The Hyperproduction of Polyhydroxybutyrate Using Bacillus mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cardboard Sample Preparation
2.3. Enzymatic Degradation of Cardboard Samples
- (a)
- Determination of optimum incubation time
- (b)
- Weight loss
- (c)
- Determination of reducing sugars
- (d)
- Determination of liberated glucose
2.4. Collection of Samples, Growth Conditions, and Screening of PHA Producing Isolates
2.5. Identification and Characterization of PHA Producing Isolates
2.6. PHA Production Extraction and Purification
- (a)
- MSM supplemented with glucose as a carbon source
- (b)
- Cardboard hydrolysate as a whole medium
- (c)
- Modified MSM with cardboard hydrolysate
- (d)
- Nitrogen depleted MSM with cardboard hydrolysate
- (e)
- Extraction of PHA
2.7. Characterization of the Produced Polymer
- (a)
- Fourier Transform Infrared (FTIR)
- (b)
- 1H and 13C NMR
- (c)
- TGA, DTG, and DTA
- (d)
- XRD
2.8. Statistical Analysis
3. Results and Discussion
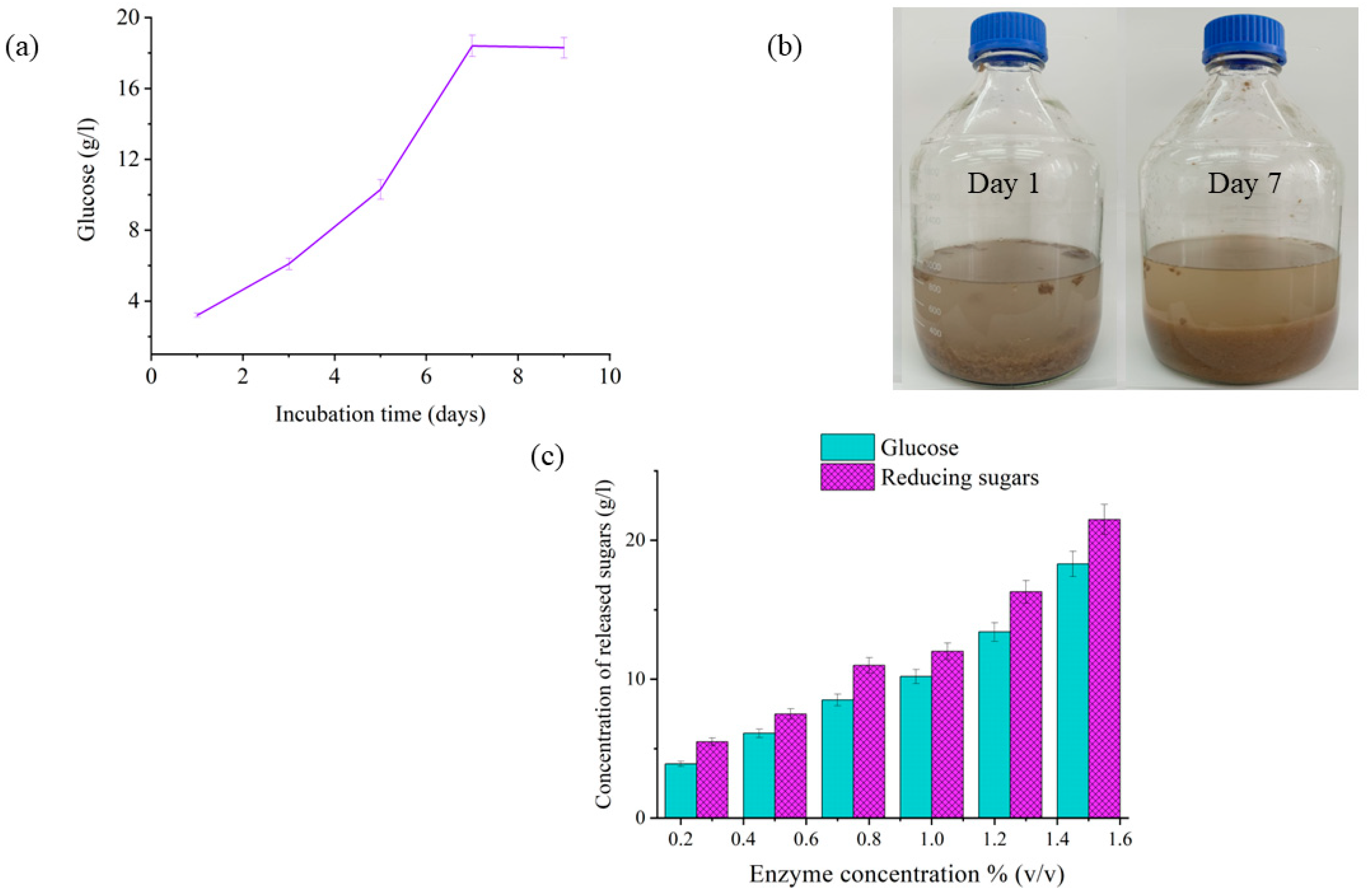
3.1. Enzymatic Degradation of Cardboard Samples
3.2. Molecular Identification of the PHA Producer and Phylogenetic Analysis
3.3. Nucleotides Accession Numbers
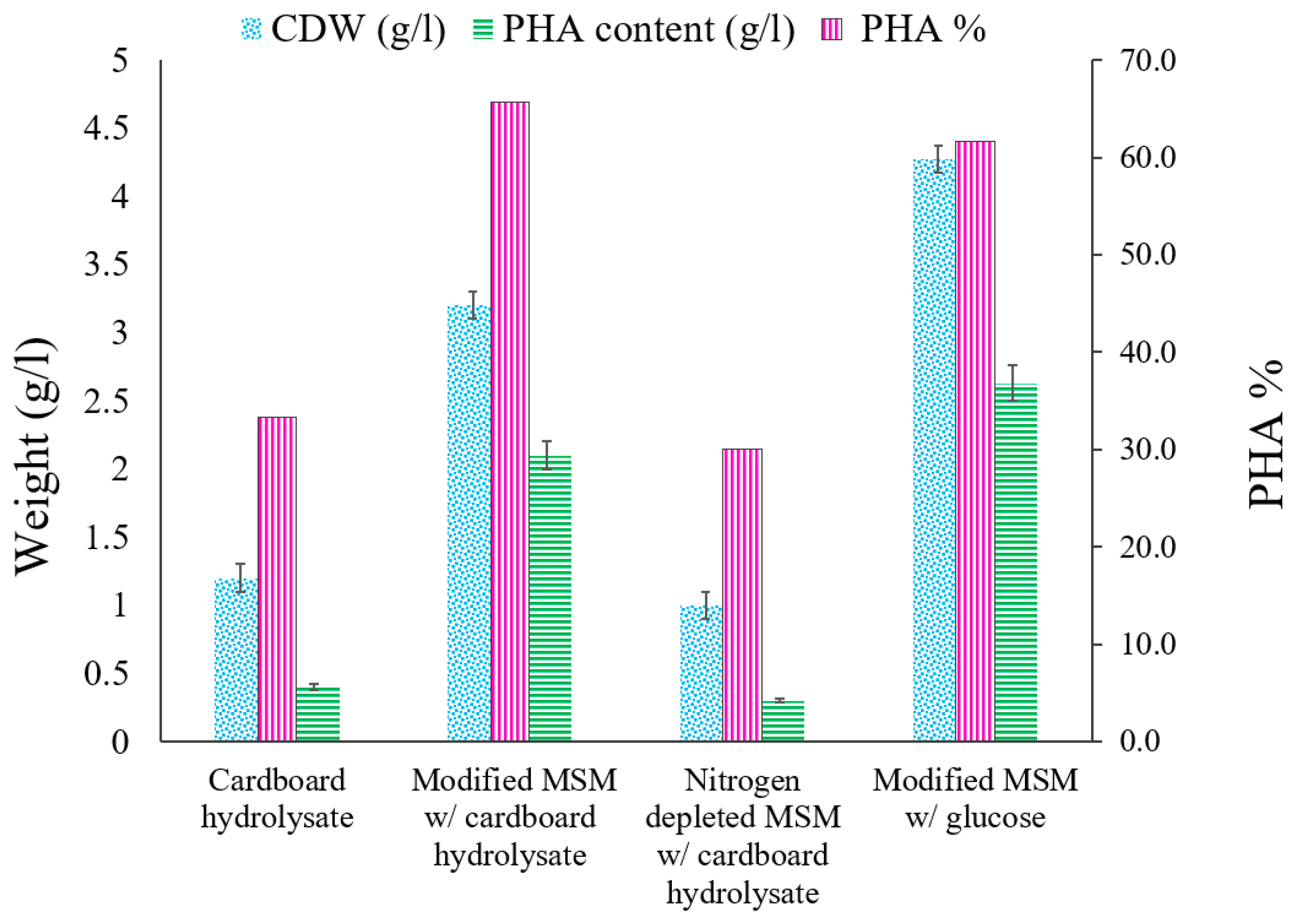
3.4. MSM Supplemented with Glucose as a Carbon Source
3.5. Cardboard Hydrolysate as a Whole Medium
3.6. Modified MSM with Cardboard Hydrolysate
3.7. Nitrogen Depleted MSM with Cardboard Hydrolysate
3.8. Analysis and Characterisation of the Purified Polymer
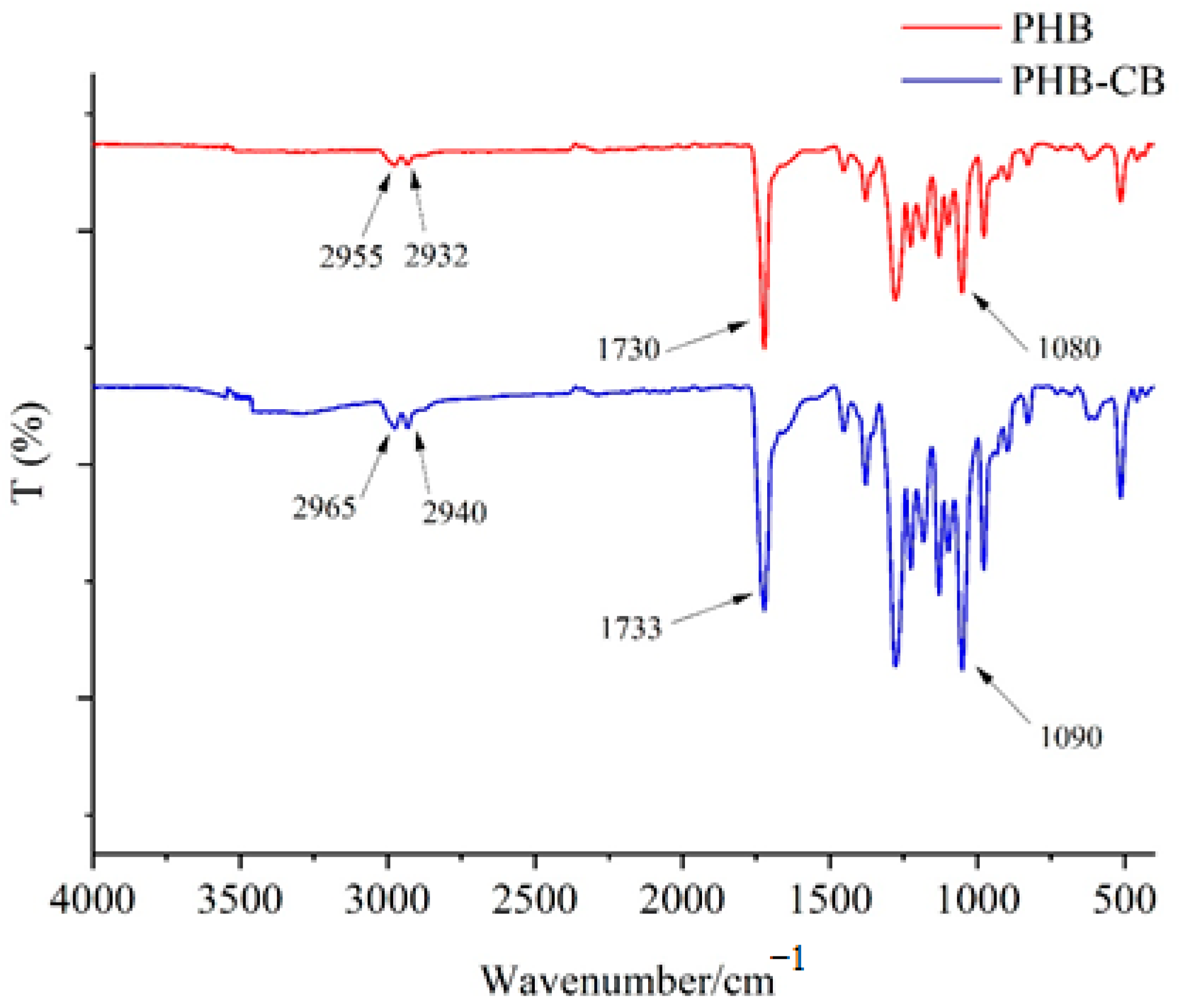
3.8.1. FTIR
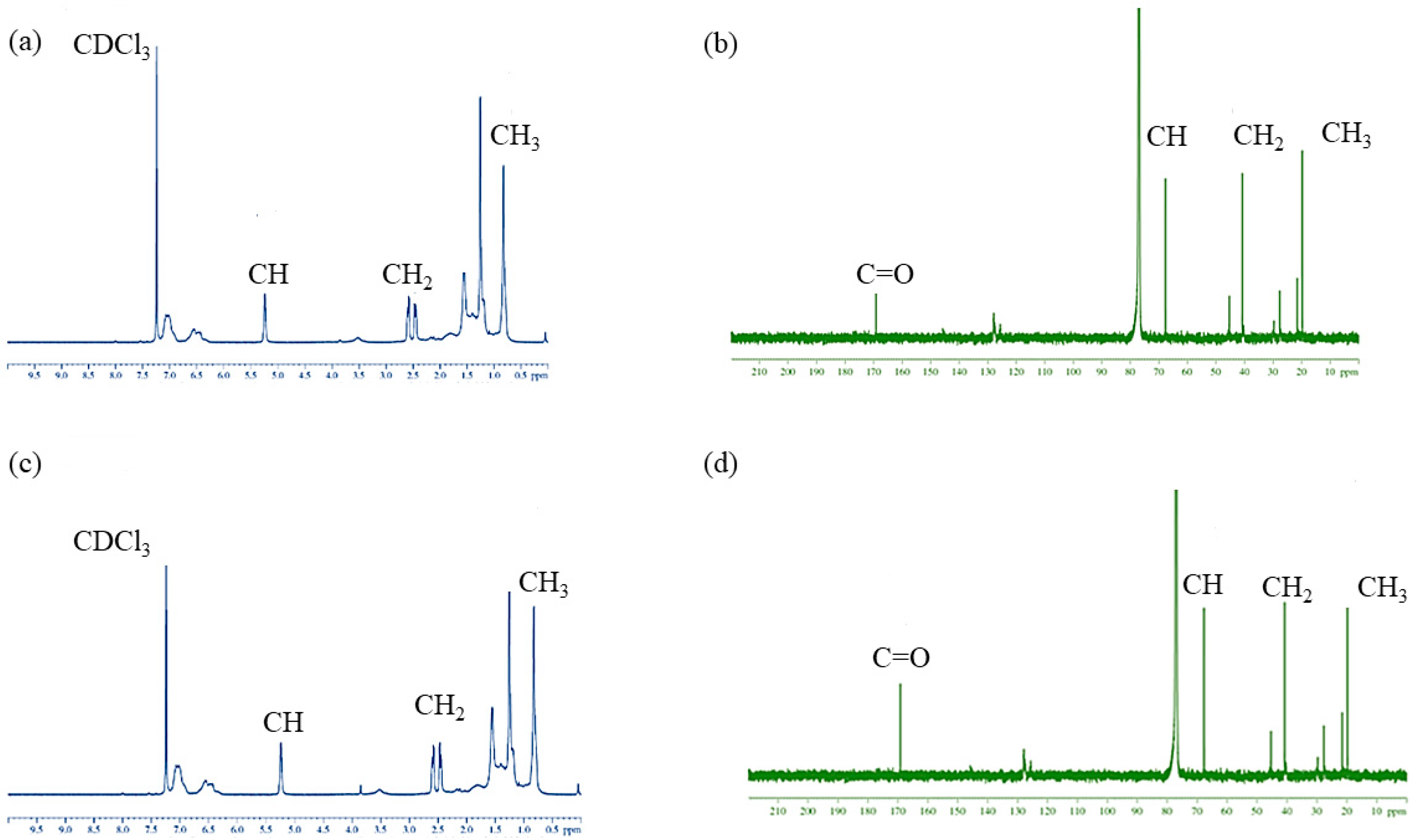
3.8.2. NMR
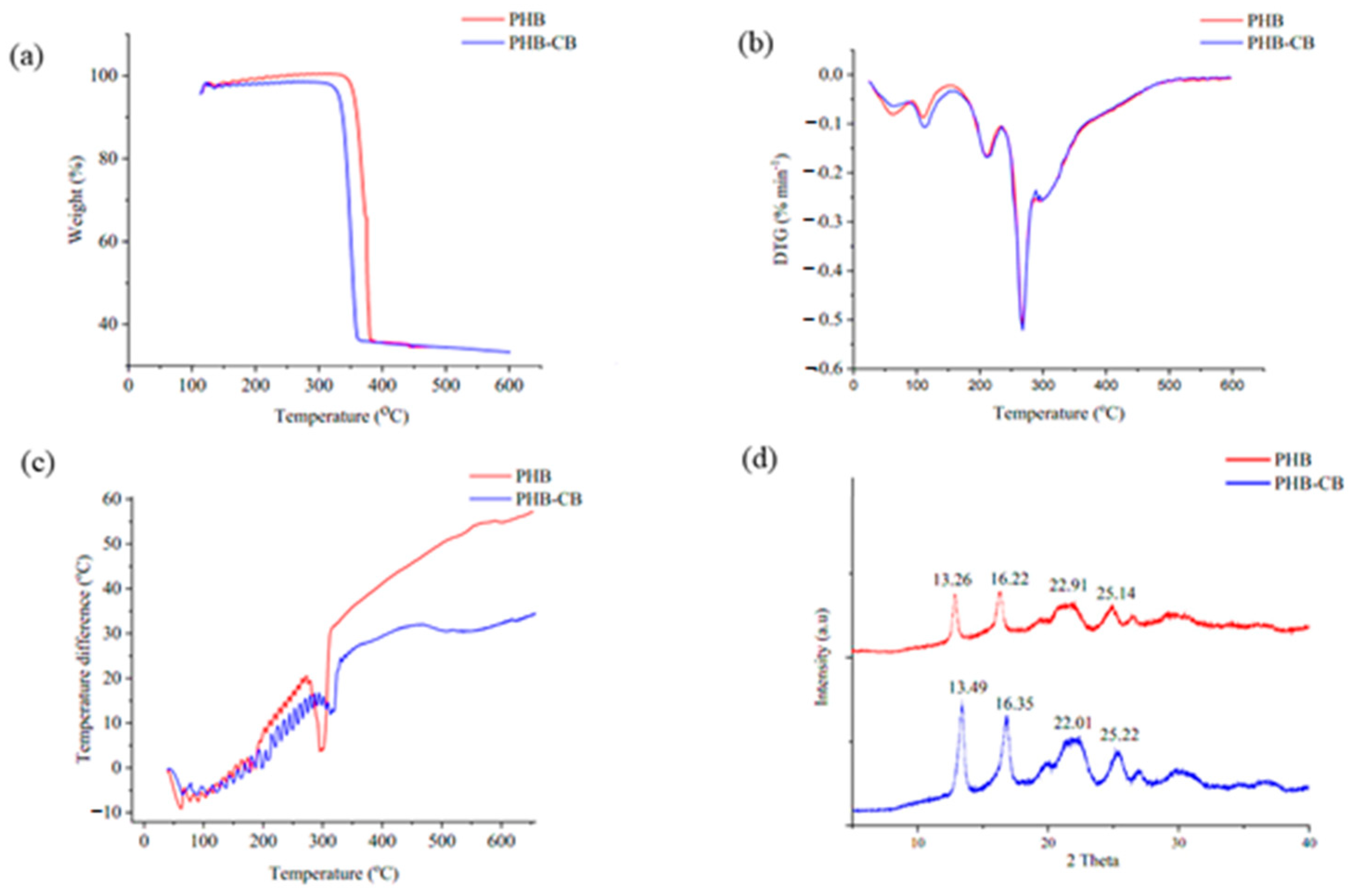
3.8.3. TGA and DTG
3.8.4. DTA
3.8.5. XRD
4. Conclusions
