Insights into the Design of Polyurethane Dressings Suitable for the Stages of Skin Wound-Healing: A Systematic Review
Abstract
:1. Introduction
- Is it possible that a single polyurethane dressing can help each stage of the wound-healing process?
- Should the polyurethane wound dressing design be based on each stage of the wound-healing process?
- What properties or characteristics should a polyurethane dressing have to perform well in each stage of the skin wound-healing process?
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
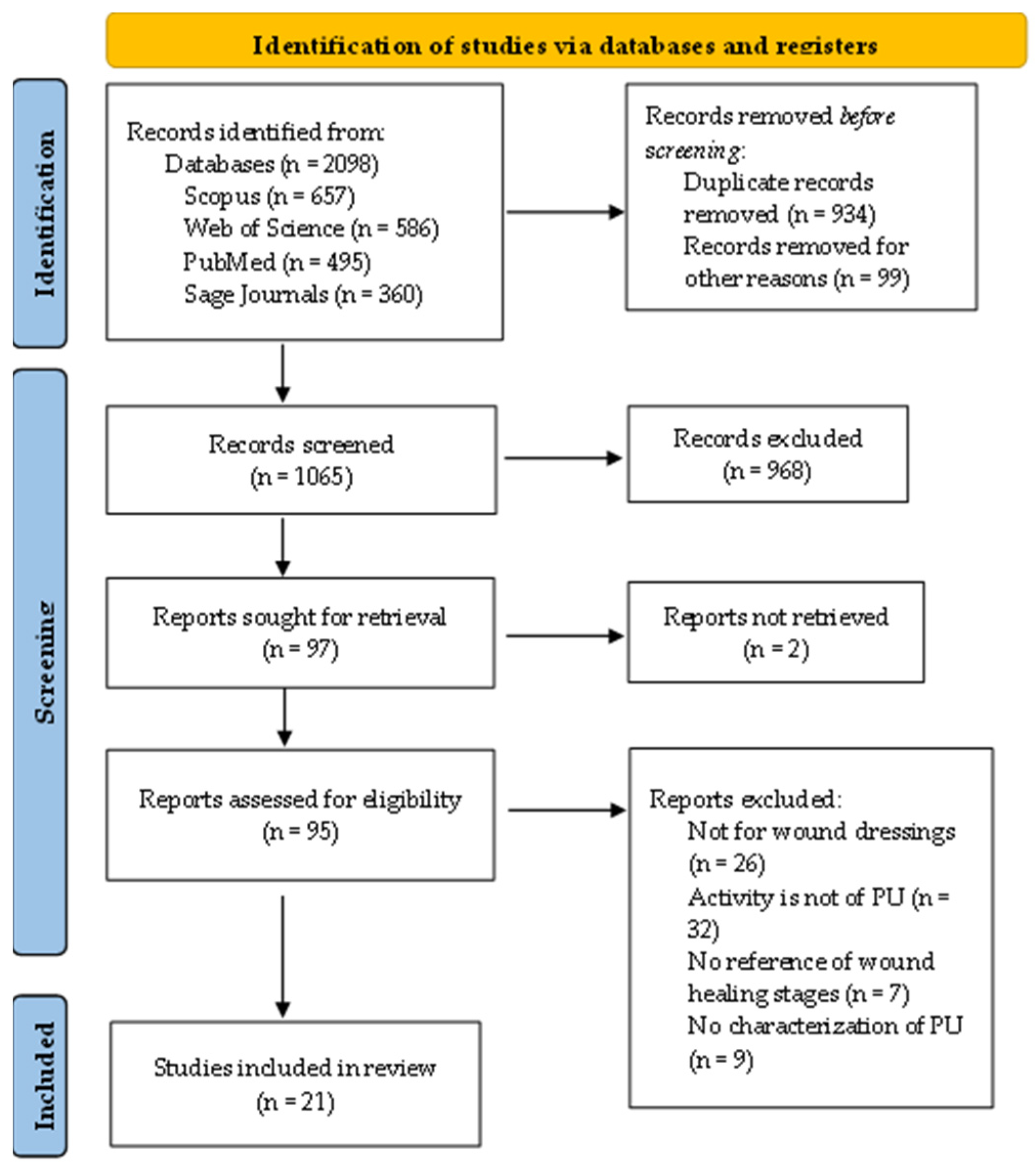
2.3. Selection and Data Collection Process
3. Results
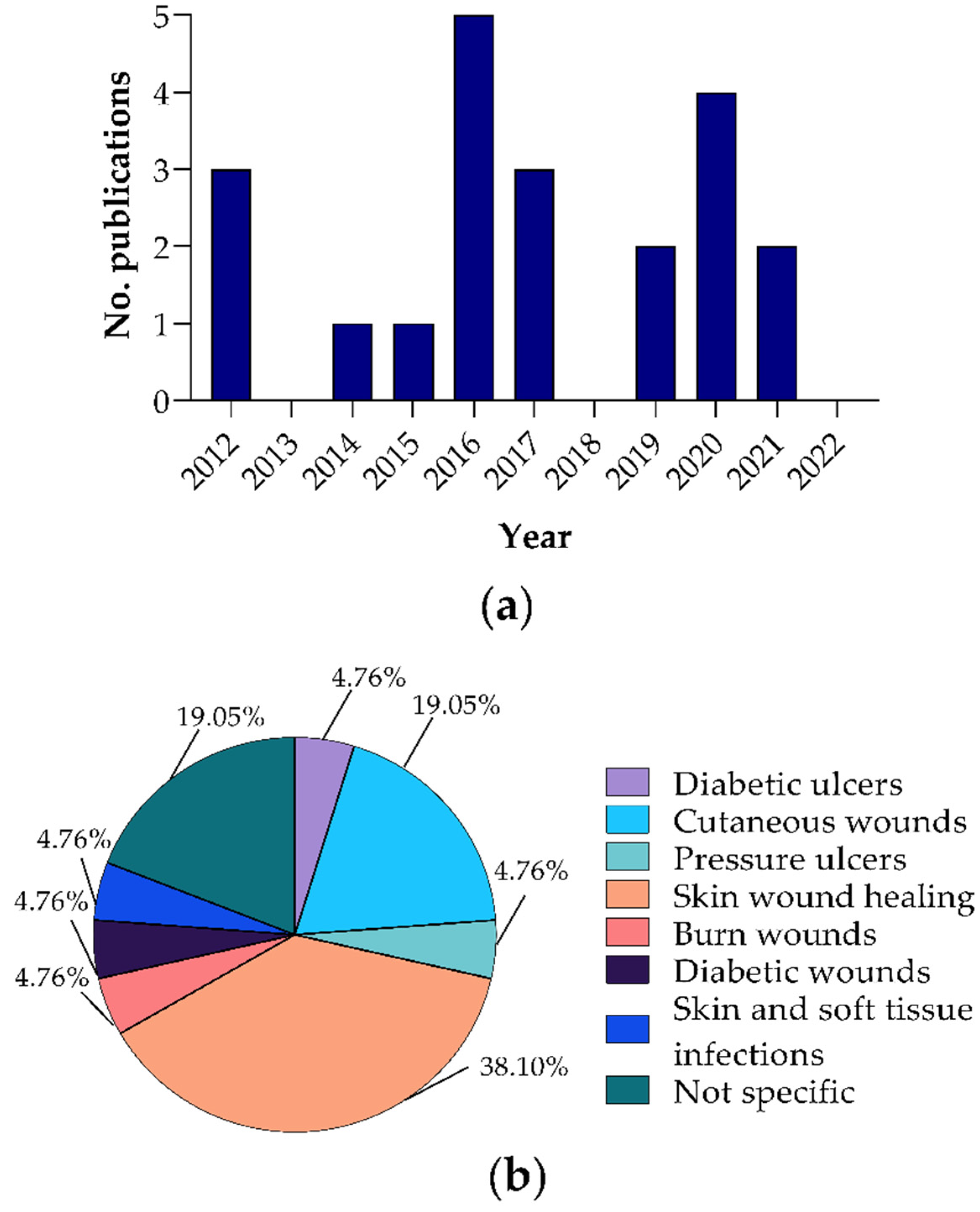
3.1. Selection and General Characteristics of the Included Reports
3.2. Polyurethane Dressings
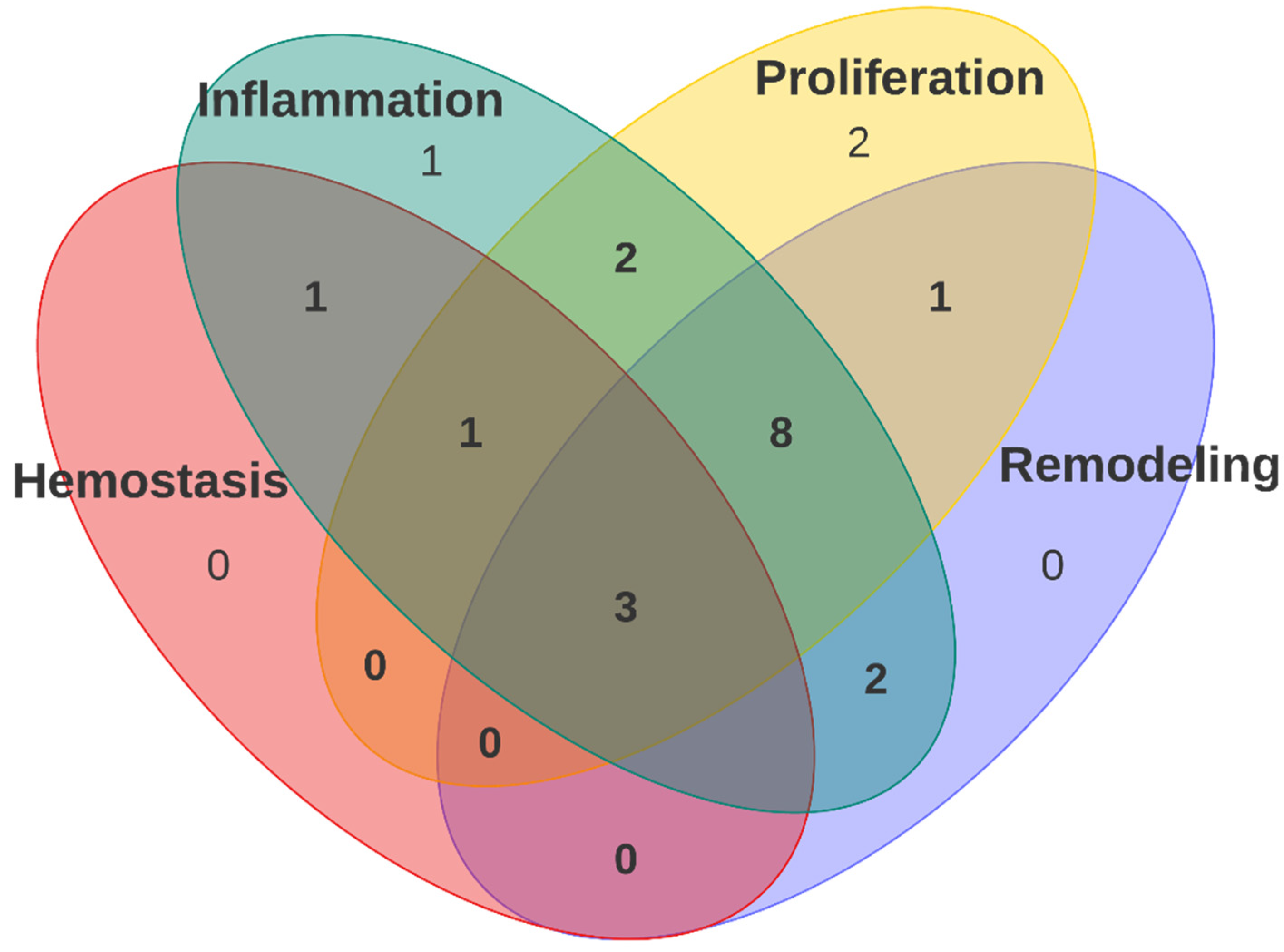
3.3. Polyurethane Dressing in Wound Healing Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun Polyurethane/Keratin/AgNP Biocomposite Mats for Biocompatible and Antibacterial Wound Dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Simões, S.; Ascenso, A.; Reis, C.P. Therapeutic Advances in Wound Healing. J. Dermatolog. Treat. 2022, 33, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A.M. Emerging Treatment Strategies in Wound Care. Int. Wound J. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef]
- Kasi, G.; Gnanasekar, S.; Zhang, K.; Tang Kang, E.; Qun Xu, L. Polyurethane-Based Composites with Promising Antibacterial Properties. J. Appl. Polym. Sci. 2022, 139, 52181. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Díaz, L.E.; Valero, M.F. In Vitro and in Vivo Biocompatibility of Polyurethanes Synthesized with Castor Oil Polyols for Biomedical Devices. J. Mater. Res. 2019, 34, 519–531. [Google Scholar] [CrossRef]
- Morales-Gonzalez, M.; Arévalo-Alquichire, S.; Diaz, L.E.; Sans, J.Á.; Vilariño-Feltrer, G.; Gómez-Tejedor, J.A.; Valero, M.F. Hydrolytic Stability and Biocompatibility on Smooth Muscle Cells of Polyethylene Glycol–Polycaprolactone-Based Polyurethanes. J. Mater. Res. 2020, 35, 3276–3285. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/Carboxymethylcellulose Nanofibers Containing Malva Sylvestris Extract for Healing Diabetic Wounds: Preparation, Characterization, in Vitro and in Vivo Studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Maity, P.P.; Goswami, P.; Datta, P.; Ghosh, S.K.; Mitra, A.; Dhara, S. Accelerated Healing of Full Thickness Dermal Wounds by Macroporous Waterborne Polyurethane-Chitosan Hydrogel Scaffolds. Mater. Sci. Eng. C 2017, 81, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Li, Z.; Xu, R.; Wang, Y.; Li, H.; Wang, Y.; Liu, M.; Yang, S.; Zhan, R.; Zhao, J.; et al. Biomimetic Thermoplastic Polyurethane Porous Membrane with Hierarchical Structure Accelerates Wound Healing by Enhancing Granulation Tissue Formation and Angiogenesis. RSC Adv. 2016, 6, 99595–99603. [Google Scholar] [CrossRef]
- Mousavi, M.A.; Abdi, Z.; Khavasi, N.; Sardari, S.; Tofangchiha, S. Bromelain-Ferula Gum-Loaded Polyurethane Nanofibers for Bedsore Healing in Rats. Eur. J. Plast. Surg. 2021, 44, 563–568. [Google Scholar] [CrossRef]
- Pahlevanneshan, Z.; Deypour, M.; Kefayat, A.; Rafienia, M.; Sajkiewicz, P.; Esmaeely Neisiany, R.; Enayati, M.S. Polyurethane-Nanolignin Composite Foam Coated with Propolis as a Platform for Wound Dressing: Synthesis and Characterization. Polymers 2021, 13, 3191. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled Water Vapor Transmission Rate Promotes Wound-Healing via Wound Re-Epithelialization and Contraction Enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [Green Version]
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In Vitro and in Vivo Performance of a Propolis-Coated Polyurethane Wound Dressing with High Porosity and Antibacterial Efficacy. Colloids Surf. B Biointerfaces 2019, 178, 177–184. [Google Scholar] [CrossRef]
