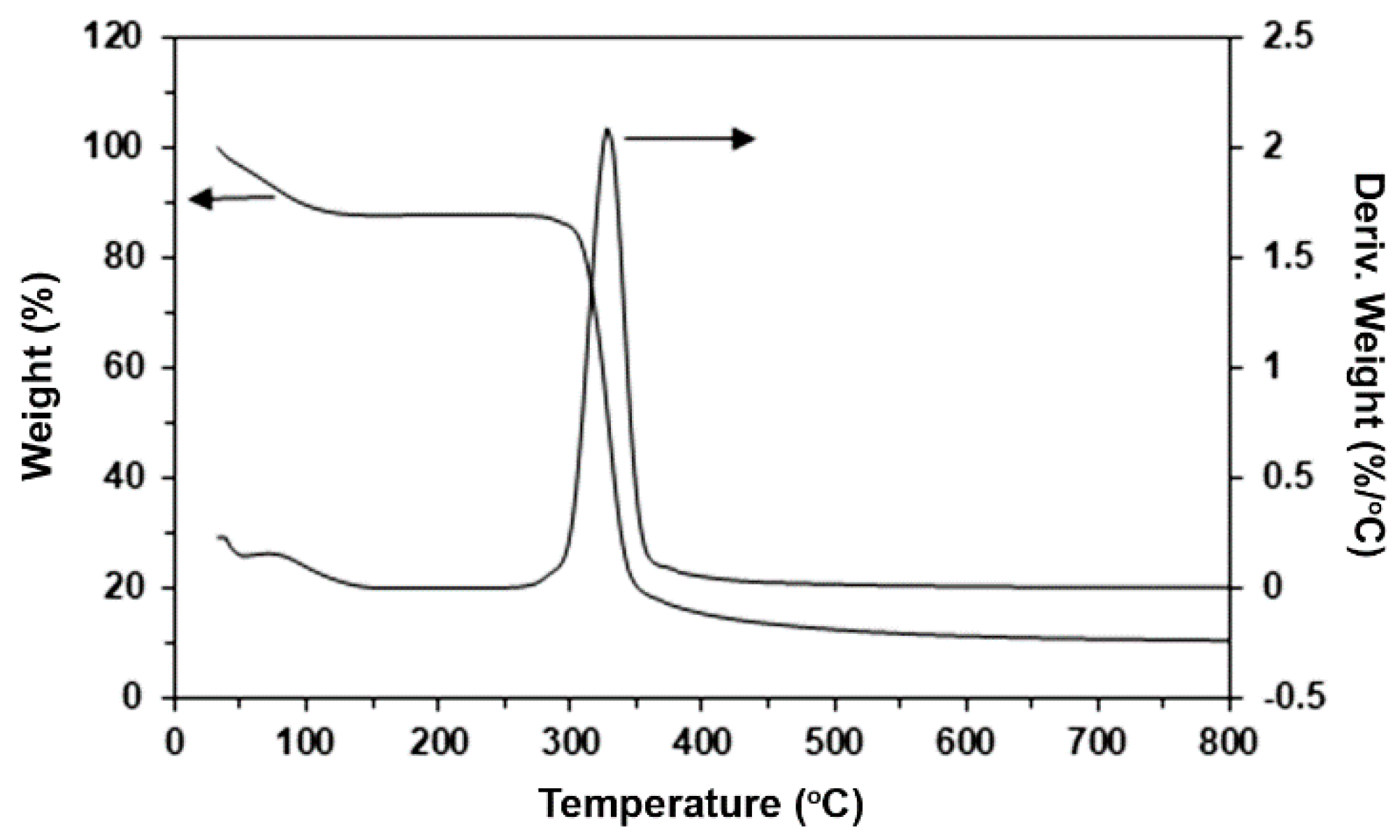
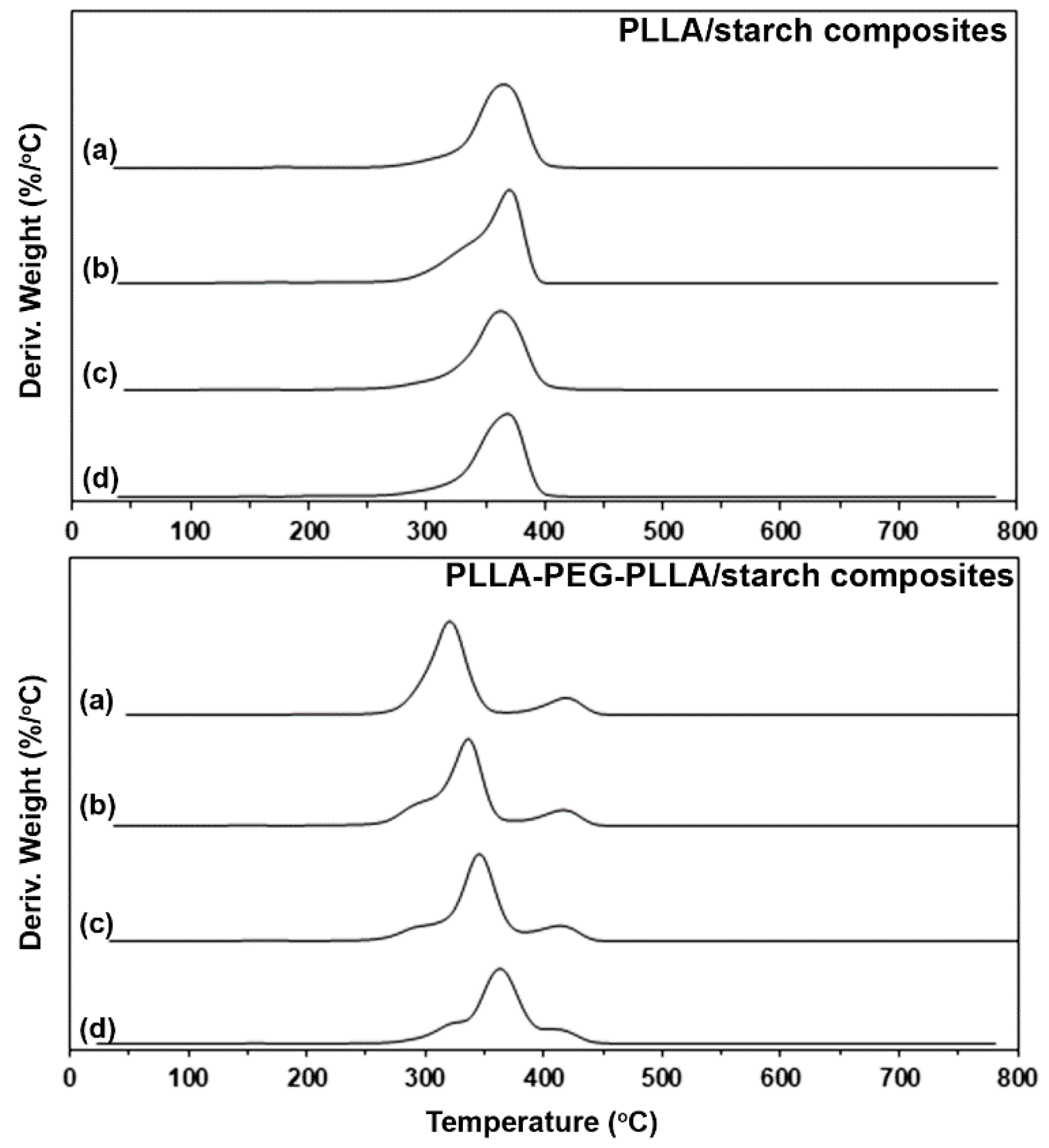
3.2. Thermal Decomposition Behaviors
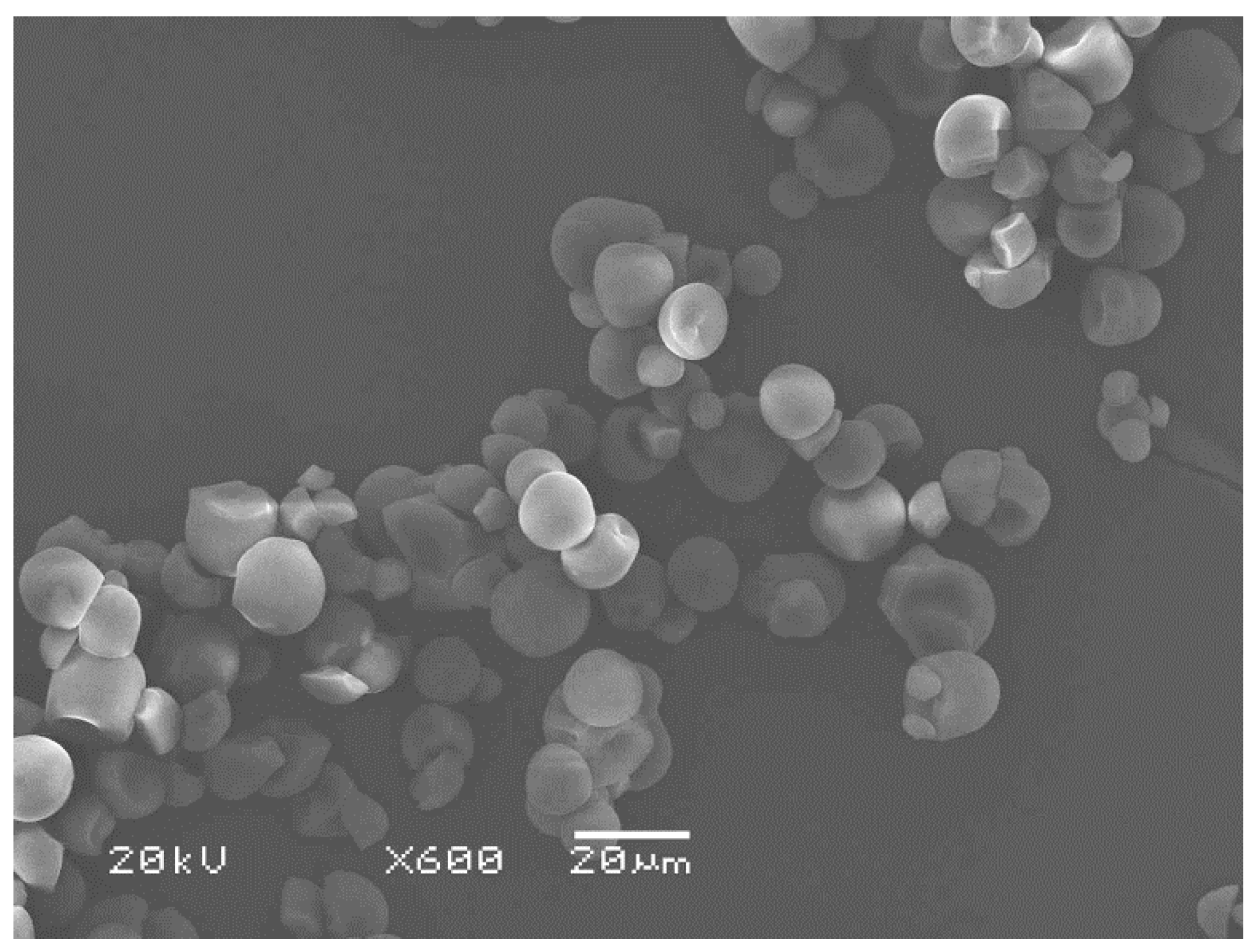
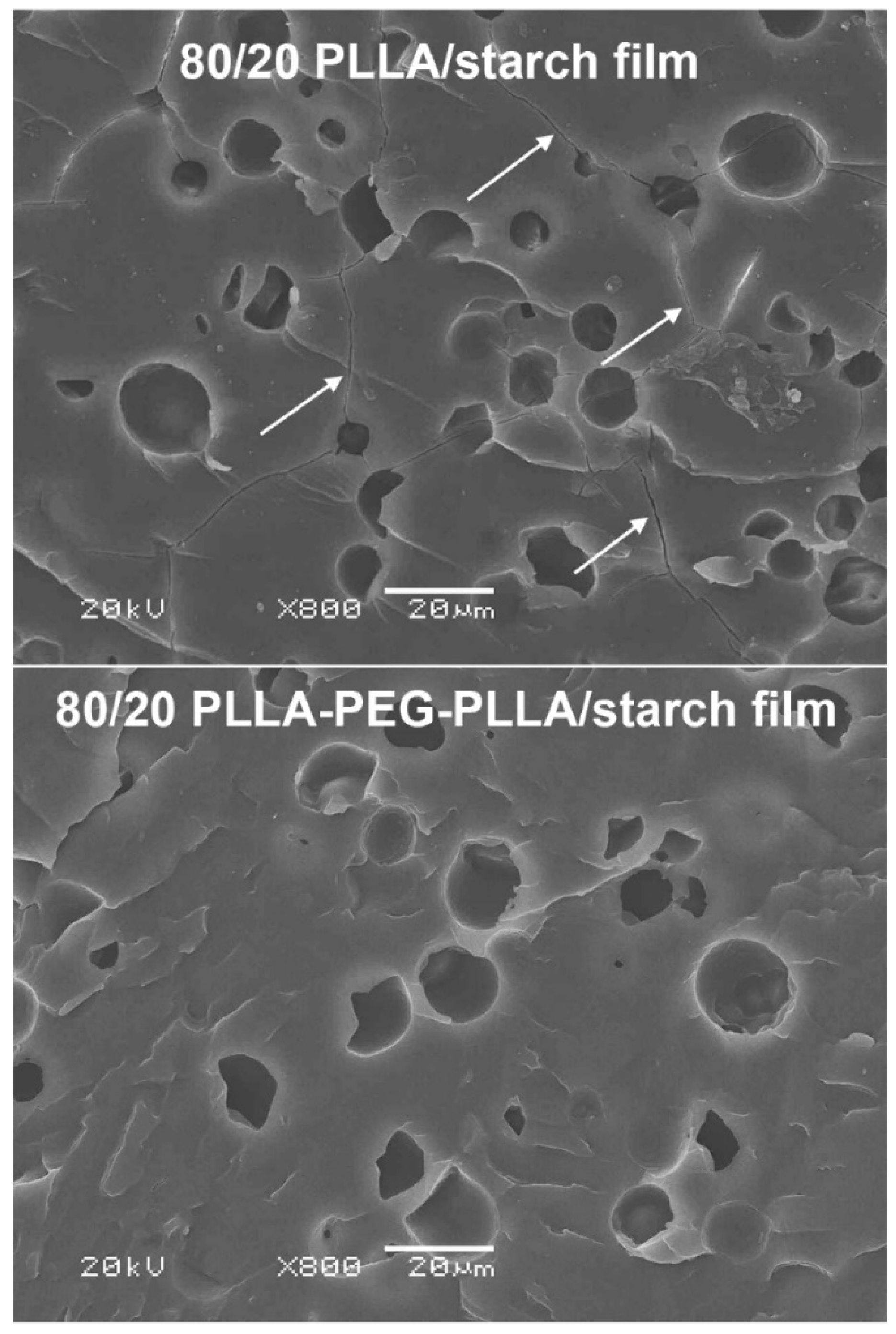
3.3. Phase Morphology
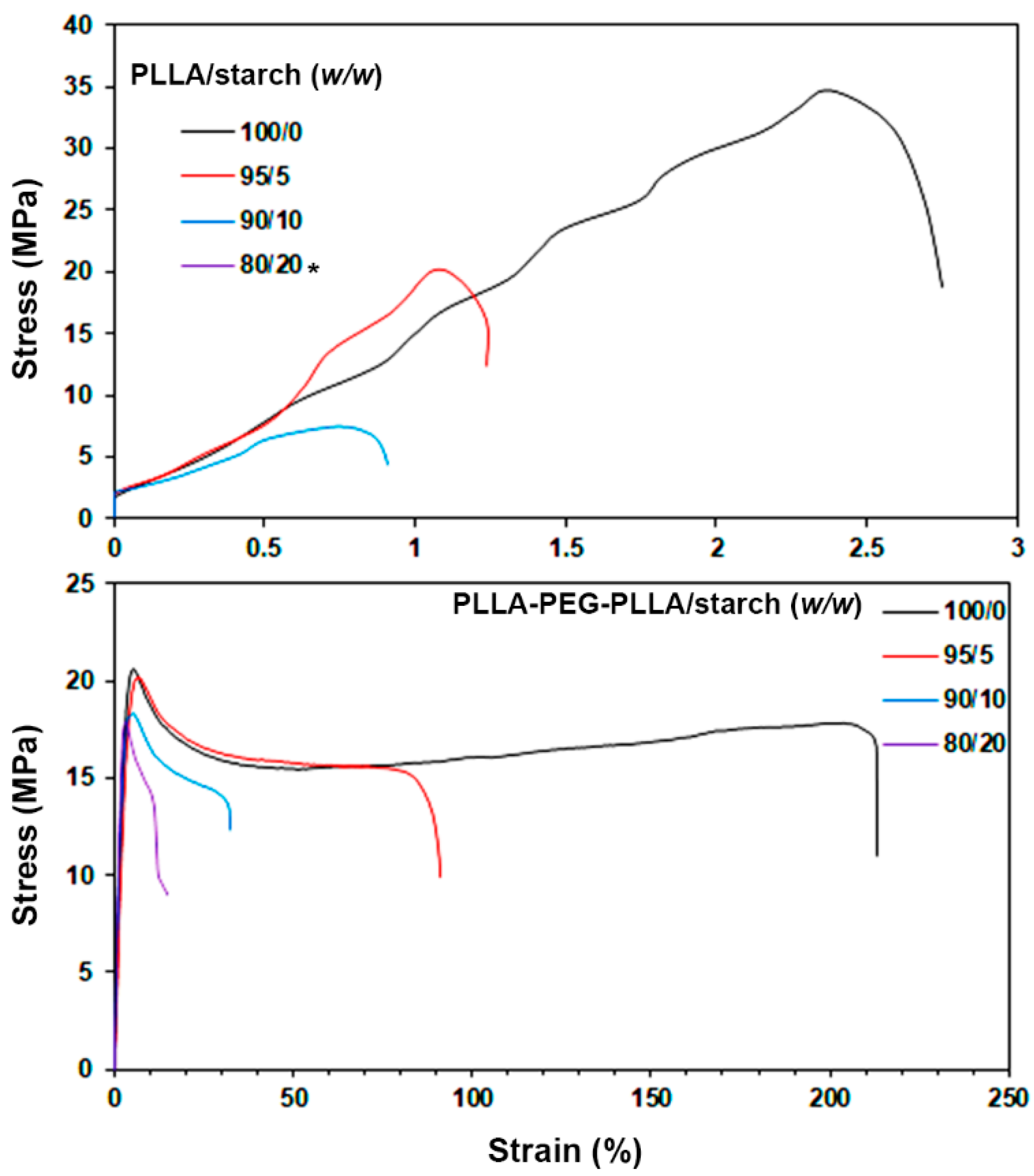
3.4. Tensile Properties
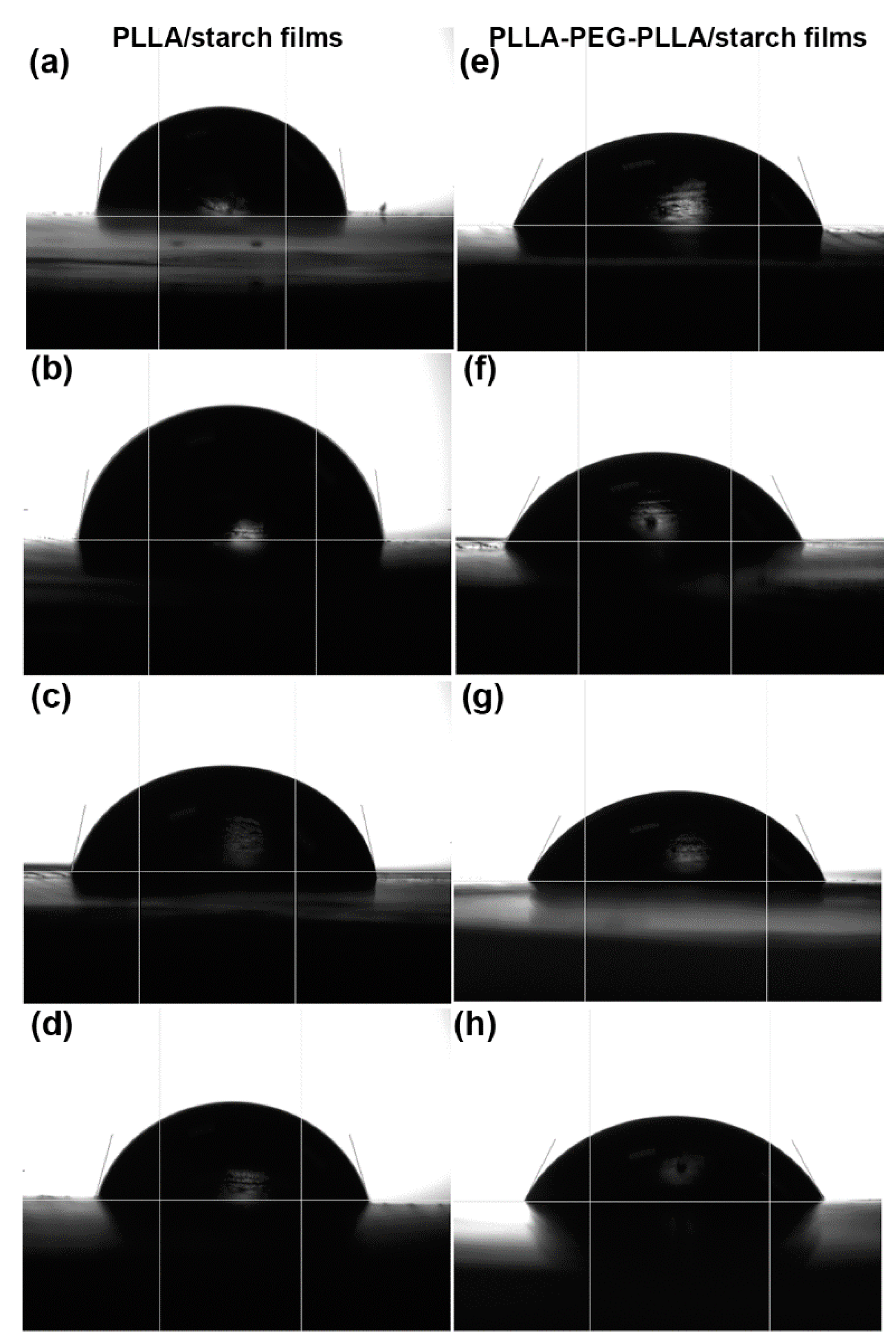
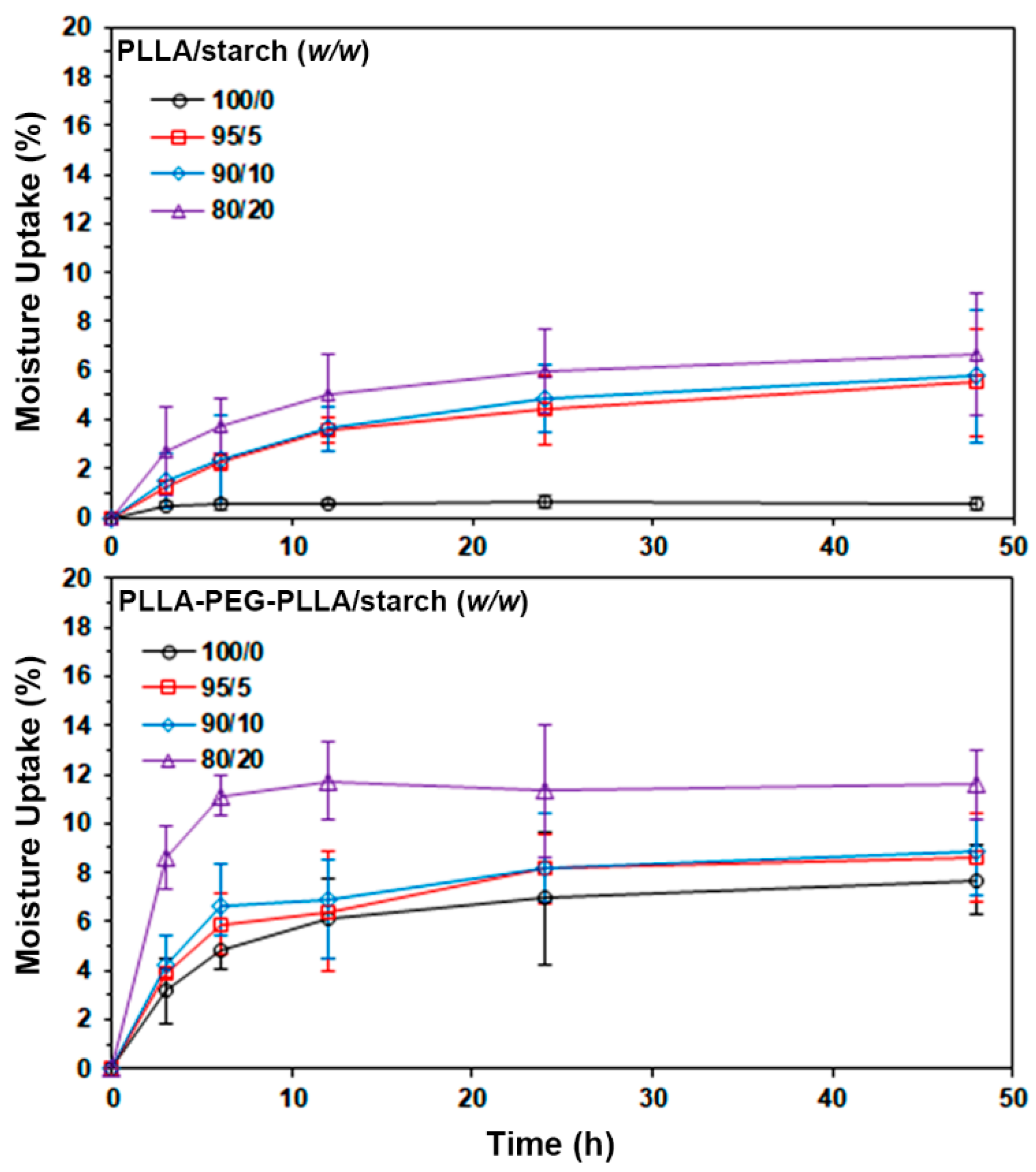
3.5. Water Contact Angle and Moisture Uptake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Hu, X.L.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, L.J.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Angelova, L.; Filipov, E.; Aceti, D.; Mincheva, R.; Carrete, X.; Kerdjoudj, H.; Dubus, M.; Chevrier, J.; Trifonov, A.; et al. Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior. Polymers 2021, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Ruzaidi, D.A.; Mahat, M.M.; Shafiee, S.A.; Mohamed Sofian, Z.; Mohmad Sabere, A.S.; Ramli, R.; Osman, H.; Hamzah, H.H.; Zainal Ariffin, Z.; Sadasivuni, K.K. Advocating Electrically Conductive Scaffolds with Low Immunogenicity for Biomedical Applications: A Review. Polymers 2021, 13, 3395. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, S.H.; Rayung, M.; Abu, F.; Ahmad, S.; Fadil, F.; Karim, A.A.; Norizan, M.N.; Sarifuddin, N.; Mat Desa, M.S.Z.; Mohd Basri, M.S.; et al. A Review on Antimicrobial Packaging from Biodegradable Polymer Composites. Polymers 2022, 14, 174. [Google Scholar] [CrossRef]
