Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Solution Preparation
2.3. Qualitative Determination of Hydrolysis
2.4. Arrhenius Analysis
2.5. Principle of Method for Determination of Hydrolysis Rate
2.6. Research Methods of Hydrolysis Mechanism
3. Results and Discussion
3.1. Plot of Standard Curve for Determination of Hydrolysis Rate
3.2. Study on Hydrolysis Properties of PMAPTAC
3.2.1. Effects of Temperature and pH on Hydrolysis Performance of PMAPTAC
3.2.2. Effect of Molecular Weight of PMAPTAC on Its Hydrolysis Properties
3.3. Comparison with Hydrolysis Performance of PDMC
3.4. Hydrolysis Mechanism of PMAPTAC
3.4.1. FTIR Spectra
3.4.2. Structural Characterization of Small Molecules Released by PMAPTAC Hydrolysis
3.4.3. Hydrolysis Mechanism Description of PMAPTAC
3.5. Hydrolysis Mechanism of PDMC
3.5.1. Structural Characterization of PDMC Polymers
3.5.2. Structural Characterization of Small Molecules Released from PDMC Hydrolysis
4. Conclusions
- (1)
- Qualitative analysis showed that the apparent viscosity of PMAPTAC solution decreased with hydrolysis time at different temperatures and pH. The lower the pH and the higher the temperature, the greater the apparent viscosity loss of the PMAPTAC solution. Quantitative analysis showed that the hydrolysis rate of PMAPTAC sample solution increased with the increase in temperature and pH. For example, the hydrolysis rates over 96 d at 30℃, 55 ℃ and 80 ℃ at pH 11 were 5.88%, 8.32% and 22.07%, respectively. In addition, the hydrolysis rates of PMAPTAC samples with different [η] were not much different, indicating that [η] had little effect on the hydrolytic stability of PMAPTAC.
- (2)
- By analyzing the viscosity curves at different pH and temperatures by Arrhenius analysis, the Arrhenius equations for pH 3, pH 7 and pH 11 were 1/τ = 200.34e^((−25.04)/RT), 1/τ = 9127.07e^((−38.90)/RT) and 1/τ = 4683.03e^((−39.89)/RT), respectively. PMAPTAC solutions were projected to maintain at least half their original viscosity for over 11 days at pH 3, 21 days at pH 7, and 56 days at pH 11, which were predicted according to the formula.
- (3)
- The apparent viscosity of the PDMC polymer decreased with aging time. The viscosity retention rates were 50.89%, 60.17% and 44.40%, and the hydrolysis rates of PDMC solution were 17.97%, 7.81% and 67.03% in pH 3, pH 7 and pH 11 solutions after 96 d at 55 ℃, respectively, indicating that the hydrolysis rate of PDMC was the fastest under alkaline conditions. In contrast, PMAPTAC had significantly superior hydrolytic stability under the same conditions
- (4)
- The mechanism of the hydrolyzed polymer was studied by FTIR and 13 CNMR, which showed that the carbonyl group of PMAPTAC in solution was hydrolyzed into a carboxyl group, and the small molecule (3-aminopropyl) trimethylammonium chloride was generated, while the ester group of PDMC was hydrolyzed into the carboxyl group, and choline chloride was released. The above results can provide a theoretical basis for the application of PMAPTAC in some high-temperature and acid–base environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moradi-Araghi, A.; Doe, P.H. Hydrolysis and precipitation of polyacrylamides in hard brines at elevated temperatures. SPE Reserv. Eng. 1987, 2, 189–198. [Google Scholar] [CrossRef]