- Guo, R.; Merkel, A.R.; Sterling, J.A.; Davidson, J.M.; Guelcher, S.A. Substrate Modulus of 3D-Printed Scaffolds Regulates the Regenerative Response in Subcutaneous Implants through the Macrophage Phenotype and Wnt Signaling. Biomaterials 2015, 73, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Adolph, E.J.; Pollins, A.C.; Cardwell, N.L.; Davidson, J.M.; Guelcher, S.A.; Nanney, L.B. Biodegradable Lysine-Derived Polyurethane Scaffolds Promote Healing in a Porcine Full-Thickness Excisional Wound Model. J. Biomater. Sci. Polym. Ed. 2014, 25, 1973–1985. [Google Scholar] [CrossRef] [Green Version]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A Propolis Enriched Polyurethane-Hyaluronic Acid Nanofibrous Wound Dressing with Remarkable Antibacterial and Wound Healing Activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive Anti-Oxidant Polyurethane Elastomers with Shape Memory Property as Non-Adherent Wound Dressing to Enhance Wound Healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Rezaei Hosseinabadi, S.; Parsapour, A.; Nouri Khorasani, S.; Razavi, S.M.; Hashemibeni, B.; Heidari, F.; Khalili, S. Wound Dressing Application of Castor Oil- and CAPA-Based Polyurethane Membranes. Polym. Bull. 2020, 77, 2945–2964. [Google Scholar] [CrossRef]
- Hao, H.; Shao, J.; Deng, Y.; He, S.; Luo, F.; Wu, Y.; Li, J.; Tan, H.; Li, J.; Fu, Q. Synthesis and Characterization of Biodegradable Lysine-Based Waterborne Polyurethane for Soft Tissue Engineering Applications. Biomater. Sci. 2016, 4, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, Y.; Chen, K.C.; Chen, S. Rapid Hemostatic and Mild Polyurethane-Urea Foam Wound Dressing for Promoting Wound Healing. Mater. Sci. Eng. C 2017, 71, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Li, Z.S.; Dong, S.W.; Chen, W.J.; Deng, L.; Wang, Y.F.; Ying, D.J. Piezoelectric PU/PVDF Electrospun Scaffolds for Wound Healing Applications. Colloids Surf. B Biointerfaces 2012, 96, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, R.; Wang, X.; Li, X.; Peng, F.; Jin, Z.; Gao, X.; Yu, J.; Wang, C. Electrospun Mupirocin Loaded Polyurethane Fiber Mats for Anti-Infection Burn Wound Dressing Application. J. Biomater. Sci. Polym. Ed. 2017, 28, 162–176. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Niu, Y.; Ye, J.; Huang, S.; Liu, C.; Xu, K. Synthesis and Wound Healing of Alternating Block Polyurethanes Based on Poly(Lactic Acid) (PLA) and Poly(Ethylene Glycol) (PEG). J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1200–1209. [Google Scholar] [CrossRef]
- Khandwekar, A.; Rho, C.K. Modulation of Cellular Responses on Engineered Polyurethane Implants. J. Biomed. Mater. Res. Part A 2012, 100A, 2211–2222. [Google Scholar] [CrossRef]
- Heit, Y.I.; Dastouri, P.; Helm, D.L.; Pietramaggiori, G.; Younan, G.; Erba, P.; Münster, S.; Orgill, D.P.; Scherer, S.S. Foam Pore Size Is a Critical Interface Parameter of Suction-Based Wound Healing Devices. Plast. Reconstr. Surg. 2012, 129, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Adolph, E.J.; Guo, R.; Pollins, A.C.; Zienkiewicz, K.; Cardwell, N.; Davidson, J.M.; Guelcher, S.A.; Nanney, L.B. Injected Biodegradable Polyurethane Scaffolds Support Tissue Infiltration and Delay Wound Contraction in a Porcine Excisional Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1679–1690. [Google Scholar] [CrossRef]
