Finding a Benign Plasticizer to Enhance the Microbial Degradation of Polyhydroxybutyrate (PHB) Evaluated by PHB Degrader Microbulbifer sp. SOL66
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Toxicity Test of Various Plasticizers to the Growth of Microbulbifer sp. SOL66
2.3. Degradation of PHB Containing Various Plasticizers under the Liquid Condition
2.4. GC-MS Analysis
2.5. Chemical and Mechanical Analysis
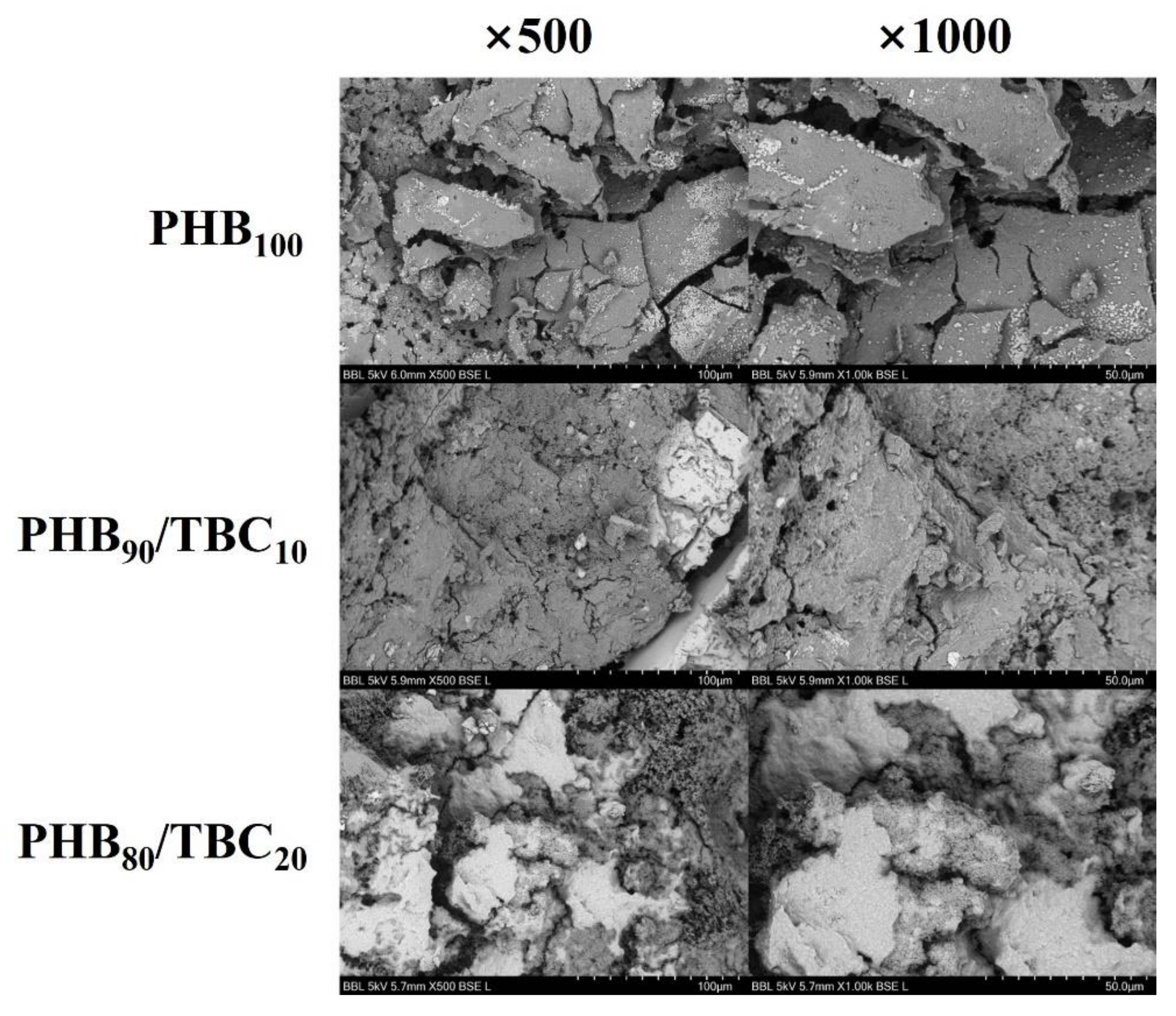
2.6. SEM Analysis
2.7. GPC Analysis
3. Results
3.1. Comparison of Properties of Various Plasticizers
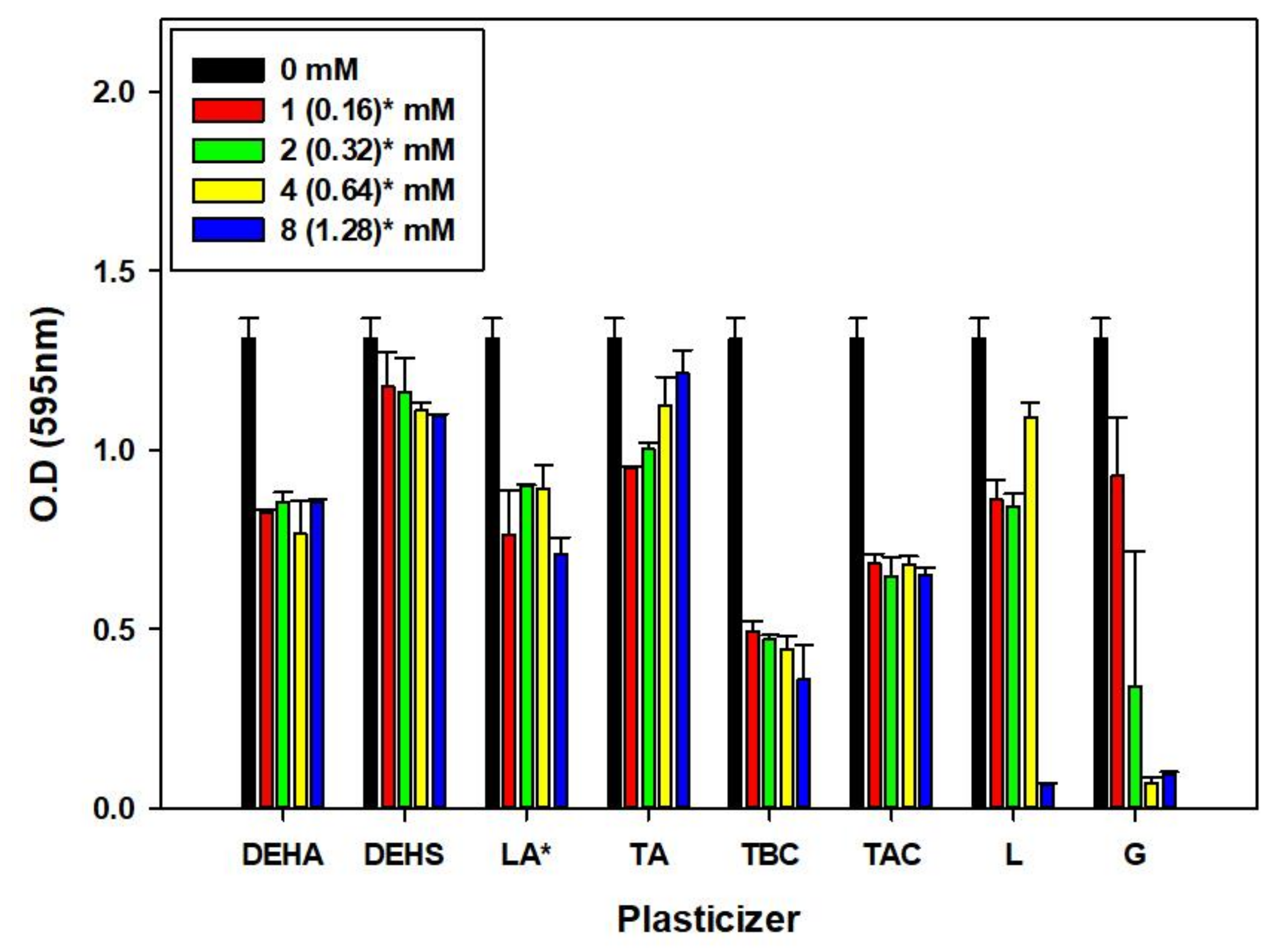
3.2. Effect of Various Plasticizers on Microbulbifer sp. SOL66 Cell
3.3. Thermal Properties Analyzed by DSC
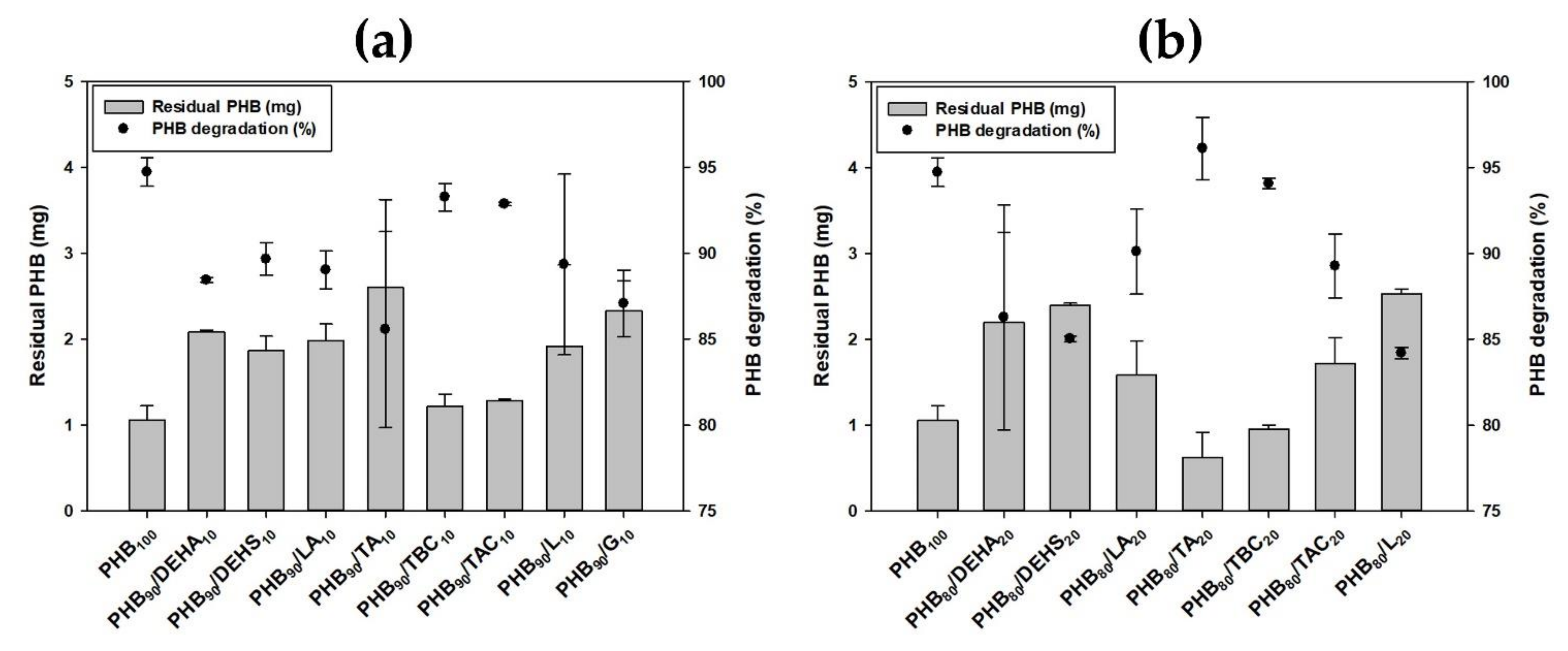
3.4. Effect of Various Plasticizers on the Biodegradability of PHB by Microbulbifer sp. SOL66
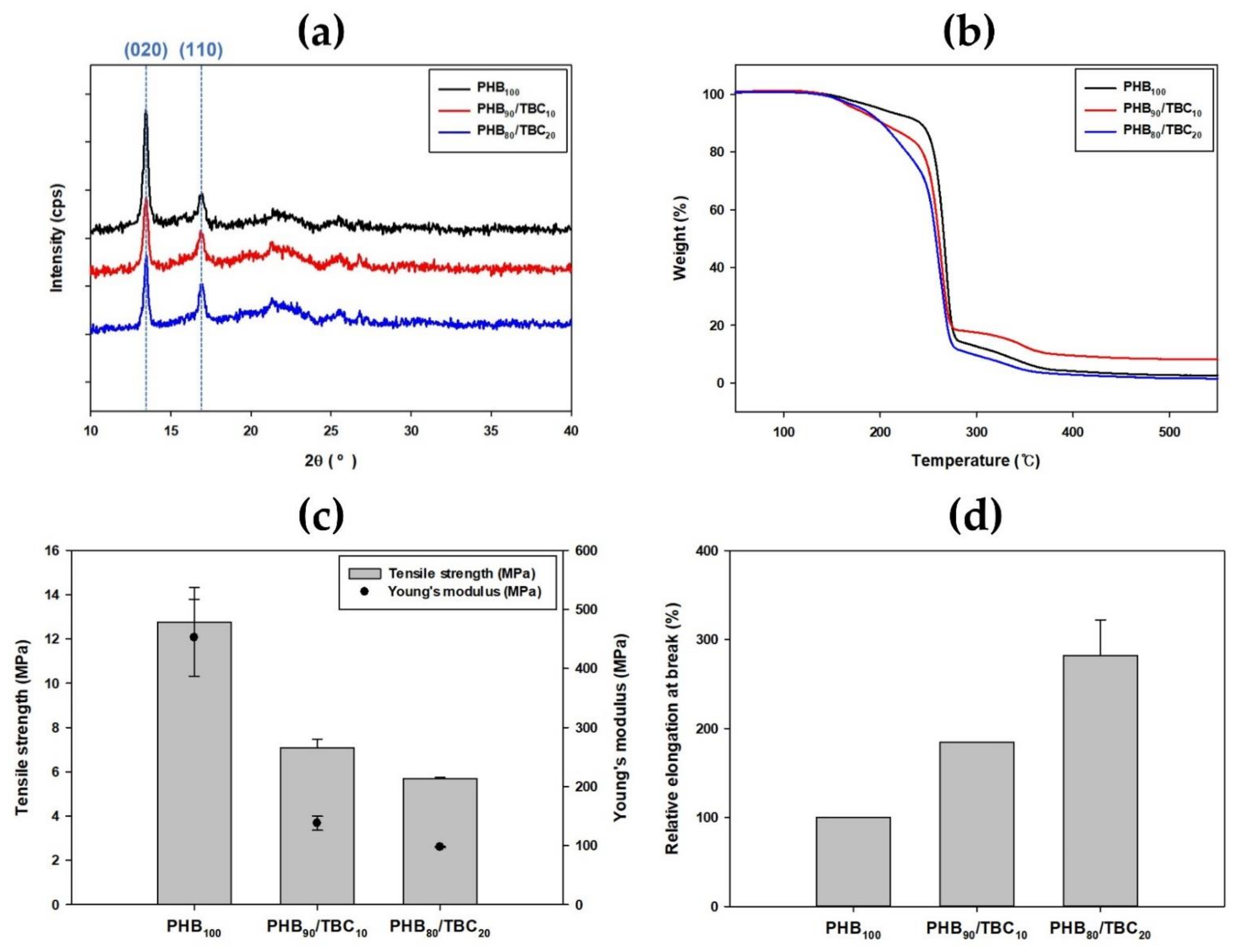
3.5. Comparison of Other Properties with Using Various Analytical Instruments
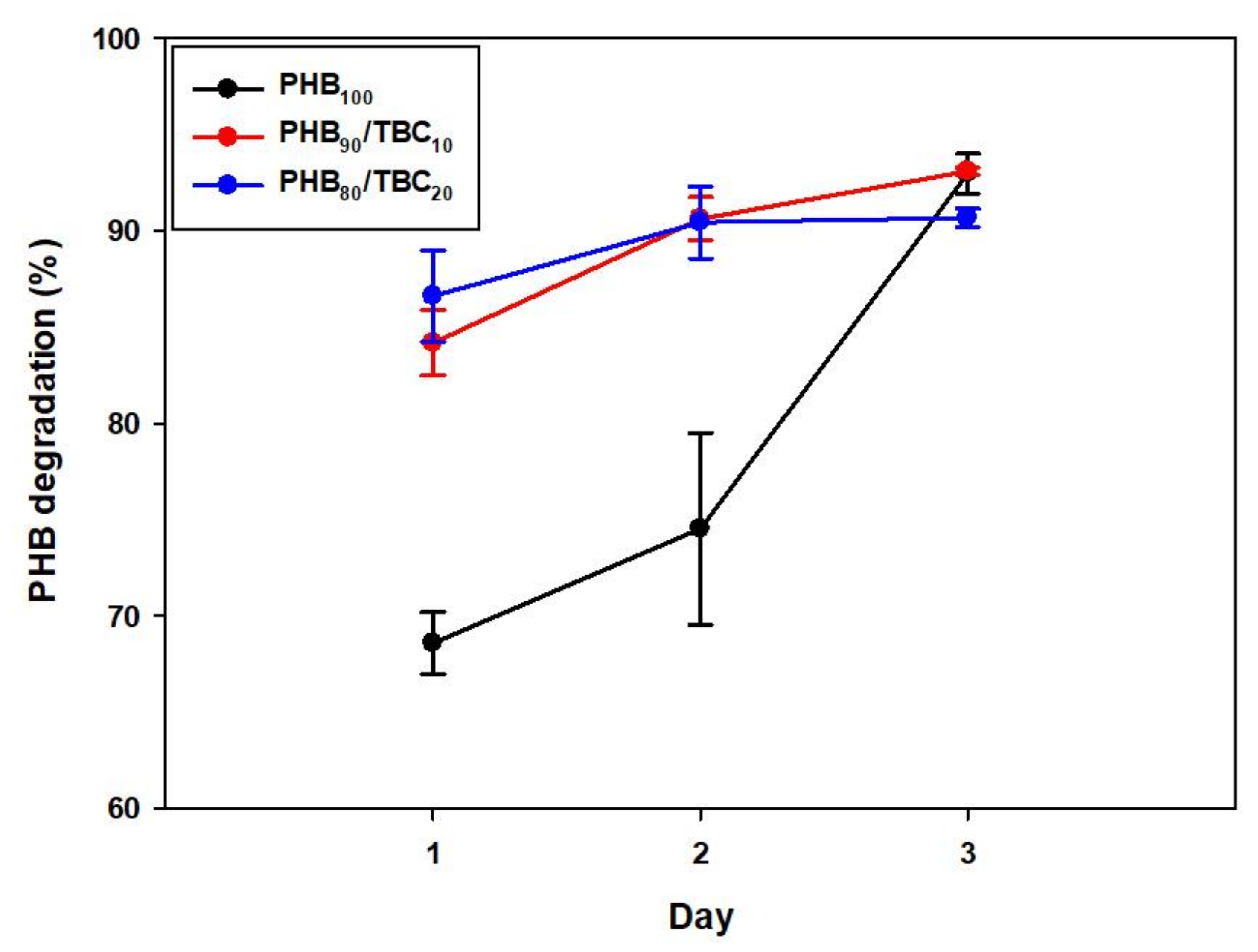
3.6. Time-Dependent Analysis of PHB Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jõgi, K.; Bhat, R. Valorization of food processing wastes and by-products for bioplastic production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Bharti, S.N.; Swetha, G. Need for Bioplastics and Role of Biopolymer PHB: A Short Review. J. Pet. Environ. Biotechnol. 2016, 7, 5–8. [Google Scholar] [CrossRef]