- Silva, D.D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Rotiman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Rihayat, T.; Hadi, A.E.; Aidy, N.; Safitri, A.; Siregar, J.P.; Cionita, T.; Irawan, A.P.; Hamdan, M.H.M.; Fitriyana, D.F. Biodegradation of Polylactic Acid-Based Bio Composites Reinforced with Chitosan and Essential Oils as Anti-Microbial Material for Food Packaging. Polymers 2021, 13, 4019. [Google Scholar] [CrossRef]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Tsou, C.-H.; Tsai, M.-L.; Guo, J.; De Guzman, M.R.; Yang, T.; Gao, C.; Lei, Y.; Gan, P.-W.; Chen, S.; et al. Barrier Properties and Hydrophobicity of Biodegradable Poly(lactic acid) Composites Reinforced with Recycled Chinese Spirits Distiller’s Grains. Polymers 2021, 13, 2861. [Google Scholar] [CrossRef]
- Li, M.-X.; Ren, Y.; Lee, D.; Choi, S.-W. Crystallization Behavior and Electrical Properties of Nanoparticle-Reinforced Poly(lactic Acid)-Based Films. Polymers 2022, 14, 177. [Google Scholar] [CrossRef]
- Yun, X.; Li, X.; Jin, Y.; Sun, W.; Dong, T. Fast crystallization and toughening of poly(L-lactic acid) by incorporating with poly(ethylene glycol) as a middle block chain. Polym. Sci. Ser. A 2018, 60, 141–155. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymorama, N. Improvement in melt flow property and flexibility of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) by chain extension reaction for potential use as flexible bioplastics. Mater. Des. 2018, 154, 73–80. [Google Scholar] [CrossRef]
- Baimark, Y.; Srisuwan, Y. Thermal and mechanical properties of highly flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastics: Effects of poly(ethylene glycol) block length and chain extender. J. Elastomers Plast. 2020, 52, 142–158. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Improvement in crystallization and toughness of poly(L-lactide) by melt blending with poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide) in the presence of chain extender. Polym. Sci. Ser. A 2021, 63, S34–S45. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Baimark, Y. Thermal, morphological and mechanical properties of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide)/thermoplastic starch blends. Carbohydr. Polym. 2022, 283, 119155. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly(lactic acid) and starch for biodegradable food packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Loginova, A.A.; Ivanushkina, N.E.; Vladimirov, L.V.; Prut, E.V.; Berlin, A.A. Influence of PEG on mechanical properties and biodegradability of composites based on PLA and starch. Starch 2018, 70, 1700268. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Prut, E.V.; Aleksanyan, K.V.; Krasheninnikov, V.G.; Perepelitsina, E.; Shashkin, D.P.; Berlin, A.A. Composites based on starch and polylactide. Polym. Sci. Ser. B 2019, 61, 334–340. [Google Scholar] [CrossRef]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure characterization and biodegradation rate of poly(ε-caprolactone)/starch blends. Front. Mater. 2020, 7, 141. [Google Scholar] [CrossRef]
- Adorna, J.A.; Aleman, C.K.A.; Gonzaga, I.L.E.; Pangasinan, J.N.; Sisican, K.M.D.; Dang, V.D.; Doong, R.-A.; Ventura, R.L.G.; Ventura, J.-R.S. Effect of lauric acid on the thermal and mechanical properties of polyhydroxybutyrate (PHB)/starch composite biofilms. Int. J. Polym. Sci. 2020, 2020, 7947019. [Google Scholar] [CrossRef]
- Lago, R.C.; Oliveira, A.L.M.; Santos, A.A.; Zitha, E.Z.; Carvalho, E.E.N.; Tonoli, G.H.D.; Boas, E.V.B.V. Addition of wheat straw nanofibrils to improve the mechanical and barrier properties of cassava starch–based bionanocomposites. Ind. Crops Prod. 2021, 170, 113816. [Google Scholar] [CrossRef]
- Noivoil, N.; Yoksan, R. Oligo(lactic acid)-grafted starch: A compatibilizer for poly(lactic acid)/thermoplastic starch blend. Int. J. Biol. Macromol. 