A Review on the Delivery of Plant-Based Antidiabetic Agents Using Nanocarriers: Current Status and Their Role in Combatting Hyperglycaemia
Abstract
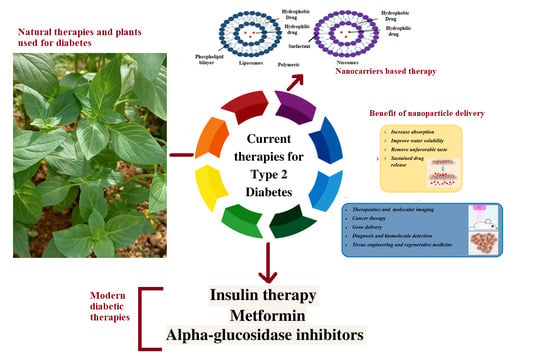
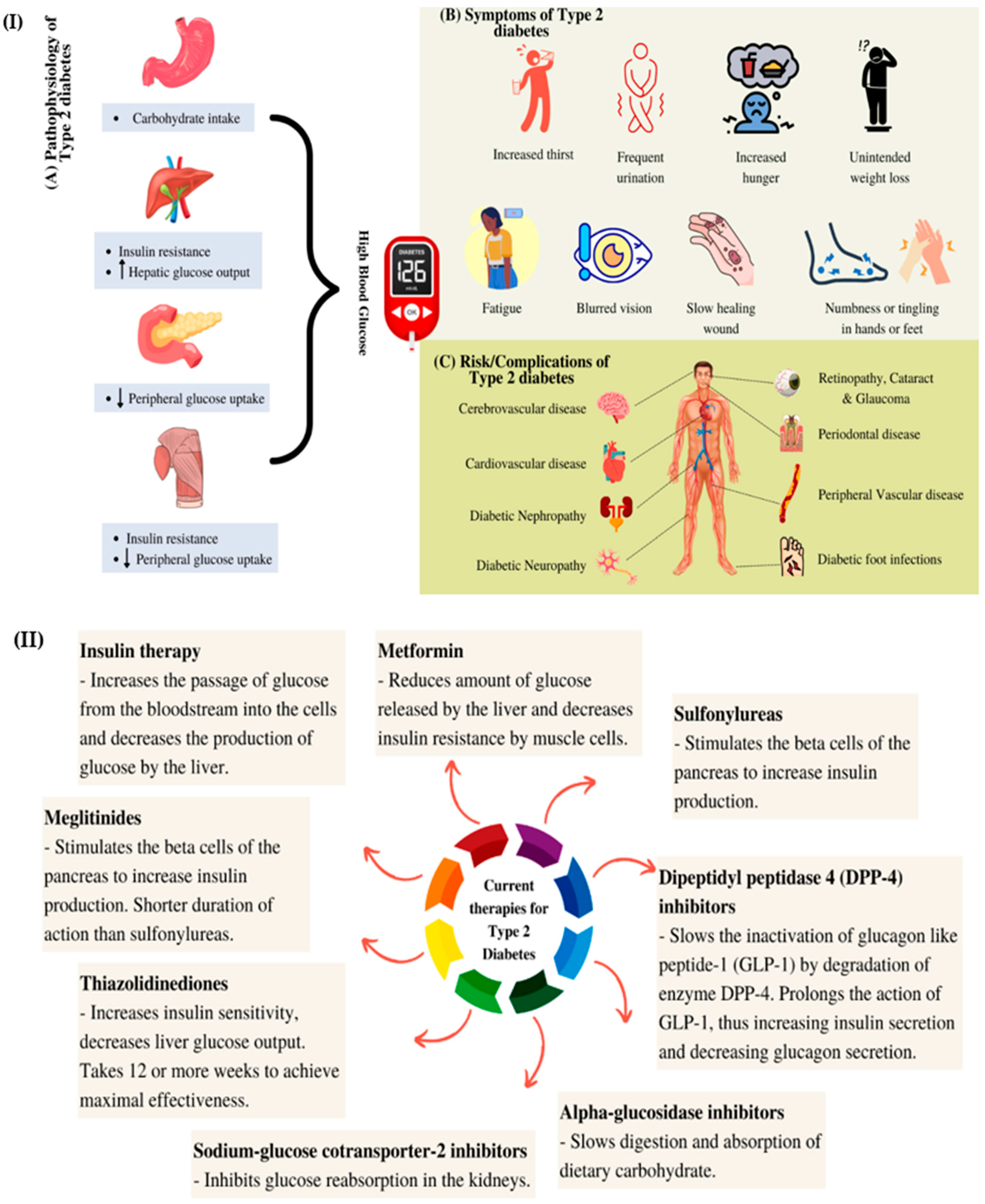
:1. Introduction
2. Natural Active Agents in Nanocarriers
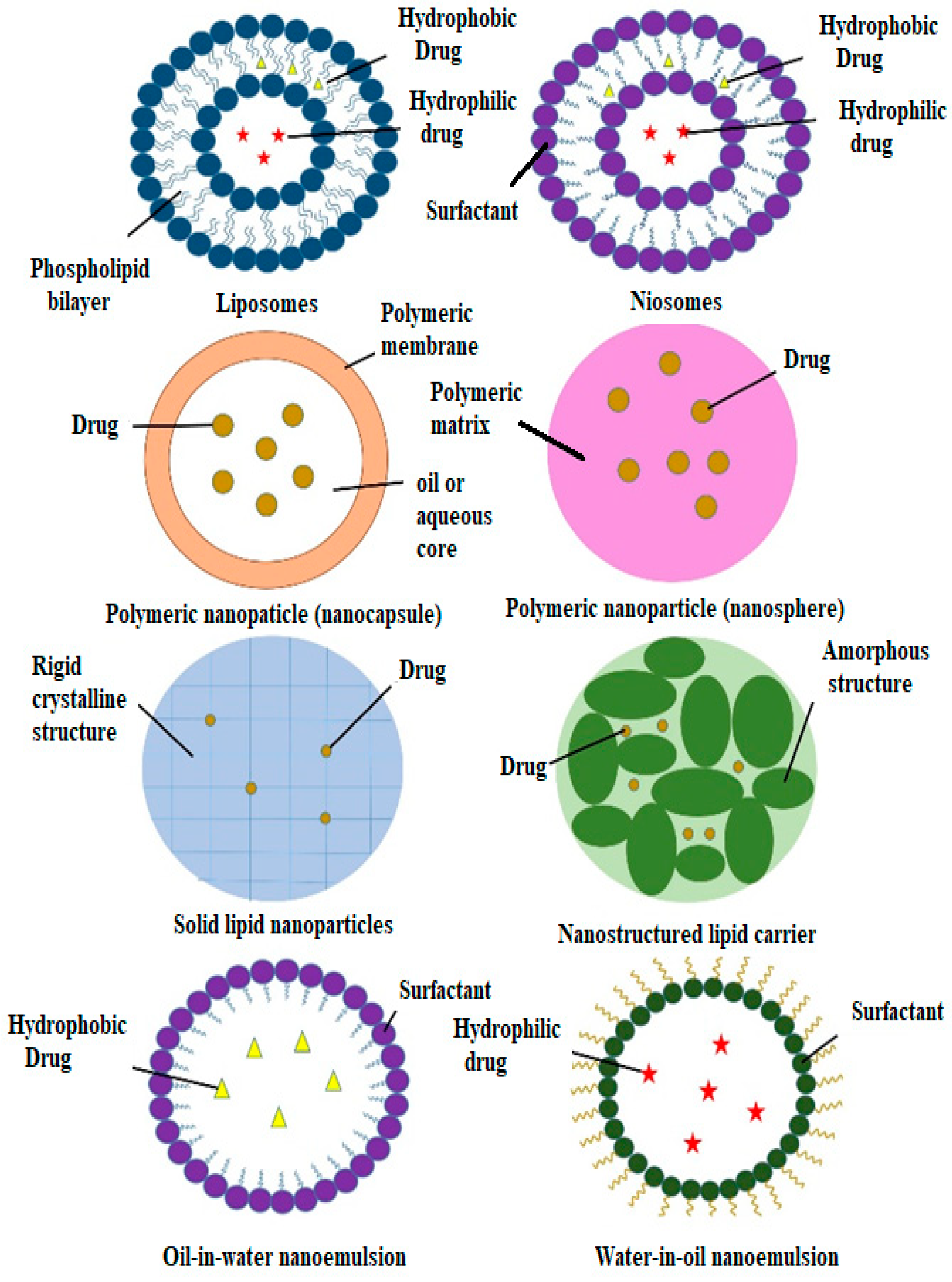
3. Types of Nanocarriers for Plant-Based Antidiabetic Extracts/Active Agents
3.1. Liposomes
| Type of Nanocarrier | Formulation (Ratio) | Active Compound | Model | Size Range (nm) | Remark | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | Lecithin | Betanin | Streptozotocin-induced diabetic rats | 40.06 ± 6.21 | Increased hypoglycaemic activity; antihyperlipidemic activity; decreased oxidative stress | [65] |
| DPPC, PEG-2000-DSPE and cholesterol (9.5:0.5:1) | Curcumin | Streptozotocin-induced diabetic rats | 140 | Increased hypoglycaemic activity; hepatoprotective effects; decreased oxidative stress | [66] | |
| Phosphatidylcholine and cholesterol (8:2) | Momordica charantia, Trigonella foenum-graecum and Withania somnifera extracts | Albino Wistar rats | 1176 ± 5.6 | Increased hypoglycaemic activity; antihyperlipidemic activity | [69] | |
| Niosomes | Span 60 and cholesterol (1:1) | Lycopene | Alloxan-induced diabetic rats | 202 ± 41 | Increased hypoglycaemic activity; antihyperlipidemic activity | [70] |
| Span 60, phospholipid 90G and cholesterol (9:4:1) | Embelin | Streptozotocin-induced diabetic rats | 609–734 | Increased hypoglycaemic activity; antioxidant efficacy | [71] | |
| Span 40 and cholesterol (1:2) | Gymnema sylvestre extract | Alloxan-induced diabetic rats | 229.5 ± 30 | Increased hypoglycaemic activity | [72] | |
| Polymeric Nanoparticles | Poly-(ε-caprolactone) (PCL) and PLGA-PEG-COOH | Fisetin | In vitro assays | 140–200 | Better α-glucosidase inhibition than acarbose; scavenging capacity | [73] |
| Eudragit RS100 | Phoenix dactylifera seed oil | In vitro assays | 207 | α-amylase and α-glucosidase inhibition | [74] | |
| Chitosan | Curcumin | In vitro assays | 74 | Increased GLUT-4 levels | [75] | |
| Chitosan and alginate (3:1) | Naringenin | Streptozotocin-induced diabetic rats | 150–300 | Increased hypoglycaemic activity | [76] | |
| Chitosan and alginate (1:3) | Quercetin | Streptozotocin-induced diabetic rats | 91.58 | Increased hypoglycaemic activity | [77] | |
| Chitosan and gum arabic | Glycyrrhizin | Streptozotocin-induced diabetic rats | 165.3 | Increased hypoglycaemic activity | ||
| Chitosan and tripolyphosphate (4:1) | Ferulic acid | Streptozotocin-induced diabetic rats | 51.2 ± 1.7 | Increased hypoglycaemic activity; increased body weight | [78] | |
| Chitosan, gum Arabic and Tween 60 | Glycyrrhizin | Streptozotocin- and nicotinamide-induced diabetic rats | 181.4 | Increased hypoglycaemic activity; reduced body weight and lipid levels | [79] | |
| Polyvinyl alcohol (PVA), Tween 80, gum-rosin polymer and oleic acid | Thymoquinone | Streptozotocin- and nicotinamide-induced diabetic rats | 70.21 | Increased hypoglycaemic activity; reduced body weight and lipid levels | [80] | |
| Gum rosin, PVA and lecithin | Thymoquinone | Streptozotocin-induced diabetic rats | 36.83 ± 0.32 | Increased hypoglycaemic activity | [81] | |
| PLGA | Quercetin | Streptozotocin-induced diabetic rats | 179.9 ± 11.2 | Increased hypoglycaemic activity; increased levels of catalase and superoxide dismutase | [82] | |
| PLGA | Pelargonidin | Streptozotocin-induced diabetic rats | 91.47 ± 2.89 | Increased hypoglycaemic activity; antihyperlipidemic activity | [83] | |
| PLGA, Pluronic F-127 and chitosan | Silybin | Streptozotocin-induced diabetic rats | 184.6 | Increased hypoglycaemic activity | [84] | |
| PLGA and PVA | Ethyl acetate | In vitro assays | 365.7 | α-amylase and α-glucosidase inhibition | [85] | |
| Tween 20 and propylene glycol | Foeniculum vulgare Mill. essential oil | Streptozotocin-induced diabetic rats | 44–105 | Increased hypoglycaemic activity | [86] | |
| Nanoemulsions | Tween 20 and polyethylene (PEG) 400 | Ipomoea reptans extract | - | 15.5 ± 0.8 | - | [87] |
| Lecithin | Resveratrol | Streptozotocin + nicotinamide-induced diabetic rats | 248 | Increased hypoglycaemic activity; prevention of weight loss | [88] | |
| Solid Lipid Nanoparticles | Compritol, Tween 80 and Span 20 | Myricitrin | Streptozotocin + nicotinamide-induced diabetic rats | 76.1 | Increased hypoglycaemic activity; antioxidant and anti-apoptotic effects | [89] |
| Glycerol tripalmitate and soybean phospholipid | Berberine | Male rats | 76.8 | Increased hypoglycaemic activity; prevention of weight gain | [90] | |
| Nanostructured Lipid Carriers | Precirol and miglyol (5:2) | Baicalin | Streptozotocin-induced diabetic rats | 92 ± 3.1 | Increased hypoglycaemic activity | [91] |
3.2. Niosomes
3.3. Polymeric Nanoparticles
3.4. Nanoemulsions
3.5. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
4. Metallic Nanoparticles
5. Characterisation of Nanocarriers
5.1. Drug Release Study
5.2. Compatibility and Stability Study