- Mohd Ishak, Z.A.; Ahmad Thirmizir, M.Z. Editorial corner-a personal view producing green composites via polymer blending. Express Polym. Lett. 2021, 15, 910–911. [Google Scholar] [CrossRef]
- Popa, M.S.; Frone, A.N.; Panaitescu, D.M. Polyhydroxybutyrate blends: A solution for biodegradable packaging? Int. J. Biol. Macromol. 2022, 207, 263–277. [Google Scholar] [CrossRef]
- Choi, B.; Yoo, S.; Park, S. Il Carbon footprint of packaging films made from LDPE, PLA, and PLA/PBAT blends in South Korea. Sustainability 2018, 10, 2369. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Park, Y.L.; Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Song, H.S.; Park, J.Y.; Han, Y.H.; Lee, S.M.; Park, S.L.; et al. Fructose based hyper production of poly-3-hydroxybutyrate from Halomonas sp. YLGW01 and impact of carbon sources on bacteria morphologies. Int. J. Biol. Macromol. 2020, 154, 929–936. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Karbasi, S.; Toloue, E.B. Modified poly(3-hydroxybutyrate)-based scaffolds in tissue engineering applications: A review. Int. J. Biol. Macromol. 2021, 166, 986–998. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Güney, A.; Özdilek, C.; Kangal, M.O.; Burat, F. Flotation characterization of PET and PVC in the presence of different plasticizers. Sep. Purif. Technol. 2015, 151, 47–56. [Google Scholar] [CrossRef]
