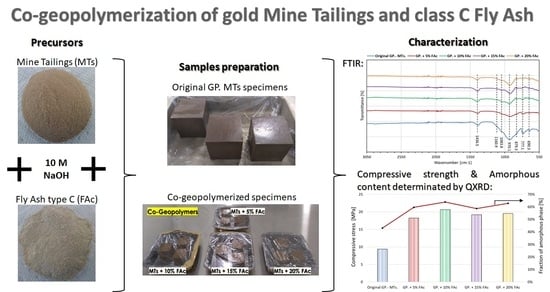
Effect of the Class C Fly Ash on Low-Reactive Gold Mine Tailing Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
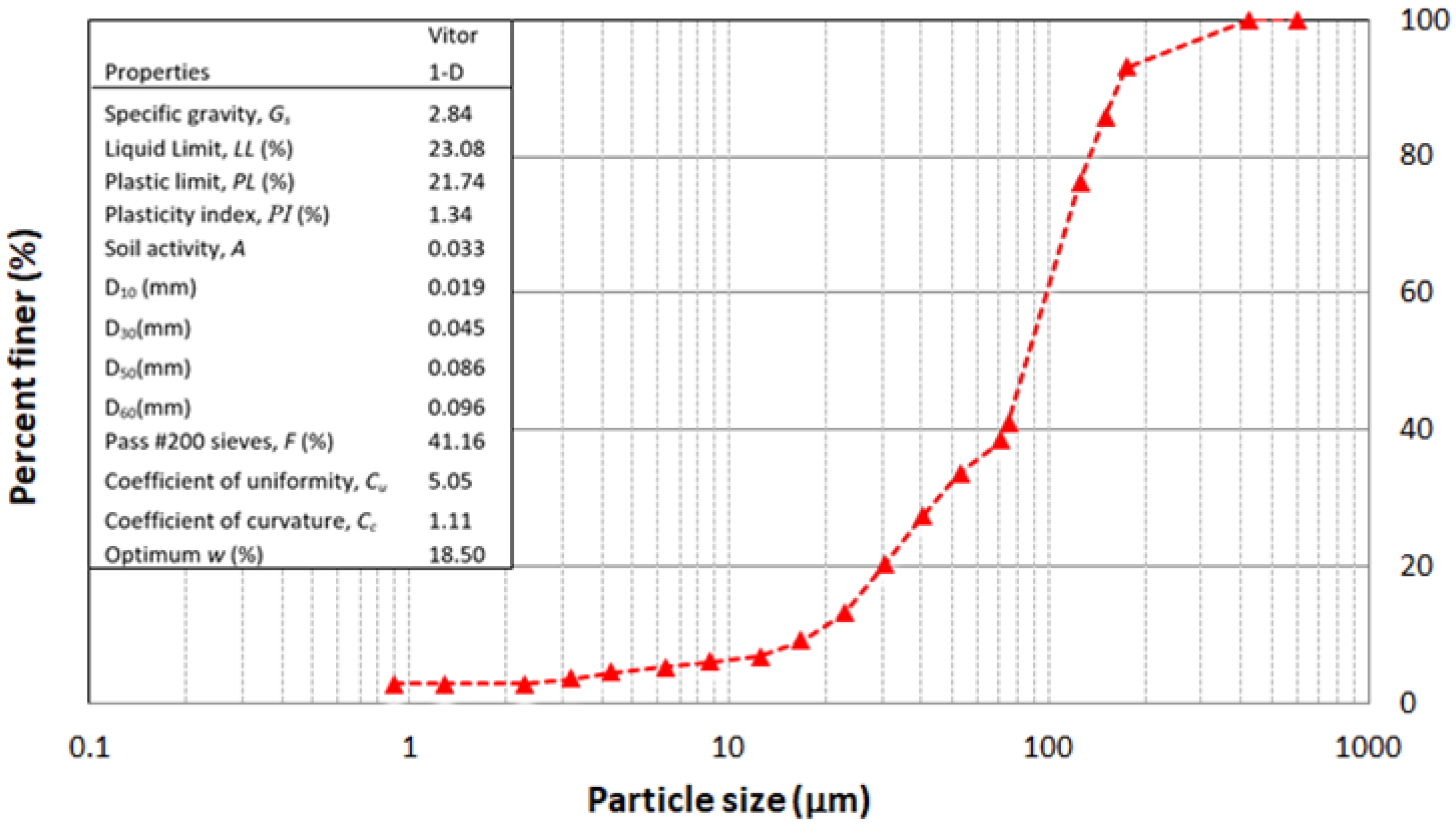
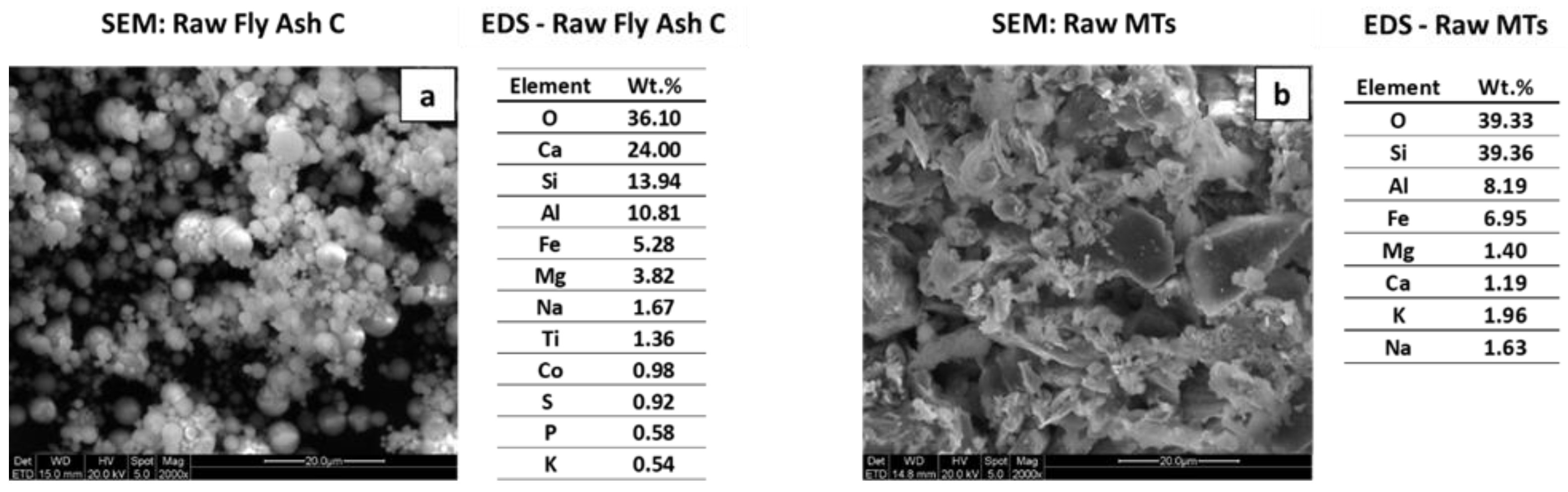
2.1. Raw Materials Characterization
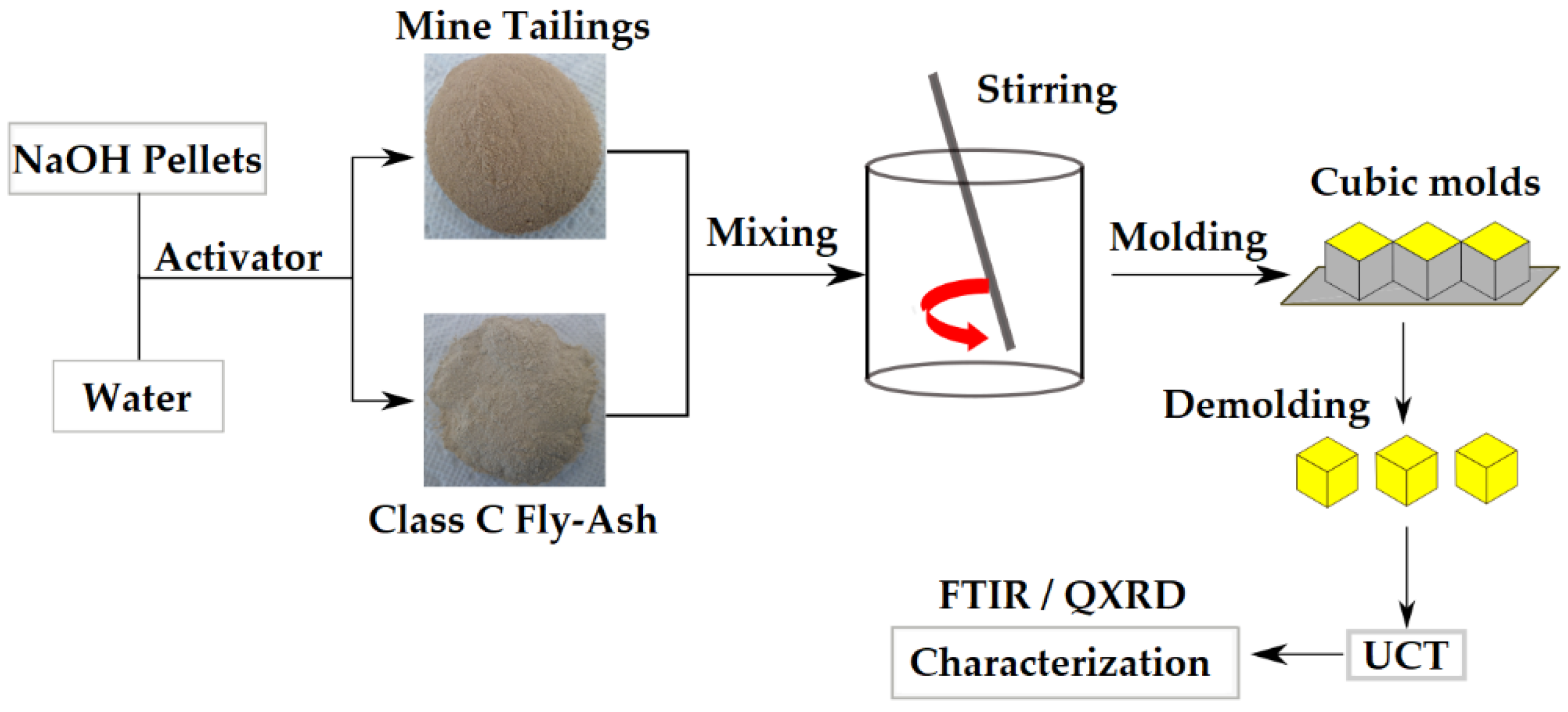
2.2. Production of Geopolymer Specimens
2.3. Characterization Techniques
2.3.1. Quantitative X-ray Diffraction (QXRD)
2.3.2. Fourier Transform Infrared (FTIR)
2.3.3. Uniaxial Compressive Tests
2.3.4. Scanning Electron Microscopy/Energy Dispersive Spectroscopy (SEM/EDS)
3. Results and Discussion
4. Conclusions
- (1)
- Based on the FTIR and QXRD results, it was found that both raw materials (raw MTs and raw FAc) could contribute to the additional amorphous phases with the presence of low quantity formed C-A-S-H crystalline phase in some of the generated specimens through the dissolution of their original aluminosilicate phases during alkali activation.