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Gupta, M.; Popli, H.; Aggarwal, G. Diabetes mellitus treatment using herbal drugs. Int. J. Phytomedicine 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Saberzadeh-Ardestani, B.; Karamzadeh, R.; Basiri, M.; Hajizadeh-Saffar, E.; Farhadi, A.; Shapiro, A.M.J.; Tahamtani, Y.; Baharvand, H. Type 1 diabetes mellitus: Cellular and molecular pathophysiology at a glance. Cell J. 2018, 20, 294–301. [Google Scholar] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic complications of diabetes mellitus: A mini review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dungan, K.; Grossman, A.; Hershman, J.M.; Hofland, H.J.; Kaltsas, G.; et al. Approach to the Patient with Dyslipidemia. In Endotext; Feingold, K.R., Ed.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Lai, W.F. Development of hydrogels with self-healing properties for delivery of bioactive agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.F. Multi-component hydrogel beads incorporated with reduced graphene oxide for ph-responsive and controlled co-delivery of multiple agents. Pharmaceutics 2021, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Ahmad, I.; Khan, M.S.A.; Ahmad, I.; Chattopadhyay, D. New Look to Phytomedicine, 1st ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–13. [Google Scholar]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and predictors of herbal medicine use among adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Nooreen, Z.; Rai, V.K.; Yadav, N.P. Phytopharmaceuticals: A new class of drug in India. Ann. Phytomed. 2018, 7, 27–37. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharm. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Bonifácio, B.V.; da Silva, P.B.; Ramos, M.A.D.S.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- William, T.C.; Jianping, Y.; Zhong, Q.W. Efficacy of Dietary Supplementation with Botanicals on Carbohydrate Metabolism in Humans. Endocr. Metab. Immune Disord Drug Targets 2008, 8, 78–81. [Google Scholar]
- Vats, V.; Grover, J.K.; Rathi, S.S. Evaluation of anti-hyperglycemic and hypoglycemic effect of Trigonella foenum-graecum Linn, Ocimum sanctum Linn and Pterocarpus marsupium Linn in normal and alloxanized diabetic rats. J. Ethnopharmacol. 2002, 79, 95–100. [Google Scholar] [CrossRef]
- Leung, L.; Birtwhistle, R.; Kotecha, J.; Hannah, S.; Cuthbertson, S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): A mini review. Br. J. Nutr. 2009, 102, 1703–1708. [Google Scholar] [CrossRef] [Green Version]
- Mall, G.K.; Mishra, P.K.; Prakash, V. Antidiabetic and hypolipidemic activity of Gymnema sylvestre in alloxan induced diabetic rats. Glob. J. Biotechnol. Biochem. 2009, 4, 37–42. [Google Scholar]
- Mostofa, M.; Choudhury, M.E.; Hossain, M.A.; Islam, M.Z.; Islam, M.S.; Sumon, M.H. Effects of Catharanthus roseus, Azadirachta indica, Allium sativum and glimepride in experimentally diabetic induced rat. Bangladesh J. Vet. Med. 2007, 5, 99–102. [Google Scholar]
- Mohamed, E.A.K. Antidiabetic, antihypercholestermic and antioxidative effect of Aloe vera gel extract in alloxan induced diabetic rats. Aust. J. Basic Appl. Sci. 2011, 5, 1321–1327. [Google Scholar]
- Kalaycıoğlu, Z.; Gazioğlu, I.; Erim, F.B. Comparison of antioxidant, anticholinesterase, and antidiabetic activities of three curcuminoids isolated from Curcuma longa L. Nat. Prod. Res. 2017, 31, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 2011, 18, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Deshmukh, R.; Rahman, M.A. Nanoemulsion: Promising nanocarrier system for delivery of herbal bioactives. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal delivery of natural product: A promising approach in health research. Trends Food Sci. Technol. 2019, 85, 177–200. [Google Scholar] [CrossRef]
- Gondim, B.L.C.; Oshiro-Júnior, J.A.; Fernanandes, F.H.A.; Nóbrega, F.P.; Castellano, L.R.C.; Medeiros, A.C.D. Plant extracts loaded in nanostructured drug delivery systems for treating parasitic and antimicrobial diseases. Curr. Pharm. Des. 2019, 25, 1604–1615. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Guccione, C.; Manconi, M.; Fadda, A.M.; Sinico, C.J. Vesicles and micelles: Two versatile vectors for the delivery of natural products. Drug Deliv. Sci. Technol. 2016, 32, 241–255. [Google Scholar] [CrossRef]
- Da Silva, F.L.O.; Marques, M.B.D.F.; Kato, K.C.; Carneiro, G. Nanonization techniques to overcome poor water-solubility with drugs. Expert Opin. Drug Discov. 2020, 15, 853–864. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, V.K.; Tewari, R.P.; Agarwal, V. Nanoparticle based drug delivery system: Advantages and applications. Nanoparticle based drug delivery system: Advantages and applications. Indian J. Sci. Technol. 2011, 4, 177–180. [Google Scholar] [CrossRef]