- Gholami, H.; Yeganeh, H. Vegetable Oil-Based Polyurethanes as Antimicrobial Wound Dressings: In Vitro and in Vivo Evaluation. Biomed. Mater. 2020, 15, 045001. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational Design of Biodegradable Thermoplastic Polyurethanes for Tissue Repair. Bioact. Mater. 2022, 15, 250–271. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Loos, K. Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins. Polymers 2019, 11, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.Y.; Beckman, E.J.; Hu, J.; Yang, G.G.; Agarwal, S.; Hollinger, J.O. Synthesis, Biodegradability, and Biocompatibility of Lysine Diisocyanate–Glucose Polymers. Tissue Eng. 2004, 8, 771–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreader, K.J.; Bayer, I.S.; Milner, D.J.; Loth, E.; Jasiuk, I. A Polyurethane-Based Nanocomposite Biocompatible Bone Adhesive. J. Appl. Polym. Sci. 2013, 127, 4974–4982. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and Stabilization of Polyurethane Elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Krsko, P.; Libera, M. Hydrogels Poly (Ethylene Glycol), or PEG, Is Used Extensively in Biomedical Device. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Horakova, J.; Mikes, P.; Saman, A.; Jencova, V.; Klapstova, A.; Svarcova, T.; Ackermann, M.; Novotny, V.; Suchy, T.; Lukas, D. The Effect of Ethylene Oxide Sterilization on Electrospun Vascular Grafts Made from Biodegradable Polyesters. Mater. Sci. Eng. C 2018, 92, 132–142. [Google Scholar] [CrossRef]
- Džunuzović, J.V.; Stefanović, I.S.; Džunuzović, E.S.; Dapčević, A.; Šešlija, S.I.; Balanč, B.D.; Lama, G.C. Polyurethane Networks Based on Polycaprolactone and Hyperbranched Polyester: Structural, Thermal and Mechanical Investigation. Prog. Org. Coat. 2019, 137, 105305. [Google Scholar] [CrossRef]
- Liu, X.; Xia, Y.; Liu, L.; Zhang, D.; Hou, Z. Synthesis of a Novel Biomedical Poly(Ester Urethane) Based on Aliphatic Uniform-Size Diisocyanate and the Blood Compatibility of PEG-Grafted Surfaces. J. Biomater. Appl. 2018, 32, 1329–1342. [Google Scholar] [CrossRef]
- Asadpour, S.; Ai, J.; Davoudi, P.; Ghorbani, M.; Jalali Monfared, M.; Ghanbari, H. In Vitro Physical and Biological Characterization of Biodegradable Elastic Polyurethane Containing Ferulic Acid for Small-Caliber Vascular Grafts. Biomed. Mater. 2018, 13, 035007. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Bu, Q.; Tao, N.; Chen, M.; Liu, H.; Zhou, J.; Liu, J.; Deng, B.; Kong, N.; Zhang, X.; et al. A Facile and General Method for Synthesis of Antibiotic-Free Protein-Based Hydrogel: Wound Dressing for the Eradication of Drug-Resistant Bacteria and Biofilms. Bioact. Mater. 2022, 18, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-A.; Qu, Z.; Lu, M.; Meng, H.; Zhan, Y.; Chen, B.; Wu, K.; Shi, J. Effect of Rosin on the Antibacterial Activity against S.Aureus and Adhesion Properties of UV-Curable Polyurethane/Polysiloxane Pressure-Sensitive Adhesive. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126146. [Google Scholar] [CrossRef]
- Wisessombat, S.; Tayeh, M. In Vitro Wound Healing Potential and Antimicrobial Activity of Clerodendrum Inerme Leave Extracts. Pharmacogn. J. 2021, 13, 1542–1548. [Google Scholar] [CrossRef]
- Zindle, J.K.; Wolinsky, E.; Bogie, K.M. A Review of Animal Models from 2015 to 2020 for Preclinical Chronic Wounds Relevant to Human Health. J. Tissue Viability 2021, 30, 291–300. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, K.P.; Wilhelm, D.; Bielfeldt, S. Models of Wound Healing: An Emphasis on Clinical Studies. Ski. Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef]
- Bailey, K.L.; Carlson, M.A. Porcine Models of Pancreatic Cancer. Front. Oncol. 2019, 9, 144. [Google Scholar] [CrossRef]

| Authors | Polyurethane | Monomers | Synthesis Technique | Modification | Wound Type | Dressing Type | Study Design | Wound Area | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Almasian et al., 2020 | Polyurethane/carboxymethyl cellulose (PU/CMC) composite | Polyurethane (MW = 110,000) and carboxymethyl cellulose (CMC) | Electrospinning | Malva sylvestris extract | Diabetic ulcers | Nanofibers | In vitro In vivo | Circle: 1.5 cm diameter | [11] |