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd El-Malek, F.; Rofeal, M.; Zabed, H.M.; Nizami, A.-S.; Rehan, M.; Qi, X. Microorganism-mediated algal biomass processing for clean products manufacturing: Current status, challenges and future outlook. Fuel 2022, 311, 122612. [Google Scholar] [CrossRef]
- Rofeal, M.; Abdelmalek, F.; Steinbüchel, A. Naturally-Sourced Antibacterial Polymeric Nanomaterials with Special Reference to Modified Polymer Variants. Int. J. Mol. Sci. 2022, 23, 4101. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates (PHAs)—Production, Properties, and Biodegradation. In Biodegradable Polymers in the Circular Plastics Economy; Wiley: Hoboken, NJ, USA, 2022; pp. 145–204. [Google Scholar] [CrossRef]
- El-malek, F.A.; Khairy, H.; Farag, A.; Omar, S. The sustainability of microbial bioplastics, production and applications. Int. J. Biol. Macromol. 2020, 157, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Rofeal, M.; Abd El-Malek, F.; Qi, X. In vitro assessment of green polyhydroxybutyrate/chitosan blend loaded with kaempferol nanocrystals as a potential dressing for infected wounds. Nanotechnology 2021, 32, 375102. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Abd El-malek, F.; Rofeal, M.; Farag, A.; Omar, S.; Khairy, H. Polyhydroxyalkanoate nanoparticles produced by marine bacteria cultivated on cost effective Mediterranean algal hydrolysate media. J. Biotechnol. 2021, 328, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef] [PubMed]
- Revelles, O.; Beneroso, D.; Menendez, J.A.; Arenillas, A.; García, J.L.; Prieto, M.A. Syngas obtained by microwave pyrolysis of household wastes as feedstock for polyhydroxyalkanoate production in Rhodospirillum rubrum. Microb. Biotechnol. 2017, 10, 1412–1417. [Google Scholar] [CrossRef] [Green Version]