- Dexter, R.W.; Ryles, R.G. Effect of anionic comonomers on the hydrolytic stability of polyacrylamides at high temperatures in alkaline solution. ACS Symp. Ser. 1989, 396, 102–110. [Google Scholar]
- Levitt, D.B.; Pope, G.A.; Jouenne, S. Chemical degradation of polyacrylamide polymers under alkaline conditions. SPE Reservoir Eval. Eng. 2011, 14, 281–286. [Google Scholar] [CrossRef]
- Pei, Y.; Zhao, L.; Du, G.; Li, N.; Xu, K.; Yang, H. Investigation of the degradation and stability of acrylamide-based polymers in acid solution: Functional monomer modified polyacrylamide. Pet. Sci. Technol. 2016, 2, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Muller, G.; Fenyo, J.C.; Selegny, E. High molecular weight hydrolyzed polyacrylamides. III. Effect of temperature on chemical stability. J. Appl. Polym. Sci. 1980, 25, 627–633. [Google Scholar] [CrossRef]
- Kheradmand, H.; Francois, J.; Plazanet, V. Hydrolysis of polyacrylamide and acrylic acid-acrylamide copolymers at neutral pH and high temperature. Polymer 1988, 29, 860–870. [Google Scholar] [CrossRef]
- Moradi-Araghi, A.; Hsieh, E.T.; Westerman, I.J. Role of imidization in thermal hydrolysis of polyacrylamides. In Water-Soluble Polymers for Petroleum Recovery; Springer: Boston, MA, USA, 1988; pp. 271–278. [Google Scholar]
- Sawant, S.; Morawetz, H. Reaction of polyacrylamide provides evidence for some head-to-head addition of monomer residues. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 385–388. [Google Scholar] [CrossRef]
- Lafuma, F.; Durand, G. Carbon-13 NMR spectroscopy of cationic copolymers of acrylamide. Polym. Bull. (Berlin) 1989, 21, 315–318. [Google Scholar] [CrossRef]
- Aksberg, R.; Waagberg, L. Hydrolysis of cationic polyacrylamides. J. Appl. Polym. Sci. 1989, 38, 297–304. [Google Scholar] [CrossRef]
- Fernandez, I.J. Evaluation of cationic water-soluble polymers with improved thermal stability. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Wu, J.Y.; Huang, C.W.; Tsai, P.S. Preparation of poly [3-(methacryloylamino) propyl] trimethylammonium chloride coated mesh for oil-water separation. Desalin. Water Treat. 2019, 158, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y. Preparation Method of Poly(Methacrylamidopropyltrimethylammonium) Chloride with Serialized Characteristic Viscosity. Chinese Patent CN109912739B, 4 May 2021. [Google Scholar]
- de Morais, S.C.; Cardoso, O.R.; de Carvalho Balaban, R. Thermal stability of water-soluble polymers in solution. J. Mol. Liq. 2018, 265, 818–823. [Google Scholar] [CrossRef]
- Seright, R.S.; Campbell, A.R.; Mozley, P.S.; Han, P. Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE J. 2010, 15, 341–348. [Google Scholar] [CrossRef]