- Subramanian, A.P.; Jaganathan, S.K.; Manikandan, A.; Pandiaraj, K.N.; Gomathi, N.; Upriyanto, E. Recent trends in nano-based drug delivery systems for efficient delivery of phytochemicals in chemotherapy. RSC Adv. 2016, 6, 48294–48314. [Google Scholar] [CrossRef]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-based antidiabetic nanoformulations: The emerging paradigm for effective therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Pandey, H.; Rani, R.; Agarwal, V.; Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59, 1–10. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta. Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Hua, S.; Wu, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharm. 2013, 4, 143. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Sarwa, K.K.; Mazumder, B.J. Fluidity enhancement: A critical factor for performance of liposomal transdermal drug delivery system. Liposome Res. 2014, 24, 83–89. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of various types of liposomes in drug delivery systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Valenzuela, S.M. Liposome Techniques for Synthesis of Biomimetic Lipid Membranes. In Nanobiotechnology of Biomimetic Membranes; Martin, D.K., Ed.; Springer: Boston, MA, USA, 2007; pp. 75–87. [Google Scholar]
- Greish, K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: Are we there yet? Drug Discov. Today Technol. 2012, 9, e161–e166. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.M.; da Rosa Zavareze, E.; Prentice-Hernández, C.; de Souza-Soares, L.A. Revisão: Características de nanopartículas e potenciais aplicações em alimentos. Braz. J. Food Technol. 2012, 15, 99–109. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H.J. Phosphatidylcholine-rutin complex as a potential nanocarrier for food applications. Funct. Foods 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Camilo, C.J.J.; Leite, D.O.D.; Silva, A.R.A.; Menezes, I.R.A.; Coutinho, H.D.M.; Costa, J.G.M. Lipid vesicles: Applications, principal components and methods used in their formulations. A review. Acta Biol. Colomb. 2020, 25, 339–352. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Hu, S.; Niu, M.; Hu, F.; Lu, Y.; Qi, J.; Yin, Z.; Wu, W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 2013, 441, 693–700. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Barea, M.J.; Jenkins, M.J.; Gaber, M.H.; Bridson, R.H. Evaluation of liposomes coated with a pH responsive polymer. Int. J. Pharm. 2010, 402, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M.; Ahmed, O.A.A.; Al-Abdali, R.T. Enteric-coated alendronate sodium nanoliposomes: A novel formula to overcome barriers for the treatment of osteoporosis. Expert Opin. Drug Deliv. 2013, 10, 741–746. [Google Scholar] [CrossRef]
- Kazakov, S. Liposome-nanogel structures for future pharmaceutical applications: An updated review. Curr. Pharm. Des. 2016, 22, 1391–1413. [Google Scholar] [CrossRef]
- Klemetsrud, T.; Jonassen, H.; Hiorth, M.; Kjøniksen, A.-L.; Smistad, G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf. B. Biointerfaces 2013, 103, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, J.; Hofhaus, G.; Thomas, S.; Cuesta, L.C.; Gropp, F.; Schröder, R.; Hartmann, K.; Fricker, G.J. Improved oral bioavailability of human growth hormone by a combination of liposomes containing bio-enhancers and tetraether lipids and omeprazole. Pharm. Sci. 2014, 103, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Lizondo, M.; Gallardo, M.J. Enrofloxacin loaded liposomes obtained by high speed dispersion method. Chem. Pharm. Bull. 1995, 43, 983–987. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Sachse, A.; Röbling, G.; Brandl, M. Large-scale production of liposomes of defined size by a new continuous high pressure extrusion device. Drug Dev. Ind. Pharm. 1994, 20, 2787–2807. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, I.; Kathiroli, S.; Subramani, S.; Veerasamy, V. Ameliorating effect of betanin, a natural chromoalkaloid by modulating hepatic carbohydrate metabolic enzyme activities and glycogen content in streptozotocin-nicotinamide induced experimental rats. Biomed. Pharmacother. 2017, 88, 1069–1079. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Bai, B.; Yang, X.; Han, J. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem. Toxicol. 2015, 78, 141–146. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Selig, M.J.; Celli, G.B.; Tan, C.; La, E.; Mills, E.; Webley, A.-D.; Padilla-Zakour, O.I.; Abbaspourrad, A. High pressure processing of beet extract complexed with anionic polysaccharides enhances red color thermal stability at low pH. Food Hydrocoll. 2018, 80, 292–297. [Google Scholar] [CrossRef]
- Amjadi, S.; Abbasi, M.M.; Shokouhi, B.; Ghorbani, M.; Hamishehkar, H.J. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. Funct. Foods. 2019, 59, 119–128. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Porfire, A.S.; Tefas, L.R.; Boarescu, P.M.; Bolboacă, S.D.; Stănescu, I.C.; Bulboacă, A.C.; Dogaru, G. Liposomal curcumin is better than curcumin to alleviate complications in experimental diabetic mellitus. Molecules 2019, 24, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadoglou, N.P.; Daskalopoulou, S.S.; Perrea, D.; Liapis, C.D. Matrix metalloproteinases and diabetic vascular complications. Angiology 2005, 56, 173–189. [Google Scholar] [CrossRef]