- Elain, A.; Le Grand, A.; Corre, Y.-M.; Le Fellic, M.; Hachet, N.; Le Tilly, V.; Loulergue, P.; Audic, J.-L.; Bruzaud, S. Valorisation of local agro-industrial processing waters as growth media for polyhydroxyalkanoates (PHA) production. Ind. Crops Prod. 2016, 80, 1–5. [Google Scholar] [CrossRef]
- Vigneswari, S.; Noor, M.S.M.; Amelia, T.S.M.; Balakrishnan, K.; Adnan, A.; Bhubalan, K.; Amirul, A.-A.A.; Ramakrishna, S. Recent advances in the biosynthesis of polyhydroxyalkanoates from lignocellulosic feedstocks. Life 2021, 11, 807. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; De Almeida, M.C.M.D.; Van Keulen, F.; Ferreira, B.S.; Da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, Z.; Zhang, K.; Liu, M.; Liu, D.; Yan, X.; Si, M.; Shi, Y. Valorizing waste liquor from dilute acid pretreatment of lignocellulosic biomass by Bacillus megaterium B-10. Ind. Crops Prod. 2021, 161, 113160. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suthar, S.; Kishore Singh, N. Fungal pretreatment facilitates the rapid and valuable composting of waste cardboard. Bioresour. Technol. 2022, 344, 126178. [Google Scholar] [CrossRef]
- Wang, L.; Sharifzadeh, M.; Templer, R.; Murphy, R.J. Bioethanol production from various waste papers: Economic feasibility and sensitivity analysis. Appl. Energy 2013, 111, 1172–1182. [Google Scholar] [CrossRef]
- Ramamoorthy, N.K.; Nagarajan, R.; Ravi, S.; Sahadevan, R. An innovative plasma pre-treatment process for lignocellulosic bio-ethanol production. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–15. [Google Scholar] [CrossRef]
- Mohapatra, S.; Pattnaik, S.; Maity, S.; Mohapatra, S.; Sharma, S.; Akhtar, J.; Pati, S.; Samantaray, D.P.; Varma, A. Comparative analysis of PHAs production by Bacillus megaterium OUAT 016 under submerged and solid-state fermentation. Saudi J. Biol. Sci. 2020, 27, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef] [PubMed]