| Bankoti et al., 2017 | Waterborne polyurethane-chitosan hydrogel scaffolds | Chitosan (MW 7,10,000) and polyurethane diol aqueous dispersion | Mechanical blending and casting | Chitosan | Skin wound healing | Scaffolds | In vitro In vivo | Square: 2 cm2 | [12] |
| Lei et al., 2016 | Biomimetic porous membrane composed of thermoplastic polyurethane (TPU) | Thermoplastic polyurethane granules (TPU) | Immersion precipitation and particle leaching | Na–citrate powder | Cutaneous wounds | Porous membrane | In vitro In vivo | Circle: 0.4 cm diameter | [13] |
| Mousavi et al., 2021 | PU-Br-Fg electrospun nanofibers | Biocompatible PU resin (Desmopan 9370A) and Polyvinyl alcohol (PVA 67000) | Electrospinning | Bromelain (Br) and Ferula gum (Fg) | Pressure ulcers | Nanofibers | In vitro In vivo | N/A | [14] |
| Pahlevanneshan et al., 2021 | Nanocomposite PU foam | Polyethylene glycol (PEG 400 and PEG 600), glycerol, 1,6-hexamethylene diisocyanate (HDI) | One shot and solvent-free foam preparation and PU foams coating and soaking | Nanolignin; coated with ethanolic extract of propolis | Skin wound healing | Foam | In vitro In vivo | Circle: 1.1 cm diameter | [15] |
| Xu et al., 2016 | Microporous PU membranes | Medical-grade PU | Particulate leaching method | Sodium citrate | Skin damage | Film | In vitro In vivo | Square: 1 cm2 | [16] |
| Khodabakhshi et al., 2019 | Highly porous polyurethane | Polyurethane (Tecoflex EG-80 A) | Solvent casting/particulate leaching | Coated with the water extract of propolis | Skin wounds | Foam | In vitro In vivo | Circle: 1.1 cm diameter | [17] |
| Guo et al., 2015 | Poly(ester urethane) scaffolds | ε-caprolactone, glycerol, glycolide, hexamethylene diisocyanate trimer (HDIt) | Reactive liquid molding of HDIt with the polyester triol and iron catalyst | No modification | Cutaneous wounds | Scaffolds | In vitro In vivo | N/A | [18] |
| Adolph et al., 2014 | PUR scaffold | Lysine triisocyanate (LTI) and a polyester triol (60% caprolactone, 30% glycolide, and 10% lactide) | Reactive liquid molding of the crosslinker and calcium stearate pore opener | Plasma treatment in the presence of carboxymethylcellulose (CME) | Cutaneous wounds | Scaffolds | In vitro In vivo | Square: 6.25 cm2 | [19] |
| Eskandarinia et al., 2020 | Electrospun polyurethane-hyaluronic (PU-HA) acid nanofiber | Polyurethane (Tecoflex EG-80A) | Electrospinning | Enriched with ethanolic extract of propolis (EEP) | Not specific | Nanofibers | In vitro In vivo | Circle: 1.1 cm diameter | [20] |
| Li et al., 2019 | Biodegradable electroactive polyurethane–urea elastomers | Polycaprolactone (PCL2000), polyethylene glycol (PEG2050), amine-capped (AT), 1,6-Hexanediamine (HDA), hexamethylene diisocyanate (HDI) | Two-step polymerization with stannous octoate | No modification | Skin repair | Film | In vitro In vivo | Cicle: 0.7 cm diameter | [21] |
| Hosseinabadi et al., 2020 | PU membranes films | Castor oil and CAPA polyol (CAPA 7201—Perstorp, 9051-88-1) or DEG, hexamethylene diisocyanate (HDI) | Two-step polymerization | Chain extender: diethylene glycol (DEG) | Not specific | Film | In vitro In vivo | Square: 0.64 cm2 | [22] |
| Hao et al., 2016 | Waterborne biodegradable polyurethane | PEG (Mn = 1450) and PCL (Mn = 2000), LDI, PDO and L-lysine | Two-step polymerization | Chain extender: L-lysine | Not specific | Waterborne | In vitro | N/A | [23] |
| Liu et al., 2017 | Porous polyurethane-urea foam (PUUF) | PEG, HMDI, 4, 4′-diaminodicyclohexylmethane (PACM) | Polymerization with stannous octanoate and soaking | Urea formation | Skin damage | Foam | In vitro In vivo | Square: 1 cm2 | [24] |
| Guo et al., 2012 | Electrospun PVDF/PU scaffold | PVDF powder and PU grains | Electrospinning | Piezoelectric PU | Skin wounds | Scaffolds | In vitro In vivo | N/A | [25] |
| Chen et al., 2017 | Electrospun polyurethane fiber mats | Polyurethane (Mw = 8000) | Electrospinning | Mupirocin incorportaion | Burn wounds | Scaffolds | In vitro In vivo | Insition: 0.6 cm | [26] |