- Singh, D.; Srivastava, S.K.; Chaudhuri, T.K.; Upadhyay, G. Multifaceted role of matrix metalloproteinases (MMPs). Front. Mol. Biosci. 2015, 2, 19. [Google Scholar] [CrossRef]
- Gauttam, V.K.; Kalia, A.N. Development of polyherbal antidiabetic formulation encapsulated in the phospholipids vesicle system. J. Adv. Pharm. Technol. Res. 2013, 4, 108–117. [Google Scholar]
- Sharma, P.K.; Saxena, P.; Jaswanth, A.; Balasubramaniam, A.J. Anti-diabetic activity of lycopene niosomes: Experimental observation. Pharm. Drug Dev. 2017, 4, 103. [Google Scholar]
- Alam, M.S.; Ahad, A.; Abidin, L.; Aqil, M.; Mir, S.R.; Mujeeb, M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in Wistar rats. Biomed. Pharmacother. 2018, 97, 1514–1520. [Google Scholar] [CrossRef]
- Kamble, B.; Talreja, S.; Gupta, A.; Patil, D.; Pathak, D.; Moothedath, I.; Duraiswamy, B. Development and biological evaluation of Gymnema sylvestre extract-loaded nonionic surfactant-based niosomes. Nanomedicine 2012, 8, 1295–1305. [Google Scholar] [CrossRef]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and α-glucosidase inhibition activities. Mater. Sci. Eng. C 2016, 68, 594–602. [Google Scholar] [CrossRef]
- Lammari, N.; Froiio, F.; Louaer, M.; Cristiano, M.C.; Bensouici, C.; Paolino, D.; Louaer, O.; Meniai, A.H.; Elaissari, A. Poly(ethyl acrylate-co-methyl Methacrylate-co-trimethylammoniethyl methacrylate chloride) (Eudragit RS100) Nanocapsules as Nanovector Carriers for Phoenix dactylifera L. Seeds Oil: A Versatile Antidiabetic Agent. Biomacromolecules 2020, 21, 4442–4456. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Tamrakar, A.K.; Mahajan, S.; Prasad, G.B.K.S. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018, 213, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018, 182, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H.; Kumar, S. Evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2017, 106, 220–230. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C 2018, 92, 381–392. [Google Scholar] [CrossRef]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kaushik, A.; Kim, K.-H.; Kumar, S. Antidiabetic activity enhancement in streptozotocin+ nicotinamide–induced diabetic rats through combinational polymeric nanoformulation. Int. J. Nanomed. 2019, 14, 4383–4395. [Google Scholar] [CrossRef] [Green Version]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H.; Kumar, S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem. Biol. Interact. 2018, 295, 119–132. [Google Scholar] [CrossRef]
- Chitkara, D.; Nikalje, S.; Mittal, A.; Chand, M.; Kumar, N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Deliv. Transl. Res. 2012, 2, 112–123. [Google Scholar] [CrossRef]
- Roy, M.; Pal, R.; Chakraborti, A.S. Pelargonidin-PLGA nanoparticles: Fabrication, characterization, and their effect on streptozotocin induced diabetic rats1. Indian J. Exp. Biol. 2017, 55, 819–830. [Google Scholar]
- Das, S.; Roy, P.; Pal, R.; Auddy, R.G.; Chakraborti, A.S.; Mukherjee, A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS ONE 2014, 9, e101818. [Google Scholar]
- Rashid, M.H.A.; Bharadwaj, P.V.P.D.; Mandal, V.; Pal, M.; Mandal, S.C.; Thandavarayan, R.A. Preparation and characterization of PLGA loaded nanoparticles obtained from D. melanoxylon Roxb. leaves for their antiproliferative and antidiabetic activity. Int. J. Green Pharm. 2017, 11, S438–S447. [Google Scholar]
- Mostafa, D.M.; Abd El-Alim, S.H.; Asfour, M.H.; Al-Okbi, S.Y.; Mohamed, D.A.; Awad, G. Transdermal nanoemulsions of Foeniculum vulgare Mill. essential oil: Preparation, characterization and evaluation of antidiabetic potential. J. Drug Deliv. Sci. Technol. 2015, 29, 99–106. [Google Scholar] [CrossRef]
- Jumaryatno, P.; Chabib, L.; Hayati, F.; Awaluddin, R.J. Stability study of ipomoea reptans extract self-nanoemulsifying drug delivery system (SNEDDS) as anti-diabetic therapy. Appl. Pharm. Sci. 2018, 8, 11–14. [Google Scholar]
- Mohseni, R.; ArabSadeghabadi, Z.; Ziamajidi, N.; Abbasalipourkabir, R.; RezaeiFarimani, A. Oral administration of resveratrol-loaded solid lipid nanoparticle improves insulin resistance through targeting expression of SNARE proteins in adipose and muscle tissue in rats with type 2 diabetes. Nanoscale Res. Lett. 2019, 14, 227. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid. Med. Cell. Longev. 2018, 2018, 7496936. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Yang, M.; Zhang, W.; Li, X.; Gao, D.; Ou, Z.; Li, Z.; Liu, S.; Li, X.; Yang, S. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 4677–4687. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Wei, Z.; Zhao, Y.; Xu, X. Nanostructured lipid carriers loaded with baicalin: An efficient carrier for enhanced antidiabetic effects. Pharm. Mag. 2016, 12, 198–202. [Google Scholar]
- Yeo, P.L.; Lim, C.L.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Niosomes: A review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2018, 11, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Dua, J.S.; Prasad, D.N.; Hira, S.; Monika. Advancement in novel drug delivery system: Niosomes. J. Drug Deliv. Ther. 2019, 9, 995–1001. [Google Scholar]