- Rofeal, M.; El-Malek, F.A. Valorization of Lipopeptides Biosurfactants as Anticancer Agents. Int. J. Pept. Res. Ther. 2021, 27, 447–455. [Google Scholar] [CrossRef]
- Rofeal, M.; El-Malek, F.A. Ribosomal proteins as a possible tool for blocking SARS-COV 2 virus replication for a potential prospective treatment. Med. Hypotheses 2020, 143, 109904. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J. Systems of analysis for evaluating fibrous feeds. In Standardization of Analytical Methodology for Feeds: Proceedings; IDRC: Ottawa, ON, Canada, 1979. [Google Scholar]
- Yáñez, R.; Alonso, J.L.; Parajó, J.C. Production of hemicellulosic sugars and glucose from residual corrugated cardboard. Process Biochem. 2004, 39, 1543–1551. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; Laboratory Analytical Procedure (LAP): Location Golden, CO, USA, 2008. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yousef, N.; Mawad, A.; Abeed, A. Enhancement the cellulase activity induced by endophytic bacteria using calcium nanoparticles. Curr. Microbiol. 2019, 76, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Martínez-Trujillo, M.A.; Bautista-Rangel, K.; García-Rivero, M.; Martínez-Estrada, A.; Cruz-Díaz, M.R. Enzymatic saccharification of banana peel and sequential fermentation of the reducing sugars to produce lactic acid. Bioprocess Biosyst. Eng. 2020, 43, 413–427. [Google Scholar] [CrossRef] [PubMed]
- El-malek, F.A.; Farag, A.; Omar, S.; Khairy, H. Polyhydroxyalkanoates (PHA) from Halomonas pacifica ASL10 and Halomonas salifodiane ASL11 isolated from Mariout salt lakes. Int. J. Biol. Macromol. 2020, 161, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Przydatek, G. Assessment of changes in the municipal waste accumulation in Poland. Environ. Sci. Pollut. Res. 2020, 27, 25766–25773. [Google Scholar] [CrossRef] [PubMed]