- Meloche, I.; Laidler, K.J. Substituent effects in the acid and base hydrolyses of aromatic amides. J. Am. Chem. Soc. 1951, 73, 1712–1714. [Google Scholar] [CrossRef]
- Cason, J.; Gastaldo, C.; Glusker, D.L.; Allinger, J.; Ash, L.B. Branched-chain fatty acids. XXVII. Further study of the dependence of rate of amide hydrolysis on substitution near the amide group. Relative rates of hydrolysis of nitrile to amide and amide to acid. J. Org. Chem. 1953, 18, 1129–1136. [Google Scholar] [CrossRef]
- Bender, M.L.; Ginger, R.D. Intermediates in the reactions of carboxylic acid derivatives. IV. The hydrolysis of benzamide. J. Am. Chem. Soc. 1955, 77, 348–351. [Google Scholar] [CrossRef]
- Lin, S.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5th Edition. Molecules 2001, 6, 1064–1065. [Google Scholar] [CrossRef] [Green Version]
- Parker, W., Jr.; Lezzi, A. Hydrolysis of sodium-2-acrylamido-2-methylpropanesulfonate copolymers at elevated temperature in aqueous solution via 13C nmr spectroscopy. Polymer 1993, 34, 4913–4918. [Google Scholar] [CrossRef]
- Lesutis, H.P.; Gläser, R.; Liotta, C.L.; Eckert, C.A. Acid/base-catalyzed ester hydrolysis in near-critical water. Chem. Commun. 1999, 2063–2064. [Google Scholar] [CrossRef]
- Stefanidis, D.; Jencks, W.P. General base catalysis of ester hydrolysis. J. Am. Chem. Soc. 1993, 115, 6045–6050. [Google Scholar] [CrossRef]
- Xiao, Y.D. Alkaline potassium persulfate digestion UV spectrophotometric method for determination of total nitrogen in water by the method. Guangdong Chem. Ind. 2012, 39, 165–166. [Google Scholar]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci.Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, Y.; Zhang, H.; Wang, C. New approximate formula for Arrhenius temperature integral. Thermochim. Acta 2003, 408, 39–43. [Google Scholar] [CrossRef]
- Brown, R.S.; Bennet, A.J.; Slebocka-Tilk, H. Recent perspectives concerning the mechanism of H3O+- and hydroxide-promoted amide hydrolysis. Acc. Chem. Res. 1992, 25, 481–488. [Google Scholar] [CrossRef]
- Zahn, D. Theoretical study of the mechanisms of acid-catalyzed amide hydrolysis in aqueous solution. J. Phys. Chem. B 2003, 107, 12303–12306. [Google Scholar] [CrossRef]
- Marx, D.; Tuckerman, M.E.; Hutter, J.; Parrinello, M. The nature of the hydrated excess proton in water. Nature 1999, 397, 601–604. [Google Scholar] [CrossRef]
- Men, J.; Wang, R.; Li, H.; Li, X.; Yang, S.; Liu, H.; Gao, B. Preparation of crosslinked poly (acryloyloxyethyltrimethyl ammonium chloride) microsphere and its adsorption and mechanism towards shikimic acid. Mater. Sci. Eng. C 2017, 71, 167–175. [Google Scholar] [CrossRef]
- Saeidi, A.; Katbab, A.A.; Vasheghani-Farahani, E.; Afshar, F. Formulation design, optimization, characterization and swelling behaviour of a cationic superabsorbent based on a copolymer of [3-(methacryloylamino)propyl]trimethylammonium chloride and acrylamide. Polym. Int. 2004, 53, 92–100. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Sahiner, M.; Yilmaz, S.; Al-Lohedan, H. The use of superporous p(3-acrylamidopropyl)trimethyl ammonium chloride cryogels for removal of toxic arsenate anions. J. Environ. Manag. 2015, 152, 66–74. [Google Scholar] [CrossRef]