- El-Mahdy, M.M.; Hassan, A.S.; El-Badry, M.; El-Gindy, G.E.-D.A. Performance of curcumin in nanosized carriers niosomes and ethosomes as potential anti-inflammatory delivery system for topical application. Bull. Pharm. Sci. 2020, 43, 105–122. [Google Scholar]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [PubMed] [Green Version]
- Reddy, B.S.; Padman, J.S.C.; Santosh, V. Niosomes as nanocarrier systems: A review. Int. J. Pharm. Sci. Res. 2012, 3, 1560. [Google Scholar]
- Abdelkader, H.; Alani, A.W.G.; Alany, R.G. Recent advances in non-ionic surfactant vesicles (niosomes): Self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014, 21, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Irchhaiya, R. Niosomes: A potential tool for novel drug delivery. Int. J. Pharm. Investig. 2016, 46, 195–204. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L. Lycopene and human health. Curr. Top. Nutraceutical Res. 2004, 2, 127–136. [Google Scholar]
- Lee, M.T.; Chen, B.H. Stability of lycopene during heating and illumination in a model system. Food Chem. 2002, 78, 425–432. [Google Scholar] [CrossRef]
- Pesek, C.A.; Warthesen, J.J. Photodegradation of carotenoids in a vegetable juice system. J. Food Sci. 1987, 52, 744–746. [Google Scholar] [CrossRef]
- Durg, S.; Kumar, B.N.; Vandal, R.; Dhadde, S.B.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Antipsychotic activity of embelin isolated from Embelia ribes: A preliminary study. Biomed. Pharm. 2017, 90, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Rachh, P.R.; Rachh, M.R.; Ghadiya, N.R.; Modi, D.C.; Modi, K.P.; Patel, N.M.; Rupareliya, M.T. Antihyperlipidemic activity of Gymenma sylvestre R. Br. leaf extract on rats fed with high cholesterol diet. Int. J. Pharmacol. 2010, 6, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Tsumura, Y.; Tonogai, Y.; Shibata, T. Fecal steroid excretion is increased in rats by oral administration of gymnemic acids contained in Gymnema sylvestre leaves. J. Nutr. 1999, 129, 1214–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barani, M.; Hajinezhad, M.R.; Sargazi, S.; Rahdar, A.; Shahraki, S.; Lohrasbi-Nejad, A.; Baino, F.J. In vitro and in vivo anticancer effect of pH-responsive paclitaxel-loaded niosomes. Mater. Sci. Mater. Med. 2021, 32, 147. [Google Scholar] [CrossRef]
- Nazari, V.M.; Mahmood, S.; Shah, A.M.; Al-Suede, F.S.R. Suppression of melanoma growth in a murine tumour model using orthosiphon stamineus benth. Extract loaded in ethanolic phospholipid vesicles (spherosome). Curr. Drug Metab. 2022, 23, 317–328. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Yaraki, M.T.; Ahmadi, S.; Chiani, M.; Nourouzian, D. Folic acid-functionalized niosomal nanoparticles for selective dual-drug delivery into breast cancer cells: An in-vitro investigation. Adv. Powder Technol. 2020, 31, 4064–4071. [Google Scholar] [CrossRef]
- Sivaramakrishna, D.; Prasad, M.D.; Swamy, M.J. A homologous series of apoptosis-inducing N-acylserinols: Thermotropic phase behavior, interaction with cholesterol and characterization of cationic N-myristoylserinol-cholesterol-CTAB niosomes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 504–513. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric nanoparticles for drug delivery. In Cancer Nanotechnology: Methods and Protocols; Grobmyer, S.R., Moudgil, B.M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 163–175. [Google Scholar]
- Cé, R.; Silva, R.C.; Trentin, D.S.; Marchi, J.G.B.D.; Paese, K.; Guterres, S.S.; Macedo, A.J.; Pohlmann, A.R. Galleria mellonella larvae as an in vivo model to evaluate the toxicity of polymeric nanocapsules. J. Nanosci. Nanotechnol. 2020, 20, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Colson, Y.L.; Grinstaff, M.W. Biologically responsive polymeric nanoparticles for drug delivery. Adv. Mater. 2012, 24, 3878–3886. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cabrer, P.; Campos, F. Liposomes and nanotechnology in drug development: Focus on neurological targets. Int. J. Nanomed. 2013, 8, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Belletti, D.; Grabrucker, A.M.; Pederzoli, F.; Menrath, I.; Cappello, V.; Vandelli, M.A.; Forni, F.; Tosi, G.; Ruozi, B. Exploiting the versatility of cholesterol in nanoparticles formulation. Int. J. Pharm. 2016, 511, 331–340. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Frank, L.A.; Gazzi, R.P.; de Andrade Mello, P.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Imiquimod-loaded nanocapsules improve cytotoxicity in cervical cancer cell line. Eur. J. Pharm. Biopharm. 2019, 136, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Raffin Pohlmann, A.; Weiss, V.; Mertins, O.; Pesce da Silveira, N.; Stanisçuaski Guterres, S. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres: Development, stability evaluation and nanostructure models. Eur. J. Pharm. Sci. 2002, 16, 305–312. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M. Transdermal and intravenous nano drug delivery systems: Present and future. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–550. [Google Scholar]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on insulin secretion in vitro. Bioorg. Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-A^{y} mice. Biofactors 2004, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef]