| Li et al., 2017 | Amphiphilic biodegradable block polyurethane based on PLA and PEG foam | PLA (Mw = 9 × 104), poly(ethylene glycol), 1,6-hexamethylene diisocyanate (HDI) | Freeze-drying method | Alternating block PU | Skin wound | Foam | In vitro In vivo | Square: 1 cm2 | [27] |
| Khandwekar & Rho, 2012 | PU films | Medical-grade polyurethane (Tecoflex) | Polymerization and surface modification | Cationic, anionic, and zwitterionic surfaces | Not specific | Film | In vitro In vivo | N/A | [28] |
| Heit et al., 2012 | PU Foam | Commercial foams (GranuFoam; Kinetic Concepts) | Manufacture procedure | Pore size | Diabetic wounds | Foam | In vivo | Square: 1 cm2 | [29] |
| Adolph et al., 2016 | Injected PUR scaffolds | Glycolide and D,L-lactide, lysine triisocyanate-poly(ethylene glycol) (LTI-PEG) prepolymer | Two-component reactive liquid molding of LTI–PEG prepolymer | Sucrose (40% and 70%) | Cutaneous wounds | Injectable scaffolds | In vivo | Square: 9 cm2 | [30] |
| Gholami & Yeganeh, 2020 | Vegetable oil-based polyurethanes | Cyclic carbonated soybean oil (CSBO), CO, IPDI | Polymerization with DBTDL | Quaternary ammonium salts (QASs) | Tissue damage by skin and soft tissue infections | Film | In vitro In vivo | Square: 1.5 cm2 | [31] |
| Reference | Dressing Type | Elongation at Break (%) | Tensile Strength (MPa) | Water Absorption (%) | Contact Angle (°) | Water Vapor Transmission Rate (g/m2·Day) |
|---|---|---|---|---|---|---|
| [16] | Film | - | - | - | - | PU membrane: 50.2 PU25/SC75: 4025.8 PU25/SC55: 3282.0 PU25/SC45: 2028.3 PU40/SC40: 954.8 |
| [21] | Film | - | - | Ranged from 58 to 106 | PCL-PEG-AT0: 25° PCL-PEG-AT6: 52° PCL-PEG-AT12: 66° PCL-PEG-AT18: 81° | - |
| [22] | Film | CAPA-based PU: about 550 Castor oil-based PU: about 100 | CAPA-based PU: about 4 ± 0.3 Castor oil-based PU: about 1.7 ± 0.01 | CAPA-based PU: 5.67 Castor oil-based PU: 0.76 | CAPA-based PU: 70 ± 5 Castor oil-based PU: 80 ± 5 | CAPA-based PU: 260 ± 20 Castor oil-based PU: 285 ± 20 |
| [27] | Film | PUL15-a-E60: 995.92 PUL15-a-E80: 548.01 PUL22-a-E60: 827.69 PUL22-a-E80: 169.04 PUL15-r-E60: 79.74 PUL15-r-E80: 61.56 PUL22-r-E60: 895.48 PUL22-r-E80: 844.08 | PUL15-a-E60: 5.56 PUL15-a-E80: 5.29 PUL22-a-E60: 6.89 PUL22-a-E80: 6.27 PUL15-r-E60: 3.18 PUL15-r-E80: 2.70 PUL22-r-E60: 6.33 PUL22-r-E80: 5.80 | PUL8-a-E60: up to 600 PUL8-a-E80: up to 700 PUL15-a-E60: up to 500 PUL15-a-E80: up to 700 PUL22-a-E60: up to 600 PUL22-a-E80: up to 600 PUL8-r-E60: up to 500 PUL8-r-E80: up to 600 PUL15-r-E60: up to 450 PUL15-r-E80: up to 500 PUL22-r-E60: up to 500 PUL22-r-E80: up to 500 | PUL8-a-E60: 44.1 ± 1.0 PUL8-a-E80: 41.4 ± 0.5 PUL15-a-E60: 52.9 ± 1.3 PUL15-a-E80: 48.9 ± 0.7 PUL22-a-E60: 57.8 ± 1.1 PUL22-a-E80: 55.3 ± 1.3 PUL8-r-E60: 50.5 ± 0.9 PUL8-r-E80: 46.8 ± 1.3 PUL15-r-E60: 55.7 ± 1.4 PUL15-r-E80: 49.7 ± 1.8 PUL22-r-E60: 61.8 ± 0.8 PUL22-r-E80: 60.4 ± 2.0 | - |
| [28] | Film | - | - | Base: 91.4 ± 2.2 Cationic: 64 ± 2.0 Zwitterionic: 29 ± 2.5 Anionic: 48.4 ± 2.7 | ||
| [31] | Film | PUWD2 (dry): 330.0 ± 7.1 PUWD2(wet): 394.2 ± 5.3 PUWD3 (dry): 260.4 ± 7.2 PUWD3 (wet): 350.1 ± 12.2 PUWD4 (dry): 142 ± 5.2 PUWD4 (wet): 149.3 ± 7.8 | PUWD2 (dry): 17.32 ± 0.61 PUWD2(wet): 5.41 ± 0.31 PUWD3 (dry): 14.37 ± 0.21 PUWD3 (wet): 2.89 ± 0.32 PUWD4 (dry): 0.11 ± 0.02 PUWD4 (wet): 0.11 ± 0.03 | PUWD2: 49 ± 1.1 PUWD3: 18 ± 0.8 PUWD4: 2.1 ± 0.2 | PUWD2: 37 ± 5 PUWD3: 49 ± 4 PUWD4: 85 ± 3 | PUWD2: 390 ± 9 PUWD3: 191 ± 8 PUWD4: 39 ± 5 |
| [15] | Foam | PU: 91 ± 3.5 PU-NL: 96 ± 5.6 PU-NL/EEP: 73 ± 3.9 | PU: 0.75 ± 0.08 PU-NL: 0.91 ± 0.1 PU-NL/EEP: 0.82 ± 0.09 | PU-NL: 267 PU-NL/EEP: 242 | PU: 98.3 ± 5.8° PU-NL: 51.1 ± 4.9° PU-NL/EEP: 50.1 ± 2.1° | - |