- Aguilar, J.M.; Bengoechea, C.; Pérez, E.; Guerrero, A. Effect of different polyols as plasticizers in soy based bioplastics. Ind. Crops Prod. 2020, 153, 112522. [Google Scholar] [CrossRef]
- Alonso-González, M.; Felix, M.; Romero, A. Influence of the plasticizer on rice bran-based eco-friendly bioplastics obtained by injection moulding. Ind. Crops Prod. 2022, 180, 114767. [Google Scholar] [CrossRef]
- Andrade, R.C. Biodegradation of Commonly Used Plasticizers by Pure Bacterial Cultures; McGill University: Montreal, QC, Canada, 2011; ISBN 9780494841518. [Google Scholar]
- Park, S.L.; Cho, J.Y.; Kim, S.H.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Ham, S.; Bhatia, S.K.; Gurav, R.; Park, S.-H.; et al. Novel Polyhydroxybutyrate-Degrading Activity of the Microbulbifer Genus as Confirmed by Microbulbifer sp. SOL03 from the Marine Environment. J. Microbiol. Biotechnol. 2022, 32, 27–36. [Google Scholar] [CrossRef]
- Park, S.L.; Cho, J.Y.; Kim, S.H.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; Yang, Y.H. Isolation of microbulbifer sp. Sol66 with high polyhydroxyalkanoate-degrading activity from the marine environment. Polymers 2021, 13, 4257. [Google Scholar] [CrossRef]