- Kinnarinen, T.; Häkkinen, A. Influence of enzyme loading on enzymatic hydrolysis of cardboard waste and size distribution of the resulting fiber residue. Bioresour. Technol. 2014, 159, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, W.; Luo, W.; Fang, H.; Lv, H.; Liu, R.; Niu, Q. Anaerobic co-digestion of chicken manure and cardboard waste: Focusing on methane production, microbial community analysis and energy evaluation. Bioresour. Technol. 2021, 321, 124429. [Google Scholar] [CrossRef] [PubMed]
- Sambasivarao, S.V.; Granum, D.M.; Wang, H.; Maupin, C.M. Identifying the enzymatic mode of action for cellulase enzymes by means of docking calculations and a machine learning algorithm. AIMS Mol. Sci. 2014, 1, 59–80. [Google Scholar] [CrossRef]
- Jayasekara, S.; Ratnayake, R. Microbial cellulases: An overview and applications. Cellulose 2019, 22, 92. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.H.; Zhang, K.; Prakash, G.S.; Song, D.-P.; Muniyasamy, S.; Pugazhendhi, A.; Jeyakanthan, J.; Arun, A. Optimization of media components and culture conditions for polyhydroxyalkanoates production by Bacillus megaterium. Fuel 2020, 271, 117522. [Google Scholar] [CrossRef]
- Borah, B.; Thakur, P.; Nigam, J. The influence of nutritional and environmental conditions on the accumulation of poly-β-hydroxybutyrate in Bacillus mycoides RLJ B-017. J. Appl. Microbiol. 2002, 92, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Contreras, A.; Koller, M.; Miranda-de Sousa Dias, M.; Calafell-Monfort, M.; Braunegg, G.; Marqués-Calvo, M.S. High production of poly (3-hydroxybutyrate) from a wild Bacillus megaterium Bolivian strain. J. Appl. Microbiol. 