2020, 160, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Nishikawa, K.; Inoue, Y.; Yazawa, K. Nucleation enhancement effect in poly(L-lactide) (PLLA)/poly(ε-caprolactone) (PCL) blend induced by locally activated chain mobility resulting from limited miscibility. Macromolecules 2009, 42, 8335–8342. [Google Scholar] [CrossRef]
- Zhang, K.; Ran, X.; Wang, X.; Han, C.; Han, L.; Wen, X.; Zhuang, Y.; Dong, L. Improvement in toughness and crystallization of poly(l-lactic acid) by melt blending with poly(epichlorohydrin-co-ethylene oxide). Polym. Eng. Sci. 2011, 51, 2370–2380. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Chirachanchai, S. Silane modified starch for compatible reactive blend with poly(lactic acid). Carbohydr. Polym. 2014, 106, 255–263. [Google Scholar] [CrossRef]
- Ferrarezi, M.M.F.; de Oliveira Taipina, M.; Escobar da Silv, L.C.; Goncalves, M.C. Poly(ethylene glycol) as a compatibilizer for poly(lactic acid)/thermoplastic starch blends. J. Polym. Environ. 2013, 21, 151–159. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Han, L.; Han, C.; Xu, K.; Zhou, C.; Zhang, M.; Dong, L. Improvement in toughness and crystallization of poly(L-lactic acid) by melt blending with ethylene/methyl acrylate/glycidyl methacrylate terpolymer. Polym. Eng. Sci. 2013, 53, 2498–2508. [Google Scholar] [CrossRef]