- Song, H.S.; Bhatia, S.K.; Choi, T.R.; Gurav, R.; Kim, H.J.; Lee, S.M.; Park, S.L.; Lee, H.S.; Joo, H.S.; Kim, W.; et al. Increased antibiotic resistance of methicillin-resistant staphylococcus aureus USA300 Δpsm mutants and a complementation study of Δpsm mutants using synthetic phenol-soluble modulins. J. Microbiol. Biotechnol. 2021, 31, 115–122. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, S.L.; Kim, S.H.; Jung, H.J.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; et al. Novel Poly(butylene adipate-co-terephthalate)-degrading Bacillus sp. JY35 from wastewater sludge and its broad degradation of various bioplastics. Waste Manag. 2022, 144, 1–10. [Google Scholar] [CrossRef]
- Choi, T.R.; Park, Y.L.; Song, H.S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Kim, H.J.; Bhatia, S.K.; Gurav, R.; Choi, K.Y.; et al. Fructose-based production of short-chain-length and medium-chain-length polyhydroxyalkanoate copolymer by arctic pseudomonas sp. B14-6. Polymers 2021, 13, 1398. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lu, W.; Yeh, J.-T. Effect of Plasticizer on the Crystallization Behavior of Poly(lactic acid). J. Appl. Polym. Sci. 2009, 113, 112–121. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Erceg, M.; Jozic, D. Preparation and characterization of poly(3-hydroxy-butyrate)/Cloisite25A nanocomposites. E-Polymers 2013, 13, 25. [Google Scholar] [CrossRef]
- Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low molecular weight and polymeric modifiers as toughening agents in poly(3-hydroxybutyrate) films. Polymers 2020, 12, 2446. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Trusca, R.; Ghiurea, M.; Gabor, A.R.; Mihailescu, M.; Casarica, A.; Lupescu, I. Role of bacterial cellulose and poly (3-hydroxyhexanoate-co-3-hydroxyoctanoate) in poly (3-hydroxybutyrate) blends and composites. Cellulose 2018, 25, 5569–5591. [Google Scholar] [CrossRef]
- Erceg, M.; Kovačić, T.; Klarić, I. Thermal degradation of poly(3-hydroxybutyrate) plasticized with acetyl tributyl citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- Araque, L.M.; De Morais, A.C.L.; Alves, T.S.; Azevedo, J.B.; Carvalho, L.H.; Barbosa, R. Preparation and characterization of poly(hydroxybutyrate) and hollow glass microspheres composite films: Morphological, thermal, and mechanical properties. J. Mater. Res. Technol. 2019, 8, 935–943. [Google Scholar] [CrossRef]
- Seoane, I.T.; Cerrutti, P.; Vazquez, A.; Cyras, V.P.; Manfredi, L.B. Ternary nanocomposites based on plasticized poly(3-hydroxybutyrate) and nanocellulose. Polym. Bull. 2018, 76, 967–988. [Google Scholar] [CrossRef]
- Inoue, S.; Yamaguchi, S.; Uyama, H.; Yamashiro, T.; Imazato, S. Influence of constant strain on the elasticity of thermoplastic orthodontic materials. Dent. Mater. J. 2020, 39, 415–421. [Google Scholar] [CrossRef]
- Chen, S.; Hori, N.; Kajiyama, M.; Takemura, A. Compatibilities and properties of poly lactide/poly (methyl acrylate) grafted chicken feather composite: Effects of graft chain length. J. Appl. Polym. Sci. 2020, 137, 48981. [Google Scholar] [CrossRef]