2013, 114, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Jamil, N. Biosynthesis and characterization of poly3-hydroxyalkanote (PHA) from newly isolated bacterium Bacillus sp. AZR-1. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 371–378. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Mokhtari, Z.-B.; Amai, T.; Tanaka, K. Microbial production of poly (hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Sangkharak, K.; Prasertsan, P. Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron. J. Biotechnol. 2008, 11, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Al-Battashi, H.; Annamalai, N.; Al-Kindi, S.; Nair, A.S.; Al-Bahry, S.; Verma, J.P.; Sivakumar, N. Production of bioplastic (poly-3-hydroxybutyrate) using waste paper as a feedstock: Optimization of enzymatic hydrolysis and fermentation employing Burkholderia sacchari. J. Clean. Prod. 2019, 214, 236–247. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Chen, G.Q.; Chen, X.Y.; Wu, F.Q.; Chen, J.C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Rauzi, J.; Tschirner, U. Enzymatic Glucose and Xylose Production from Paper Mill Rejects. Recycling 2022, 7, 24. [Google Scholar] [CrossRef]
- Abd El-malek, F.; Steinbüchel, A. Post-Synthetic Enzymatic and Chemical Modifications for Novel Sustainable Polyesters. Front. Bioeng. Biotechnol. 2021, 9, 817023. [Google Scholar] [CrossRef]
- Ibrahim, H.G.; Ouiminga, S.K.; Yonli, A.; Sanogo, O.; Daho, T.; Koulidiati, J. Study of temperature fields and heavy metal content in the ash and flue gas produced by the combustion of briquettes coming from paper and cardboard waste. Recycling 2018, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Dikshit, P.K.; Moholkar, V.S. Production, ultrasonic extraction, and characterization of poly (3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym. Adv. Technol. 