- Mothe, C.G.; Azevedo, A.D.; Drumond, W.S.; Wang, S.H. Thermal properties of amphiphilic biodegradable triblock copolymer of L,L-lactide and ethylene glycol. J. Therm. Anal. Calorim. 2010, 101, 229–233. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Gollveia, R.F.; de Carvalho, G.M.; Rubira, A.F.; Muniz, E.C. Polymer blends based on PEO and starch: Miscibility and spherulite growth rate evaluated through DSC and optical microscopy. Mater. Sci. Eng. C 2009, 29, 499–504. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Vu, T.T.; Grillet, A.-C.; Ha Thuc, H.; Ha Thuc, C.N. Effect of organoclay on morphology and properties of linear low density polyethylene and Vietnamese cassava starch biobased blend. Carbohydr. Polym. 2016, 136, 163–170. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Raquez, J.-M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly(lactic acid) (PLA)/thermoplastic starch (TPS) blends. Polym. Plast. Technol. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R.; Altstädt, V. Poly(lactic acid)/coplasticized thermoplastic starch blend: Effect of plasticizer migration on rheological and mechanical properties. Polym. Adv. Technol. 2019, 30, 839–851. [Google Scholar] [CrossRef]
- Xu, G.; Chen, S.; Yan, X.; Yang, C.; Chen, Z. Synthesis and hydrophilic performance of poly(lactic acid)-poly(ethylene glycol) block copolymers. Am. J. Anal. Chem. 2016, 7, 299–305. [Google Scholar] [CrossRef][Green Version]