- Rehman, S.U.; Siddiq, M.; Al-Lohedan, H.; Aktas, N.; Sahiner, M.; Demirci, S.; Sahiner, N. Fast removal of high quantities of toxic arsenate via cationic p(APTMACl) microgels. J. Environ. Manag. 2016, 166, 217–226. [Google Scholar] [CrossRef]
- Li, Q.; Yi, Y.; Wang, Y.-n.; Li, J.; Shi, B. Effect of cationic monomer structure on the aggregation behavior of amphoteric acrylic polymer around isoelectric point. J. Leather Sci.Eng. 2022, 4, 1–9. [Google Scholar] [CrossRef]
- O’Connor, C. Acidic and basic amide hydrolysis. Quart. Rev. Chem. Soc. 1970, 24, 553–564. [Google Scholar] [CrossRef]
- Smith, C.R.; Yates, K. Kinetic evidence for predominant oxygen protonation of amides. Can. J. Chem. 1972, 50, 771–773. [Google Scholar] [CrossRef]
- Zahn, D. On the role of water in amide hydrolysis. Eur. J. Org. Chem. 2004, 4020–4023. [Google Scholar] [CrossRef]
- Lobaugh, J.; Voth, G.A. The quantum dynamics of an excess proton in water. J. Chem. Phys. 1996, 104, 2056–2069. [Google Scholar] [CrossRef] [Green Version]
- Vuilleumier, R.; Borgis, D. Quantum dynamics of an excess proton in water using an extended empirical valence-bond Hamiltonian. J. Phys. Chem. B 1998, 102, 4261–4264. [Google Scholar] [CrossRef]
- Agmon, N. Proton solvation and proton mobility. Isr. J. Chem. 1999, 39, 493–502. [Google Scholar] [CrossRef]
- Zahn, D. Car–Parrinello molecular dynamics simulation of base-catalyzed amide hydrolysis in aqueous solution. Chem. Phys. Lett. 2004, 383, 134–137. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, D.; Xu, L.; Kong, F.; Wang, S.; Yang, G. Preparation and characterization of softwood kraft lignin copolymers as a paper strength additive. Polymers 2018, 10, 743. [Google Scholar] [CrossRef] [Green Version]
- Geyik, G.; Işıklan, N. Synthesis, characterization and swelling performance of a temperature/pH-sensitive κ-carrageenan graft copolymer. Int. J. Biol. Macromol. 2020, 152, 359–370. [Google Scholar] [CrossRef]














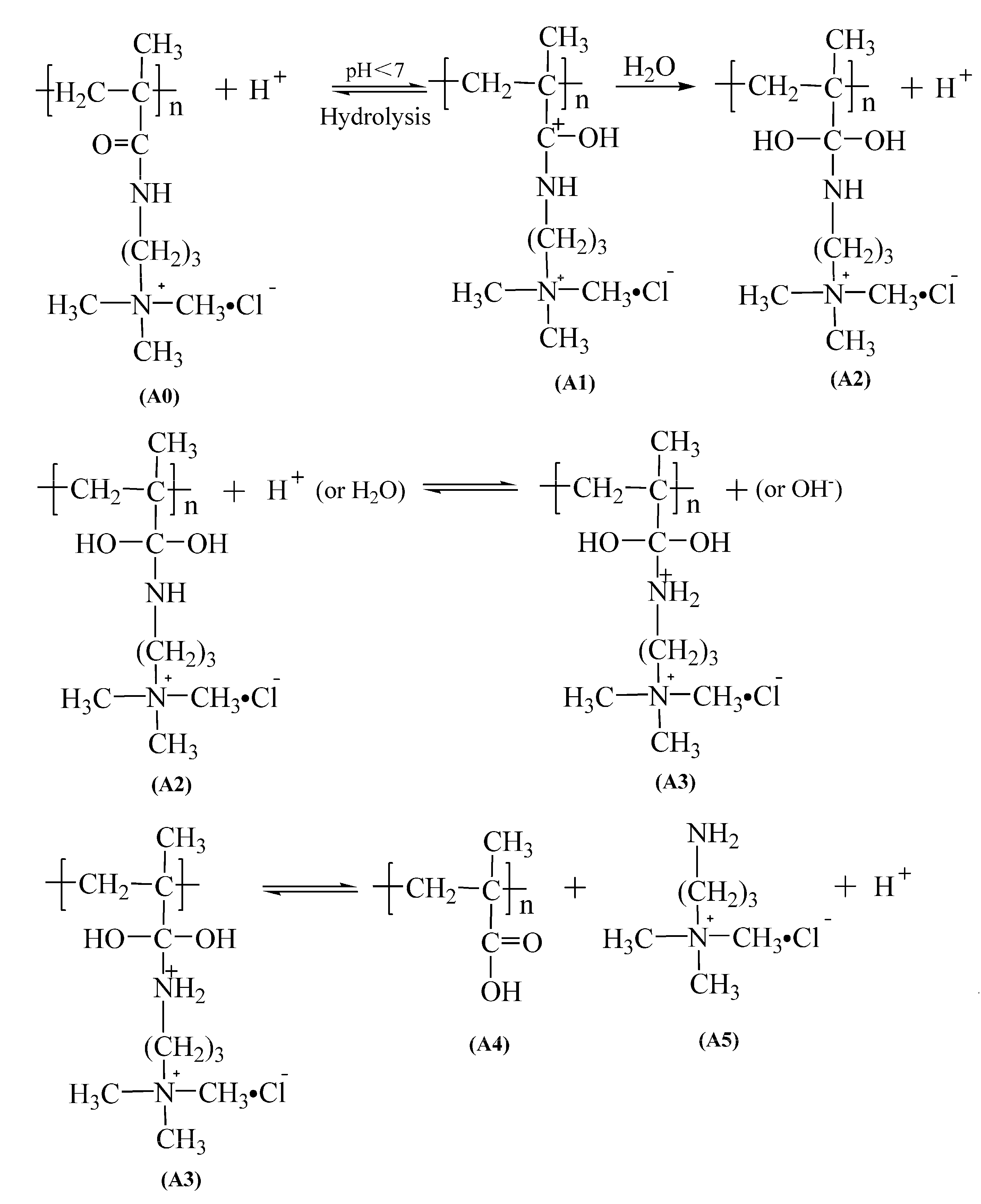




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jia, X.; Zhang, Y. Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution. Polymers 2022, 14, 2811. https://doi.org/10.3390/polym14142811
Wang Y, Jia X, Zhang Y. Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution. Polymers. 2022; 14(14):2811. https://doi.org/10.3390/polym14142811
Chicago/Turabian StyleWang, Yongji, Xu Jia, and Yuejun Zhang. 2022. "Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution" Polymers 14, no. 14: 2811. https://doi.org/10.3390/polym14142811
APA StyleWang, Y., Jia, X., & Zhang, Y. (2022). Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution. Polymers, 14(14), 2811. https://doi.org/10.3390/polym14142811