2018, 29, 2392–2400. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Y.; Wang, X.; Yang, J.; Gao, Y.; Zi, X.; Zhang, X.; Gao, H.; Hu, N. Psychrotrophic Pseudomonas mandelii CBS-1 produces high levels of poly-β-hydroxybutyrate. Springerplus 2013, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravuri, J.; Galla, M. Assessment of marine microbial polyhydroxybutyrate by employing high-performance liquid chromatography and nuclear magnetic resonance techniques. Int. J. Environ. Sci. Technol. 2022, 19, 85–94. [Google Scholar] [CrossRef]
- Hablot, E.; Bordes, P.; Pollet, E.; Avérous, L. Thermal and thermo-mechanical degradation of poly (3-hydroxybutyrate)-based multiphase systems. Polym. Degrad. Stab. 2008, 93, 413–421. [Google Scholar] [CrossRef]
- Mahansaria, R.; Dhara, A.; Saha, A.; Haldar, S.; Mukherjee, J. Production enhancement and characterization of the polyhydroxyalkanoate produced by Natrinema ajinwuensis (as synonym) ≡ Natrinema altunense strain RM-G10. Int. J. Biol. Macromol. 2018, 107, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Bakhiet, E.K.; Ali, S.G.; Hussien, H.R. Production and characterization of polyhydroxybutyrate (PHB) produced by Bacillus sp. isolated from Egypt. J. Appl. Pharm. Sci. 2016, 6, 046–051. [Google Scholar] [CrossRef] [Green Version]
- Saranya, V.; Shenbagarathai, R. Production and characterization of PHA from recombinant E. coli harbouring phaC1 gene of indigenous Pseudomonas sp. LDC-5 using molasses. Braz. J. Microbiol. 2011, 42, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Škrbić, Z.; Divjaković, V. Temperature influence on changes of parameters of the unit cell of biopolymer PHB. Polymer 1996, 37, 505–507. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Production of poly-hydroxyalkanoate as secondary metabolite with main focus on sustainable energy. Renew. Sust. Energ. Rev. 2017, 72, 95–104. [Google Scholar] [CrossRef]

| Cardboard Samples Weights (g) | 100 | 80 | 50 | 20 |
|---|---|---|---|---|
| Enzyme Concentration (%) | Weight Loss (%) | |||
| 0.25 | 20 ± 0.5 a | 23 ± 0.3 a | 26 ± 0.5 a | 30 ± 0.1 a |
| 0.5 | 32 ± 0.2 b | 33 ± 0.9 b | 36 ± 0.5 b | 39 ± 0.3 b |
| 0.75 | 51 ± 0.1 c | 50 ± 0.5 c | 50 ± 0.3 c | 52 ± 0.9 c |
| 1 | 60 ± 0.2 d | 61 ± 0.4 d | 64 ± 0.1 d | 66 ± 0.1 d |
| 1.25 | 65 ± 0.3 e | 67 ± 0.1 d | 68 ± 0.1 d | 70 ± 0.6 e |
| 1.5 | 69 ± 0.3 e | 69 ± 0.2 d | 70 ± 0.3 d | 73 ± 0.3 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmalek, F.; Steinbüchel, A.; Rofeal, M. The Hyperproduction of Polyhydroxybutyrate Using Bacillus mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard. Polymers 2022, 14, 2810. https://doi.org/10.3390/polym14142810
Abdelmalek F, Steinbüchel A, Rofeal M. The Hyperproduction of Polyhydroxybutyrate Using Bacillus mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard. Polymers. 2022; 14(14):2810. https://doi.org/10.3390/polym14142810
Chicago/Turabian StyleAbdelmalek, Fady, Alexander Steinbüchel, and Marian Rofeal. 2022. "The Hyperproduction of Polyhydroxybutyrate Using Bacillus mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard" Polymers 14, no. 14: 2810. https://doi.org/10.3390/polym14142810
APA StyleAbdelmalek, F., Steinbüchel, A., & Rofeal, M. (2022). The Hyperproduction of Polyhydroxybutyrate Using Bacillus mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard. Polymers, 14(14), 2810. https://doi.org/10.3390/polym14142810