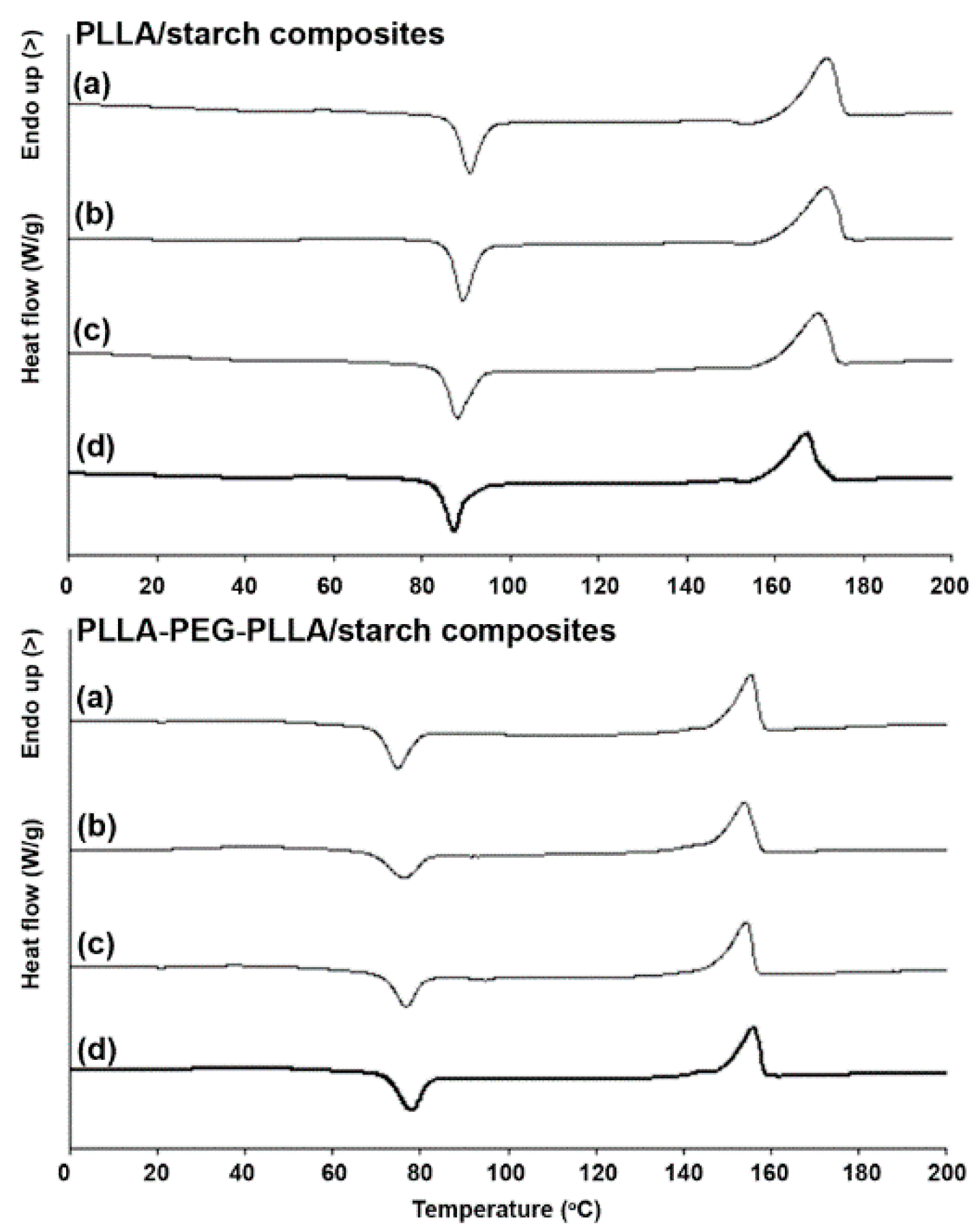

| Sample | Tg (°C) a | Tcc (°C) b | ΔHcc (J/g) c | Tm (°C) d | ΔHm (J/g) e | Xc (%) f |
|---|---|---|---|---|---|---|
| PLLA/starch (w/w) | ||||||
| 100/0 | 53 | 90 | 29.5 | 172 | 51.6 | 23.6 |
| 95/5 | 53 | 90 | 29.6 | 172 | 53.7 | 27.1 |
| 90/10 | 52 | 88 | 29.5 | 170 | 48.5 | 22.6 |
| 80/20 | 52 | 88 | 26.2 | 168 | 40.2 | 18.7 |
| PLLA-PEG-PLLA/starch (w/w) | ||||||
| 100/0 | 32 | 76 | 17.9 | 155 | 29.8 | 15.3 |
| 95/5 | 32 | 78 | 14.7 | 154 | 29.7 | 20.3 |
| 90/10 | 32 | 77 | 15.0 | 154 | 28.6 | 19.4 |
| 80/20 | 32 | 78 | 16.6 | 155 | 26.6 | 16.1 |
| Sample | 5%-Td (°C) a | Residue ash (wt%) b | PLLA-Td,max (°C) c | PEG-Td,max (°C) d |
|---|---|---|---|---|
| PLLA/starch (w/w) | ||||
| 100/0 | 291 | 0.06 | 365 | - |
| 95/5 | 291 | 0.59 | 369 | - |
| 90/10 | 287 | 1.64 | 366 | - |
| 80/20 | 285 | 2.61 | 369 | - |
| PLLA-PEG-PLLA/starch (w/w) | ||||
| 100/0 | 286 | 0.11 | 319 | 421 |
| 95/5 | 278 | 0.79 | 336 | 421 |
| 90/10 | 280 | 1.74 | 349 | 418 |
| 80/20 | 300 | 3.05 | 363 | 419 |
| Film Samples | Ultimate Tensile Stress (MPa) | Strain at Break (%) | Young’s Modulus (MPa) | Water Contact Angle (°) |
|---|---|---|---|---|
| PLLA/starch (w/w) | ||||
| 100/0 | 38.4 ± 3.1 | 3.4 ± 0.7 | 957 ± 57 | 81.5 ± 3.3 |
| 95/5 | 24.6 ± 2.5 | 1.8 ±0.4 | 782 ±68 | 81.1 ± 4.1 |