| [17] | Foam | PU-control: 372 ± 12 PU/10WEP: 377 ± 14 PU/20WEP:384 ± 29 PU/30WEP: 434 ± 22 | PU-control:5.26 ± 0.40 PU/10WEP: 4.79 ± 0.21 PU/20WEP: 2.91 ± 0.47 PU/30WEP: 2.99 ± 0.1 | PU-control: 243 PU/10WEP:229 PU/20WEP:218 PU/30WEP: 207 | PU-control: 114.52 ± 2.31 PU/10WEP: 52.41 ± 1.82 PU/20WEP: 48.81 ± 3.57 PU/30WEP: 35.53 ± 1.65 | - |
| [24] | Foam | PUUF: about 97 CaduMedi: about 143 | PUUF: 0.246 CaduMedi: about 0.116 | PUU film: 88.47 in 10 min PUUF: 1310.8 in 10 min CaduMedi: 1331.69 | Rapidly spread on the surface and permeating into the wound dressing in a second time | - |
| [29] | Foam | Small pore size foam > medium and large pore size foam | Small pore size foam > medium and large pore size | - | - | - |
| [30] | Injectable scaffolds | - | - | - | - | - |
| [11] | Nanofibers | PU/CMC: 171.52 PU/CMC/5: 167.02 PU/CMC/10: 169.71 PU/CMC/15: 200.2 PU/CMC/20: 232.88 | PU/CMC: 18.5 PU/CMC/5: 21 PU/CMC/10: 22.2 PU/CMC/15: 24.9 PU/CMC/20: 26.8 | PU70/CMC30: 488.11 PU80/CMC20: 469.47 PU90/CMC10: 411.36 | - | PU: 497.28 PU70/CMC30: 1716.65–1987.01 PU80/CMC20: 1600.13–2074.62 |
| [14] | Nanofibers | - | PU-Fg: 3.4 ± 0.3 PVA-Br: 21.4 ± 0.5 Sandwich: 15.8 ± 0.2 | PU-Fg: 5.3 | - | - |
| [20] | Nanofibers | PU: 354.5 ± 15.7 PU-HA: 360.1 ± 12.2 PU-HA/0.5% EEP: 379.8 ± 23.6 PU-HA/1% EEP: 382.2 ± 14.3 PU-HA/2% EEP: 453.6 ± 38.5 | PU: 5.42 ± 1.4 PU-HA: 5.05 ± 0.8 PU-HA/0.5% EEP: 4.91 ± 0.5 PU-HA/1% EEP: 4.86 ± 0.9 PU-HA/2% EEP: 3.07 ± 1.1 | PU: 35.21 ± 9.5 PU-HA: 74.68 ± 11.8 PU-HA/0.5% EEP: 72.11 ± 5.1 PU-HA/1% EEP: 65.54 ± 8.0 PU-HA/ 2% EEP: 51.06 ± 4.2 | PU: 118.2° ± 6.2 PU-HA:43.8° ± 5.9 PU-HA/0.5% EEP: 47.6° ± 11.5 PU-HA/1% EEP: 52.2° ± 6.8 PU-HA/2% EEP: 67.2° ± 7.2 | - |
| [13] | Porous membrane | HTPM: 424.3 CTPM: 194.6 | HTPM: 2.07 CTPM: 0.21 | - | - | HTPM: 2265 g per m2 per day CTPM: 528 g per m2 per day |
| [12] | Scaffolds | Dry samples 0.8/1: 7 0.47/1: 8 Wet samples 0.8/1: 35 0.47/1: 38 | Dry samples 0.8/1: 34 0.47/1: 26 Wet samples 0.8/1: 10 0.47/1: 7 | C8P2: 118.36 ± 4.9 C7P3: 100.06 ± 5.6 | C8P2 and C7P3 80° ± 10° | - |
| [19] | Scaffolds | - | - | - | Plasma treatment significantly decreased the contact angle from 66° to 46° | - |
| [25] | Scaffolds | PU: 188.71 ± 22.40 PU/PVDF (3/1): 156.09 ± 31.72 PU/PVDF (2/1): 123.78 ± 46.56 PU/PVDF (1/1): 107.94 ± 25.80 PU/PVDF (1/2): 88.40 ± 26.41 PU/PVDF (1/3): 94.75 ± 20.00 PVDF: 76.47 ± 36.46 | PU: 9.632 ± 0.927 PU/PVDF (3/1): 7.433 ± 1.106 PU/PVDF (2/1): 6.860 ± 0.976 PU/PVDF (1/1): 5.984 ± 1.249 PU/PVDF (1/2): 5.562 ± 0.884 PU/PVDF (1/3): 4.107 ± 1.364 PVDF: 4.016 ± 0.732 | - | - | - |
| [26] | Scaffolds | Pu: 455.26 Pu/2%mu: 218.16 Pu/5%mu: 223.59 | Pu: 8.88 Pu/2%mu: 6.29 Pu/5%mu: 5.29 | - | - | Pu: 2975.13 ± 61.76 Pu/2%mu: 2810.68 ± 88.57 Pu/5%mu: 2892.89 ± 58.63 |
| [23] | Waterborne | LWPU17: 1608 ± 15 LWPU25: 2511 ± 24 LWPU33: 2120 ± 12 LWPU45: 2050 ± 21 | LWPU17: 17.8 ± 1.2 LWPU25: 12.3 ± 1.5 LWPU33: 16.8 ± 0.7 LWPU45: 15.6 ± 1.6 | - | LWPUs: 72°–90° | - |
| Reference | Animal Model | Injury Model | Dressing Change | Time (Days) | Techniques Performed | Hemostasis | Inflammation | Proliferation | Remodeling | Wound Closure (%) | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [11] | Wistar rats | Full-thickness wound model | Not specific | 14 | Histological analysis | NO | YES | YES | YES | Gauze bandage: 32.1 ± 0.2 PU/CMC: 51.4 ± 0.4 PU/CMC/5: 71 ± 0.14 PU/CMC/10: 87.64 ± 1.02 PU/CMC/15: 95.05 ± 0.24 PU/CMC/20: 95.11 ± 0.2% | A good dual anti-inflammatory–antimicrobial wound dressing candidate for improving diabetic wound-healing |
| [12] | Wistar rat | Full-thickness wound model | Not specific | 21 | Hemostasis: in vitro. Histological analysis | YES | YES | YES | YES | Control group: 82 ± 3.91% C7P3: 100 ± 4.12% | C7P3 was observed to be biocompatible on sub-cutaneous implantation, which was supported by scaffold integration with tissue and presence of blood vessels |