- Mura, C.; Nácher, A.; Merino, V.; Merino-Sanjuán, M.; Manconi, M.; Loy, G.; Fadda, A.M.; Díez-Sales, O. Design, characterization and in vitro evaluation of 5-aminosalicylic acid loaded N-succinyl-chitosan microparticles for colon specific delivery. Colloids Surf. B Biointerfaces 2012, 94, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P.J. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers–a review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Veiga, F.; Figueiras, A. Biodegradable polymeric nanostructures: Design and advances in oral drug delivery for neurodegenerative disorders. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 61–86. [Google Scholar]
- Guigas, B.; Naboulsi, R.; Villanueva, G.R.; Taleux, N.; Lopez-Novoa, J.M.; Leverve, X.M.; El-Mir, M.-Y. The flavonoid silibinin decreases glucose-6-phosphate hydrolysis in perifused rat hepatocytes by an inhibitory effect on glucose-6-phosphatase. Cell. Physiol. Biochem. 2007, 20, 925–934. [Google Scholar] [CrossRef]
- Ahmad, N.; Ramsch, R.; Llinàs, M.; Solans, C.; Hashim, R.; Tajuddin, H.A. Influence of nonionic branched-chain alkyl glycosides on a model nano-emulsion for drug delivery systems. Colloids Surf. B Biointerfaces 2014, 115, 267–274. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Halnor, V.V.; Pande, V.V.; Borawake, D.D.; Nagase, H.S. Nanoemulsion: A novel platform for drug delivery system. J. Mat. Sci. Nanotechol. 2018, 6, 104. [Google Scholar]
- Lovelyn, C.; Attama, A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Abed, K.F. Antimicrobial activity of essential oils of some medicinal plants from Saudi Arabia. Saudi J. Biol. Sci. 2007, 14, 53–60. [Google Scholar]
- El-Soud, N.; El-Laithy, N.; El-Saeed, G.; Wahby, M.; Khalil, M.; Morsy, F.; Shaffie, N. Antidiabetic activities of Foeniculum vulgare Mill. essential oil in streptozotocin-induced diabetic rats. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar]
- Hilmi, Y.; Abushama, M.F.; Abdalgadir, H.; Khalid, A.; Khalid, H. A study of antioxidant activity, enzymatic inhibition and in vitro toxicity of selected traditional sudanese plants with anti-diabetic potential. BMC Complement. Altern. Med. 2014, 14, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Hayati, F.; Widyarini, S.; Helminawati, H.J. Efek antihiperglikemik infusa kangkung darat (ipomea reptans poir) pada tikus jantan galur swiss yang diinduksi streptozotocin. ILM Farm. 2010, 7, 13–22. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.H.; Lin, Z.C.; Fang, J.Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017, 25, 219–234. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Padhi, S.J. Solid lipid nanoparticles–A review. Crit. Rev. 2016, 3, 5–12. [Google Scholar]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Becker Peres, L.; Becker Peres, L.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Rosenholm Pharm. 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Uner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie 2006, 61, 375–386. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Li, H.T.; Wu, X.D.; Davey, A.K.; Wang, J. Antihyperglycemic effects of baicalin on streptozotocin–nicotinamide induced diabetic rats. Phytother Res. 2011, 25, 189–194. [Google Scholar] [CrossRef]
- Waisundara, V.Y.; Hsu, A.; Huang, D.; Tan, B.K.-H. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am. J. Chin. Med. 2008, 36, 517–540. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Z.; Zhang, H.; Zhen, Z.; Calway, T.; Wang, Y.; Yuan, C.-S.; Wang, C.-Z. Pretreatment of baicalin and wogonoside with glycoside hydrolase: A promising approach to enhance anticancer potential. Oncol. Rep. 2013, 30, 2411–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO nanoparticles synthesized using a novel plant extract: Application to enhance physiological and biochemical traits in maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Kitture, R.; Chordiya, K.; Gaware, S.; Ghosh, S.; More, A.P.; Kulkarni, P.; Chopade, B.A.; Kale, S.N. ZnO nanoparticles-red sandalwood conjugate: A promising anti-diabetic agent. J. Nanosci. Nanotechnol. 2015, 15, 4046–4051. [Google Scholar] [CrossRef]