| 90/10 | 6.2 ± 2.2 | 0.8 ± 0.5 | 229 ± 62 | 77.8 ± 3.8 |
| 80/20 | - * | - * | - * | 75.1 ± 2.7 |
| PLLA-PEG-PLLA/starch (w/w) | ||||
| 100/0 | 20.4 ± 1.8 | 239.6 ± 15.1 | 321 ± 31 | 68.9 ± 4.2 |
| 95/5 | 20.6 ± 1.5 | 90.6 ± 12.5 | 251 ± 16 | 67.5 ± 3.1 |
| 90/10 | 19.8 ± 1.9 | 31.2 ± 8.2 | 238 ± 25 | 64.4 ± 2.5 |
| 80/20 | 17.4 ± 0.8 | 14.4 ± 5.3 | 212 ± 12 | 63.9 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisuwan, Y.; Baimark, Y. Improvement in Thermal Stability of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Blending with Native Cassava Starch. Polymers 2022, 14, 3186. https://doi.org/10.3390/polym14153186
Srisuwan Y, Baimark Y. Improvement in Thermal Stability of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Blending with Native Cassava Starch. Polymers. 2022; 14(15):3186. https://doi.org/10.3390/polym14153186
Chicago/Turabian StyleSrisuwan, Yaowalak, and Yodthong Baimark. 2022. "Improvement in Thermal Stability of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Blending with Native Cassava Starch" Polymers 14, no. 15: 3186. https://doi.org/10.3390/polym14153186
APA StyleSrisuwan, Y., & Baimark, Y. (2022). Improvement in Thermal Stability of Flexible Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Blending with Native Cassava Starch. Polymers, 14(15), 3186. https://doi.org/10.3390/polym14153186