| [13] | BALB/c mice | Full-thickness wound model | Every other day | 7 | Angiogenesis and proliferation: western blot; granulation thickness: histological analysis | NO | NO | YES | YES | Control: 60.3% Vaseline gauze: 72.4% CTPM: 79.4% HTPM: 91.9% | The membranes favored granulation tissue formation, wound re-epithelialization, and angiogenesis |
| [14] | Sprague–Dawley (SD) rats | Ischemia–reperfusion (I/R) injury | Every day | 10 | Histological analysis | NO | YES | NO | YES | No data | The dressing decreased bleeding, inflammation, and tissue infiltration in the dermis area and epidermis induced due to bedsore |
| [15] | Wistar rats | Full-thickness wound model | Not specific | 12 | Histological analysis | NO | NO | YES | NO | Control: ~60% PU: ~68% PU-NL: ~72% PU-NL/EEP: ~90% | PU-NL/EEP-promoted better skin wound-healing |
| [16] | Balb/c mice | Full-thickness wound model | Not specific | 7 | Histological analysis, immunohistochemistry and immunofluorescence and western blot | NO | YES | YES | YES | MP: ~95.6% Blank: 34.8% EHP: 53.2% HP: 73.4% LP: 59.0% ELP: 46.0% | Application of MP-PU membranes could maintain a suitable moist environment in the wound that could enhance the wound contraction and tissue regeneration, thereby accelerating wound-healing |
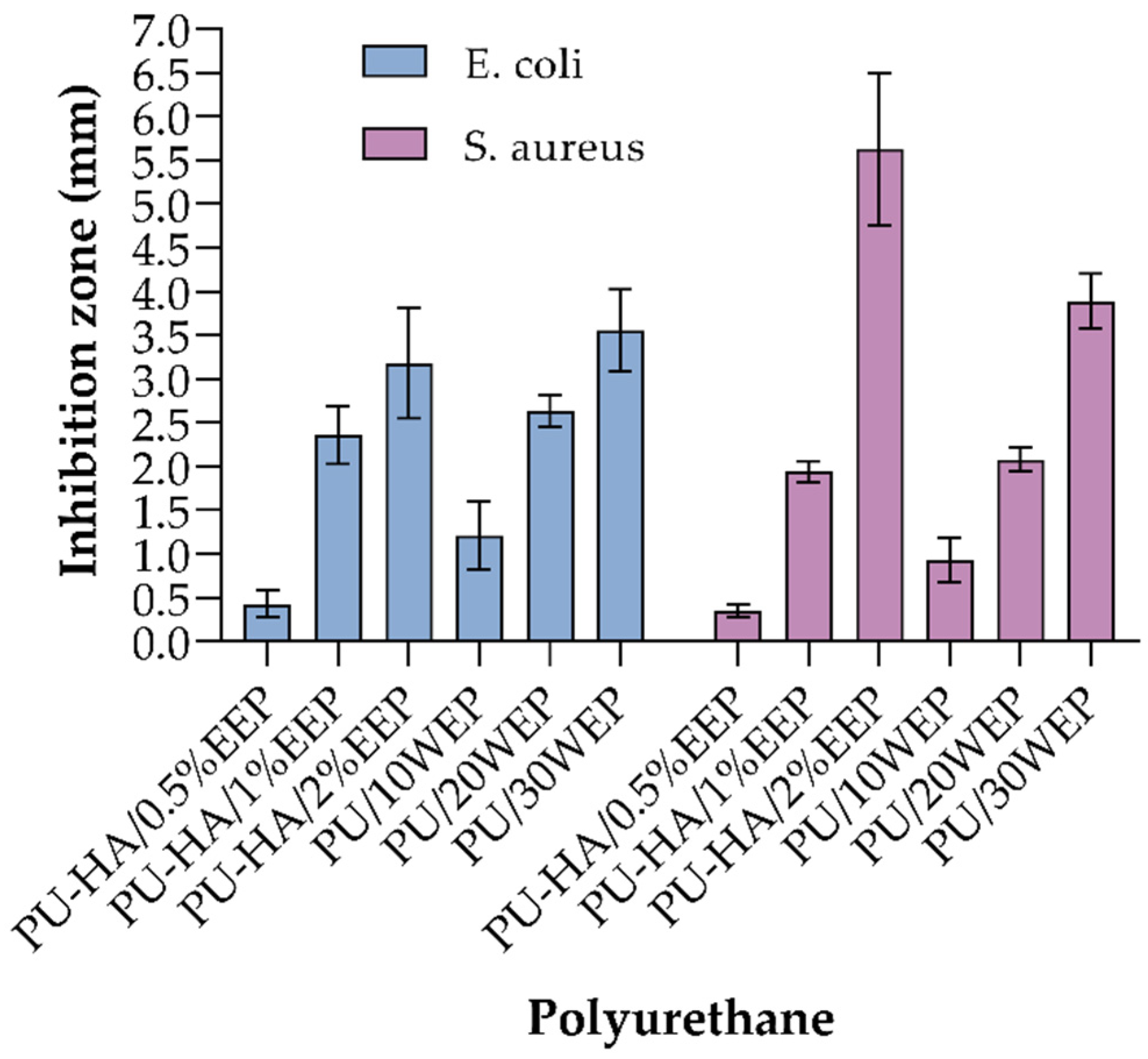
| [17] | Wistar rats | Full-thickness wound model | Not specific | 15 | Histological analysis | NO | YES | YES | NO | Control: 79.03% PU foam: 91.5% PU/30WEP: 94.32% | The increase of propolis concentration caused enhancement of the antibacterial activity against E. coli and S. aureus. The propolis-coated wound dressing exhibited significant enhancement of in vivo wound-healing |
| [18] | Sprague–Dawley (SD) rats | Cutaneous repair model | Not specific | 21 | Histological analysis, collagen: PCR; inflammation: modulation of macrophages | NO | YES | YES | YES | - | Scaffolds with a substrate modulus promoted increased deposition and random orientation of collagen, angiogenesis, and regenerative macrophage. Additionally, Wnt signaling was down-regulated on scaffolds |
| [19] | Yorkshire pigs | Full-thickness wound model | Every 2–3 days | 15 | Histological analysis | NO | YES | YES | YES | - | PUR scaffolds do not adversely affect the wound-healing process in porcine excisional wounds. The results suggest that all wounds were moving into the remodeling phase by day 15 |
| [20] | Wistar rats | Full-thickness wound model | Not specific | 21 | Histological analysis | NO | YES | YES | YES | PU: 93.89 ± 0.2% PU-HA/1% EEP: 100% | The PU-HA/1% EEP exhibited higher antibacterial activity against S. aureus and E. coli in comparison with the PU and PU-HA dressings. Besides, the PU-HA/1% EEP sample caused considerable acceleration of Wistar rat skin excision healing |
| [21] | Kunming mice | Full-thickness wound model | Not specific | 14 | Histological analysis | NO | YES | YES | YES | Tegaderm™: ~90 PCL-PEG-AT0: ~98 PCL-PEG-AT12: 100 PCL-PEG-AT12/VCM: 100 | PCL-PEG-AT12 film shows a prominent wound-healing effect |
| [22] | Wistar rats | Full-thickness wound model | Every second day | 13 | Histological analysis | NO | YES | NO | YES | Gauze: 68% Castor oil Pus: 99% CAPA-based Pus: 99% | This dressing can be used as the secondary dressing or applied to simple wounds with small amounts of exudates |
| [23] | - | - | - | - | In vitro evaluation | YES | YES | YES | NO | - | The LWPU films showed suitable mechanical properties, low cytotoxicity, good hemocompatibility and cytocompatibility. LWPUs elicited a transition of macrophages from a pro-inflammation to a wound-healing phenotype |
| [24] | Sprague–Dawley (SD) rats | Full-thickness wound model | Every second day | 13 | Histological analysis | YES | YES | YES | YES | Gauze: 35.44% CaduMedi: 98% PUUF: 98% | The results showed that PUUF can accelerate hemostasis and adsorb abundant wound exudates to build a regional moist environment beneficial for wound-healing |