- Duong, H.C.; Chuai, D.; Woo, Y.C.; Shon, H.K.; Nghiem, L.D.; Sencadas, V. A novel electrospun, hydrophobic, and elastomeric styrene-butadiene-styrene membrane for membrane distillation applications. J. Membr. Sci. 2018, 549, 420–427. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee Park, S.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Ham, S.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; et al. Polyhydroxyalkanoates (PHAs) degradation by the newly isolated marine Bacillus sp. JY14. Chemosphere 2021, 283, 131172. [Google Scholar] [CrossRef]
- Sivaprakash, G.; Raja, R.K.; Mohanrasu, K.; Dinesh, G.H.; Arun, A. Microbial nanotechnology in food industry: Antimicrobial packaging. In Handbook of Microbial Nanotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 311–329. [Google Scholar] [CrossRef]
- Behairy, A.; Abd El-Rahman, G.I.; Aly, S.S.H.; Fahmy, E.M.; Abd-Elhakim, Y.M. Di(2-ethylhexyl) adipate plasticizer triggers hepatic, brain, and cardiac injury in rats: Mitigating effect of Peganum harmala oil. Ecotoxicol. Environ. Saf. 2021, 208, 111620. [Google Scholar] [CrossRef]
- Silverstein, B.D.; White, O.J.; Brower, J.E.; Bernholc, N.M. Biological Effects Summary Report di(2-ethylhexyl) Sebacate; Brookhaven National Laboratory: Upton, NY, USA, 1983.