- Sati, S.C.; Kour, G.; Bartwal, A.S.; Sati, M.D. Biosynthesis of metal nanoparticles from leaves of Ficus palmata and evaluation of their anti-inflammatory and anti-diabetic activities. Biochemistry 2020, 59, 3019–3025. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Abdullah Alam, I.; Rizwan, M.; Hussain, Z.; Sultana, K.; Ali, Z.; Uddin, M.N. In vivo analgesic, anti-inflammatory, and anti-diabetic screening of Bacopa monnieri-synthesized copper oxide nanoparticles. ACS Omega 2022, 7, 4071–4082. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.W.A.; Qureshi, M.T.; Hussain, Z.; Ullah, I.; Kalsoom, U.E.; Rahim, F.; Rahman, S.S.U.; Sultana, N.; Khan, M.K. Antidiabetic and hypolipidemic potential of green AgNPs against diabetic mice. ACS Appl. Bio Mater. 2021, 4, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Badeggi, U.M.; Ismail, E.; Adeloye, A.O.; Botha, S.; Badmus, J.A.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A. Green synthesis ofgold nanoparticles capped with procyanidins from Leucosidea sericea as potential antidiabetic and antioxidant agents. Biomolecules 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwetha, U.R.; Latha, M.S.; Kumar, C.R.R.; Kiran, M.S.; Betageri, V.S. Facile synthesis of zinc oxide nanoparticles using novel Areca catechu leaves extract and their in vitro antidiabetic and anticancer studies. J. Inorg. Organomet. Polym. 2020, 30, 4876–4883. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Biogenic proficient synthesis of (Au-NPs) via aqueous extract of Red Dragon Pulp and seed oil: Characterization, antioxidant, cytotoxic properties, anti-diabetic anti-inflammatory, anti-Alzheimer and their anti-proliferative potential against cancer cell lines. Saudi J. Biol. Sci. 2022, 29, 2836–2855. [Google Scholar]
- Shwetha, U.R.; Latha, M.S.; Kumar, C.R.; Kiran, M.S.; Onkarappa, H.S.; Betageri, V.S. Potential antidiabetic and anticancer activity of copper oxide nanoparticles synthesised using Areca catechu leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 025008. [Google Scholar] [CrossRef]
- Ayyoub, S.; Al-Trad, B.; Aljabali, A.A.; Alshaer, W.; Al Zoubi, M.; Omari, S.; Fayyad, D.; Tambuwala, M.M. Biosynthesis of gold nanoparticles using leaf extract of Dittrichia viscosa and in vivo assessment of its anti-diabetic efficacy. Drug Deliv. Transl. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Lava, M.B.; Muddapur, U.M.; Basavegowda, N.; More, S.S.; More, V.S. Characterization, anticancer, antibacterial, anti-diabetic and anti-inflammatory activities of green synthesized silver nanoparticles using Justica wynaadensis leaves extract. Mater. Today Proc. 2021, 46, 5942–5947. [Google Scholar] [CrossRef]
- Malik, A.R.; Sharif, S.; Shaheen, F.; Khalid, M.; Iqbal, Y.; Faisal, A.; Aziz, M.H.; Atif, M.; Ahmad, S.; Fakhar-e-Alam, M.; et al. Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, antidiabetic and photocatalytic activity. Saudi Chem. Soc. 2022, 26, 101438. [Google Scholar] [CrossRef]
- Xing, H. Citrus aurantifulia extract as a capping agent to biosynthesis of gold nanoparticles: Characterization and evaluation of cytotoxicity, antioxidant, antidiabetic, anticholinergics, and anti-bladder cancer activity. Appl. Organomet. Chem. 2021, 35, e6191. [Google Scholar] [CrossRef]
- UR, S.; CR, R.K.; MS, K.; Betageri, V.S.; MS, L.; Veerapur, R.; Lamraoui, G.; Al-Kheraif, A.A.; Elgorban, A.M.; Syed, A.; et al. Biogenic synthesis of NiO nanoparticles using areca catechu leaf extract and their antidiabetic and cytotoxic effects. Molecules 2021, 26, 2448. [Google Scholar]
- Hosny, M.; Fawzy, M.; El-Fakharany, E.M.; Omer, A.M.; Abd El-Monaem, E.M.; Khalifa, R.E.; Eltaweil, A.S. Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J. Environ. Chem. Eng. 2022, 10, 106806. [Google Scholar] [CrossRef]
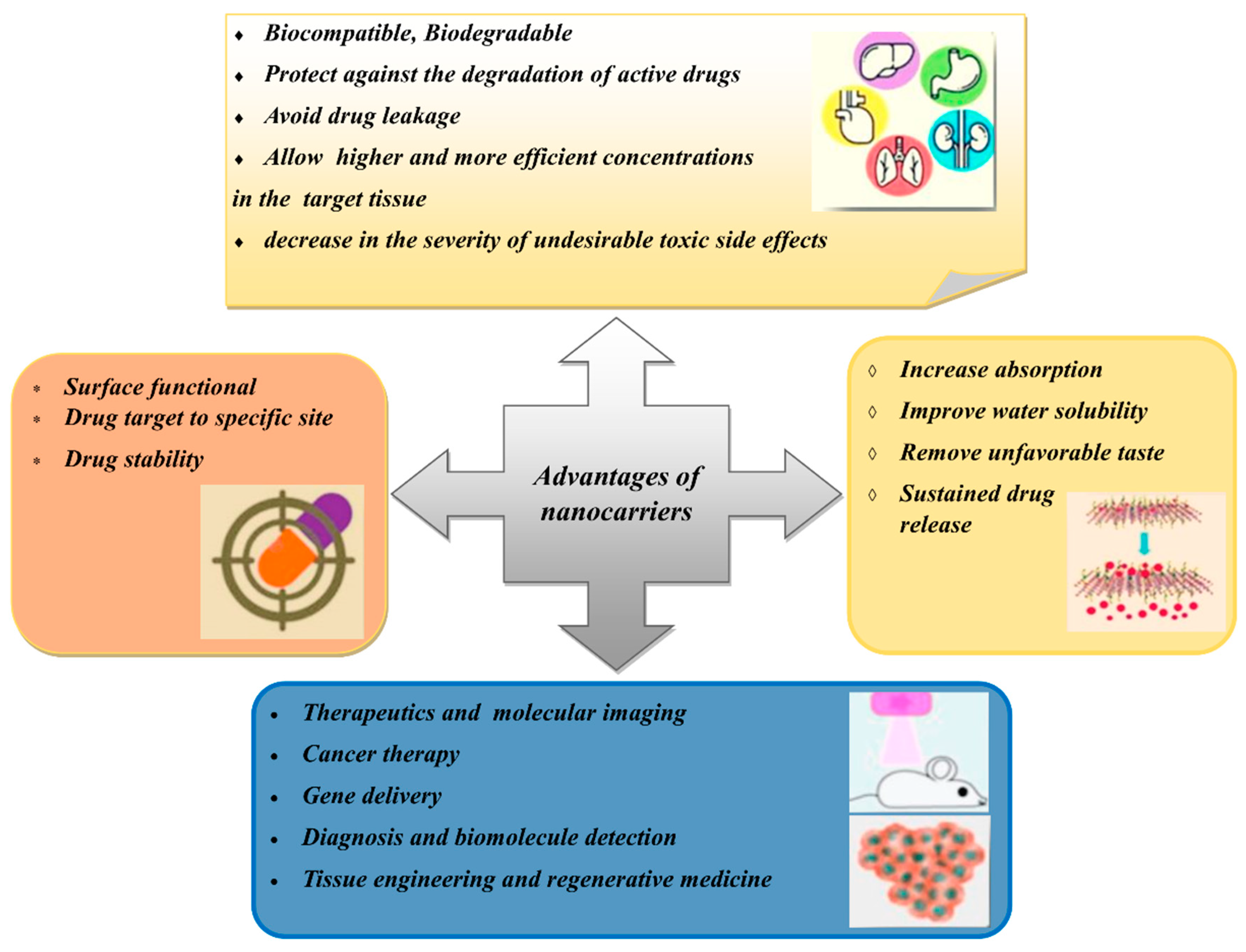
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [Green Version]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Kiong, K.C.; Tham, C.S.; Chien, T.C.; Hilles, A.R.; Venugopal, J.R. PEGylated lipid polymeric nanoparticle–encapsulated acyclovir for in vitro controlled release and ex vivo gut sac permeation. AAPS PharmSciTech 2022, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Mei, T.S.; Yee, W.X.; Hilles, A.R.; Alelwani, W.; Bannunah, A.M. Synthesis of capsaicin loaded silver nanoparticles using green approach and its anti-bacterial activity against human pathogens. J. Biomed. Nanotechnol. 2021, 17, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Chatterjee, B.; Mandal, U.K. Pharmacokinetic evaluation of the synergistic effect of raloxifene loaded transfersomes for transdermal delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102545. [Google Scholar] [CrossRef]
| Plant Part | Scientific Name | Common Name | Antidiabetic and Other Biological Activities | Ref. |
|---|---|---|---|---|
| Bark | Pterocarpus marsupium | Indian kino tree | Antidiabetic and hypoglycaemic | [20] |
| Fruit | Momordica charantia | Bitter melon | Antidiabetic and hypoglycaemic | [21] |
| Leaf | Gymnema sylvestre | Gurmar | Hypoglycaemic and hypolipidemic | [22] |
| Catharanthus roseus | Nayantara | Hypoglycaemic | [23] | |
| Azadirachta indica | Neem | Hypoglycaemic | [23] | |
| Ocimum sanctum | Holy basil | Antidiabetic and hypoglycaemic | [20] | |
| Aloe vera | Aloe vera | Antidiabetic, antihypercholesterolemic and antioxidative | [24] | |
| Rhizome | Curcuma longa | Turmeric | Antidiabetic, antioxidant and anticholinesterase | [25] |
| Seed | Allium sativum | Garlic | Hypoglycaemic | [23] |