| [25] | Sprague–Dawley (SD) rats | Subcutaneous implantation | Not specific | 14 | Histological analysis | NO | NO | YES | NO | - | The nonpiezoelectric-excited PU/PVDF scaffolds and the piezoelectric-excited PU scaffolds showed no significant differences in fibroblast activities |
| [26] | Sprague–Dawley rats | Full-thickness wound model | No | 3 | Histological analysis, cytokine expression: PCR | YES | YES | YES | YES | - | Increasing the content of mupirocin, the average of diameter did not show much change. There appears to be no obvious differences in the number of cells between PU and mixed PU/mupirocin scaffolds |
| [27] | Sprague–Dawley rats | Full-thickness wound model | Every second day | 14 | Histological analysis | NO | YES | YES | NO | Gauze: ~55% PULA-alt-PEG: ~98% PULA-ran-PEG: ~80% | The higher water absorption with gel formation of the alternating block polyurethanes would be good for wound-healing. It ensures that the dressings will not adhere to the wound tissue |
| [28] | Sprague–Dawley rats | Subcutaneous implantation-Rat cage implant system | Not specific | 21 | Cytokine: gene expression: PCR | YES | YES | NO | NO | - | The cationic surfaces promoted the highest rate of macrophage fusion. Anionic and zwitterionic surfaces could suppress the early macrophage response to fusogenic surface stimulus. Identify apoptosis of polyurethane adherent monocytes/macrophages as a mechanism for the removal of these cells without generating a prolonged inflammatory response |
| [29] | Homozygous genetically diabetic Lep/r-db/db mice (strain C57BL/KsJ-Leprdb) | Full-thickness wound model | On days 2 and 4 | 7 | Histological analysis | NO | YES | YES | YES | No data | Larger pore sizes result in greater wound deformation, granulation tissue thickness, and induction of contractile myofibroblasts. Angiogenesis seems to be largely independent of pore size, the polyurethane foam itself induces angiogenesis |
| [30] | Yorkshire pigs | Full-thickness wound model | Every 2–3 days | 30 | Histological analysis, immunohistochemistry | NO | YES | YES | YES | I40: ~90% P40: ~85% NT: ~92% | Injected PUR scaffolds facilitate wound-healing, support tissue infiltration and matrix production, delay or reduce wound contraction, and reduce scarring in a clinically relevant animal model |
| [31] | Wistar rats | Full-thickness wound model | After 7 days | 21 | - | NO | YES | NO | NO | Gauze: ~64% PUWD4: ~71% PUWD2: ~88% | PUWD2 is probably not suitable for a bandage of heavily exudative wounds due to possibility of accumulation of exudates and consequent maceration of surrounding skin tissue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-González, M.; Díaz, L.E.; Dominguez-Paz, C.; Valero, M.F. Insights into the Design of Polyurethane Dressings Suitable for the Stages of Skin Wound-Healing: A Systematic Review. Polymers 2022, 14, 2990. https://doi.org/10.3390/polym14152990
Morales-González M, Díaz LE, Dominguez-Paz C, Valero MF. Insights into the Design of Polyurethane Dressings Suitable for the Stages of Skin Wound-Healing: A Systematic Review. Polymers. 2022; 14(15):2990. https://doi.org/10.3390/polym14152990
Chicago/Turabian StyleMorales-González, Maria, Luis Eduardo Díaz, Carlos Dominguez-Paz, and Manuel F. Valero. 2022. "Insights into the Design of Polyurethane Dressings Suitable for the Stages of Skin Wound-Healing: A Systematic Review" Polymers 14, no. 15: 2990. https://doi.org/10.3390/polym14152990
APA StyleMorales-González, M., Díaz, L. E., Dominguez-Paz, C., & Valero, M. F. (2022). Insights into the Design of Polyurethane Dressings Suitable for the Stages of Skin Wound-Healing: A Systematic Review. Polymers, 14(15), 2990. https://doi.org/10.3390/polym14152990