- Chodak, I. Polyhydroxyalkanoates: Origin, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 451–477. [Google Scholar] [CrossRef]
- Adorna, J.A.; Aleman, C.K.A.; Gonzaga, I.L.E.; Pangasinan, J.N.; Sisican, K.M.D.; Dang, V.D.; Doong, R.A.; Ventura, R.L.G.; Ventura, J.R.S. Effect of Lauric Acid on the Thermal and Mechanical Properties of Polyhydroxybutyrate (PHB)/Starch Composite Biofilms. Int. J. Polym. Sci. 2020, 2020, 7947019. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Holzer, A.; Stańczyk, D.; Sabura, E. Selected Fatty Acids Esters as Potential PHB-V Bioplasticizers: Effect on Mechanical Properties of the Polymer. J. Polym. Environ. 2020, 29, 38–53. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the most important methods of improving the processing properties of starch toward non-food applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- Chaos, A.; Sangroniz, A.; Gonzalez, A.; Iriarte, M.; Sarasua, J.R.; del Río, J.; Etxeberria, A. Tributyl citrate as an effective plasticizer for biodegradable polymers: Effect of plasticizer on free volume and transport and mechanical properties. Polym. Int. 2019, 68, 125–133. [Google Scholar] [CrossRef]
- Râpă, M.; Darie-Nită, R.N.; Grosu, E.; Tănase, E.E.; Trifoi, A.R.; Pap, T.; Vasile, C. Effect of plasticizers on melt processability and properties of PHB. J. Optoelectron. Adv. Mater. 2015, 17, 1778–1784. [Google Scholar]
- Mangeon, C.; Michely, L.; Rios De Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Natural Terpenes Used as Plasticizers for Poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar] [CrossRef]
- Immergut, E.H.; Mark, H.F. Principles of Plasticization. Adv. Chem. 1965, 48, 1–26. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 403–433. [Google Scholar] [CrossRef]
- Chiulan, I.; Mihaela Panaitescu, D.; Nicoleta Frone, A.; Teodorescu, M.; Andi Nicolae, C.; Căşărică, A.; Tofan, V.; Sălăgeanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, and in vitro evaluation. J. Biomed. Mater. Res. Part A 2016, 104, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, J.; Ren, Z.; Li, H.; Sun, X.; Yan, S. A grazing incident XRD study on the structure of poly(3-hydroxybutyrate) ultrathin films sandwiched between Si wafers and amorphous polymers. Polym. Chem. 2016, 7, 3705–3713. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]

| Plasticizer | Molecular structure | Molecular formula | Molecular Weight (g/mol) | References |
|---|---|---|---|---|
| Bis(2-ethylhexyl) adipate (DEHA) | 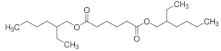 | C22H42O4 | 370.57 | [36,37] |
| Bis(2-ethylhexyl) sebacate (DEHS) |  | C26H50O4 | 426.67 | [38,39] |
| Lauric acid (LA) | 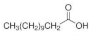 | C12H24O2 | 200.32 | [40,41] |
| Triacetin (TA) |  | C9H14O6 | 218.20 | [42,43] |
| Tributyl citrate (TBC) |  | C18H32O7 | 360.44 | [44,45] |
| Tributyl 2-acetylcitrate (TAC) | 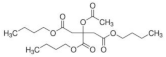 | C20H34O8 | 402.48 | [27,45] |