| Trigonella foenum-graecum | Fenugreek | Antidiabetic and hypoglycaemic | [20] | |
| Stem | Tinospora cordifolia | Giloy | Hypoglycaemic | [26] |
| Type of Nanocarrier | Plant Extract/Compound | Approximate Size Range (nm) | In Vitro/In Vivo Model | Outcome | Ref. |
|---|---|---|---|---|---|
| Zinc Oxide (ZnO) | Red sandalwood (RSW) | 20 nm | α-Amylase and α-glucosidase inhibition assays with murine pancreatic and small intestinal extracts | ZnO–RSW conjugate showed 61.93% inhibition compared to ZnO nanoparticles and RSW, which showed 21.48% and 5.90% inhibition, respectively | [163] |
| Silver Nanoparticles (Ag NPs) | Bedu (Ficus palmate) | 30 nm | α-Amylase and α-glucosidase assays | Inhibition of α-amylase IC50 showed by Ag NPs for F. palmate leaves (27 μg/mL) and inhibition of α-glucosidase IC50 showed by Ag NPs for F. palmate leaves (32 μg/mL) | [164] |
| ZnO NPs | Andrographis paniculata | Spherical 96–115 nm and hexagonal shapes of 57 ± 0.3 nm | α-Amylase inhibitory activity | IC50 values of the ZnO NPs (121.42 μg/mL) were lower than those of the A. paniculata leaf extract ZnNO3 (149.65 and 178.84 μg/mL, respectively) | [165] |
| Copper Oxide Nanoparticles (CuO NPs) | Bacopa monnieri | 22 nm | STZ-induced diabetic mice | Blood glucose levels were reduced by about 33.66 and 32.19% in groups of mice that were treated with CuO NPs and CuO NPs + insulin, respectively | [166] |
| Ag NPs | Phyllanthus emblica | 30–65 nm | Alloxan-induced diabetic mice | Oral administration of Ag NPs reduced glucose levels from 280.83 ± 4.17 to 151.17 ± 3.54 mg/dL | [167] |
| Gold Nanoparticles (Au NPs) | Leucosidea sericea total extract (LSTE) | 6, 24 and 21 nm | α-Amylase inhibitory activity | Fraction LSTE 4 (F-1) Au NPs demonstrated the highest IC50 value of 1.88 µg/mL | [168] |
| ZnO NPs | Areca catechu | 29 nm | Glucose uptake assay with yeast cells (Saccharomyces cerevisiae) | ZnO NP-treated yeast cells showed a decrease in uptake, which was attributed to antidiabetic activity | [169] |
| Au NPs | Hylocereus polyrhizus (Red Pitaya) Red Dragon Pulp | 25.31 nm | α-Amylase inhibitory activity | Significant inhibition (IC50) of 40.07 ± 0.65, 22.02 ± 0.15 and 11.34 ± 0.11 at 200, 100 and 50 µg/mL, respectively | [170] |
| Copper Oxide Nanoparticles (CuO NPs) | Areca catechu | 200–800 nm | α-Amylase (pancreatic) inhibition assay and glucose uptake with yeast cells | α-Amylase: the inhibition by CuO NP samples showed an IC50 value of 17.3049 at 100 μg/mL; Yeast model: percentage of glucose uptake by the samples showed a value of 70.81 at 250 μg/mL | [171] |
| Au NPs | Dittrichia viscosa | 20 and 50 nm | STZ-induced diabetic adult male Sprague–Dawley rats | Significantly lower levels of hepatic PEPCK enzyme activity when treated with Au NPs compared to the diabetic group (p < 0.02) | [172] |
| Silver Nanoparticles | Justicia wynaadensis | 30 nm–50 nm | Enzymatic activity of α-amylase | The IC50 value of the synthesised Ag nanoparticles was 493.87 µg/mL | [173] |
| Reduced Graphene Oxide (RGO—ZnO) | Ocimum basilicum | 31 nm | α-Amylase and α-glucosidase enzyme inhibition assays | Inhibition activity increased by 67.12% for ZnO NPs and 72.41% for RGO–ZnO NCs at 600 µg/mL | [174] |
| Au NPs | Citrus aurantifolia | 17–24 nm | α-Glycosidase inhibition results | Au NPs produced IC50 values of 43.51 and 130.32 μM | [175] |
| Nickel Oxide Nanoparticles (NiO NPs) | Areca catechu | 5.46 nm | α-Amylase and α-glucosidase enzyme inhibition assays | The results showed α-amylase enzymes with IC50 values of 268.13 µg/mL | [176] |
| Platinum Nanoparticles (Pt NPs) | Polygonum salicifolium | 1–3 nm | In vitro α-amylase and α-glucosidase inhibition | α-Amylase inhibitory IC50 was found to be 72 μg/mL; α-glucosidase inhibitory activity IC50 was found to be 53 μg/mL | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolkepli, H.; Widodo, R.T.; Mahmood, S.; Salim, N.; Awang, K.; Ahmad, N.; Othman, R. A Review on the Delivery of Plant-Based Antidiabetic Agents Using Nanocarriers: Current Status and Their Role in Combatting Hyperglycaemia. Polymers 2022, 14, 2991. https://doi.org/10.3390/polym14152991
Zolkepli H, Widodo RT, Mahmood S, Salim N, Awang K, Ahmad N, Othman R. A Review on the Delivery of Plant-Based Antidiabetic Agents Using Nanocarriers: Current Status and Their Role in Combatting Hyperglycaemia. Polymers. 2022; 14(15):2991. https://doi.org/10.3390/polym14152991
Chicago/Turabian StyleZolkepli, Husna, Riyanto Teguh Widodo, Syed Mahmood, Norazlinaliza Salim, Khalijah Awang, Noraini Ahmad, and Rozana Othman. 2022. "A Review on the Delivery of Plant-Based Antidiabetic Agents Using Nanocarriers: Current Status and Their Role in Combatting Hyperglycaemia" Polymers 14, no. 15: 2991. https://doi.org/10.3390/polym14152991
APA StyleZolkepli, H., Widodo, R. T., Mahmood, S., Salim, N., Awang, K., Ahmad, N., & Othman, R. (2022). A Review on the Delivery of Plant-Based Antidiabetic Agents Using Nanocarriers: Current Status and Their Role in Combatting Hyperglycaemia. Polymers, 14(15), 2991. https://doi.org/10.3390/polym14152991