| L-Linalool (L) |  | C10H18O | 154.25 | [46] |
| Geraniol (G) | 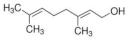 | C10H18O | 154.25 | [46] |
| Plasticizer | Concentration (%) | Tg (°C) | Tm (°C) | Tc (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| Control | - | 51.1 | 166.7 | 112.8 | 63.0 | 43.1 |
| Bis(2-ethylhexyl) adipate (DEHA) | 10 | 48.2 | 167.0 | 112.2 | 64.9 | 49.4 |
| 20 | 46.8 | 163.0 | 110.5 | 56.1 | 48.0 | |
| Bis(2-ethylhexyl) sebacate (DEHS) | 10 | 47.7 | 167.5 | 112.6 | 62.2 | 47.3 |
| 20 | 48.5 | 166.2 | 112.1 | 50.3 | 43.1 | |
| Lauric acid (LA) | 10 | 40.6 | 166.3 | 109.6 | 70.9 | 53.9 |
| 20 | 40.2 | 160.5 | 104.0 | 61.3 | 52.5 | |
| Triacetin (TA) | 10 | 44.0 | 165.0 | 110.9 | 67.7 | 51.5 |
| 20 | 42.0 | 159.1 | 107.8 | 62.4 | 53.4 | |
| Tributyl citrate (TBC) | 10 | 46.4 | 166.8 | 111.2 | 66.9 | 50.9 |
| 20 | 42.9 | 164.0 | 108.5 | 51.8 | 44.3 | |
| Tributyl 2-acetylcitrate (TAC) | 10 | 49.1 | 168.6 | 112.0 | 68.5 | 52.1 |
| 20 | 45.1 | 161.0 | 105.9 | 76.0 | 65.0 | |
| L-Linalool (L) | 10 | 49.7 | 169.0 | 113.5 | 50.5 | 38.4 |
| 20 | 52.6 | 171.4 | 116.3 | 55.9 | 47.8 | |
| Geraniol (G) | 10 | 44.3 | 166.2 | 112.1 | 61.3 | 46.7 |
| 20 | 40.7 | 155.1 | 104.5 | 60.4 | 51.7 |
| Day | TBC Concentration (%) | Mn × 105 | PDI |
|---|---|---|---|
| 0 | 0 | 5.33 | 1.46 |
| 10 | 5.39 | 1.44 | |
| 20 | 5.33 | 1.44 | |
| 2 | 0 | 0.29 | 1.56 |
| 10 | 0.27 | 1.74 | |
| 20 | 0.26 | 1.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.Y.; Kim, S.H.; Jung, H.J.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Ahn, J.; Jeon, J.-M.; Yoon, J.-J.; Lee, J.; et al. Finding a Benign Plasticizer to Enhance the Microbial Degradation of Polyhydroxybutyrate (PHB) Evaluated by PHB Degrader Microbulbifer sp. SOL66. Polymers 2022, 14, 3625. https://doi.org/10.3390/polym14173625
Cho JY, Kim SH, Jung HJ, Cho DH, Kim BC, Bhatia SK, Ahn J, Jeon J-M, Yoon J-J, Lee J, et al. Finding a Benign Plasticizer to Enhance the Microbial Degradation of Polyhydroxybutyrate (PHB) Evaluated by PHB Degrader Microbulbifer sp. SOL66. Polymers. 2022; 14(17):3625. https://doi.org/10.3390/polym14173625
Chicago/Turabian StyleCho, Jang Yeon, Su Hyun Kim, Hee Ju Jung, Do Hyun Cho, Byung Chan Kim, Shashi Kant Bhatia, Jungoh Ahn, Jong-Min Jeon, Jeong-Jun Yoon, Jongbok Lee, and et al. 2022. "Finding a Benign Plasticizer to Enhance the Microbial Degradation of Polyhydroxybutyrate (PHB) Evaluated by PHB Degrader Microbulbifer sp. SOL66" Polymers 14, no. 17: 3625. https://doi.org/10.3390/polym14173625
APA StyleCho, J. Y., Kim, S. H., Jung, H. J., Cho, D. H., Kim, B. C., Bhatia, S. K., Ahn, J., Jeon, J.-M., Yoon, J.-J., Lee, J., & Yang, Y.-H. (2022). Finding a Benign Plasticizer to Enhance the Microbial Degradation of Polyhydroxybutyrate (PHB) Evaluated by PHB Degrader Microbulbifer sp. SOL66. Polymers, 14(17), 3625. https://doi.org/10.3390/polym14173625