- (2)
- The FTIR and QXRD results also indicated an increase in the dissolution of the gold MTs (low calcium content) by the addition of the FAc (rich in calcium) into the blended systems with a maximum value of formed amorphous content reported for the system with 10 wt.% of FAc. This suggests that the dissolution of FAc contributes to a major quantity of calcium species being able to react during the co-geopolymerization step and dissolving to a major degree the MTs’ aluminosilicates phases, such as the muscovite, albite, and even quartz.
- (3)
- The final compressive strength of all co-geopolymerized systems based on gold MTs, and mixed with FAc at different concentrations, increased by 95–120% in comparison to the original MTs geopolymer system. The higher strength value was obtained by the blended system with 10 wt.%. FAc, which also showed the highest production of amorphous content after the co-geopolymerization step. This suggests that the improvement of the specimens’ strength is directly correlated to the increase in the amorphous phase production during the co-geopolymerization of these raw materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baziz, A.; Bouzidi, N.; Eliche-Quesada, D. Recycling of gold mining reject from Amesmessa mine as ceramic raw material: Microstructure and mechanical properties. Environ. Sci. Pollut. Res. Int. 2021, 28, 46738–46747. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, Q.; Liu, Y.; Xue, T.; Yu, Q.; Chen, S. Influence of new organic alkali activators on microstructure and strength of fly ash geopolymer. Ceram. Int. 2022, 48, 12442–12449. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.A.; Liew, M.S.; Haruna, A. Mechanical and microstructural properties of high calcium fly ash one-part geopolymer cement made with granular activator. Heliyon 2019, 5, e02255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Bernal, R.P.H.; Tupa, N.; Morales, I.Y.; Loza, R.S.C. On the incorporation of class F fly-ash to enhance the geopolymerization effects and splitting tensile strength of the gold mine tailings-based geopolymer. Constr. Build. Mater. 2021, 308, 125112. [Google Scholar] [CrossRef]
- Kiventerä, J.; Sreenivasan, H.; Cheeseman, C.; Kinnunen, P.; Illikainen, M. Immobilization of sulfates and heavy metals in gold mine tailings by sodium silicate and hydrated lime. J. Environ. Chem. Eng. 2018, 6, 6530–6536. [Google Scholar] [CrossRef]
- Perumal, P.; Kiventerä, J.; Illikainen, M. Influence of alkali source on properties of alkali activated silicate tailings. Mater. Chem. Phys. 2021, 271, 124932. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.; Tang, Q.; Nzeukou, A.N.; Billong, N.; Melo, U.C.; Cui, X.-M. Review on the use of volcanic ashes for engineering applications. Resour. Conserv. Recycl. 2018, 137, 177–190. [Google Scholar] [CrossRef]
- Yun-Ming, L.; Cheng-Yong, H.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar]
- Jeon, D.; Jun, Y.; Jeong, Y.; Oh, J.E. Microstructural and strength improvements through the use of Na2CO3 in a cement less Ca(OH)2-Activated Class F fly ash system. Cem. Concr. Res. 2015, 67, 215–225. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction-Recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Kamath, M.; Prashant, S.; Kumar, M. Micro-characterization of alkali activated paste with fly ash-GGBS-metakaolin binder system with ambient setting characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2021, 1, 100014. [Google Scholar]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Tupa, N.; Morales, I.Y.; Loza, R.S.C. Crack evolution in the Brazilian disks of the mine tailings-based geopolymers measured from digital image correlations: An experimental investigation considering the effects of class F fly ash additions. Ceram. Int. 2021, 47, 32382–32396. [Google Scholar] [CrossRef]
- ASTM D6913; Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D7928-17; Standard Test Method for Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedimentation (Hydrometer) Analysis. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D4318-17e1; Standard Test Methods for Liquid Limit, Plastic Limit, And Plasticity Index of Soils. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019. Available online: www.astm.org. (accessed on 5 October 2021).
- Yong-Sing, N.; Yun-Ming, L.; Cheng-Yong, H.; Al-Bakri-Abdullah, M.M.; Pakawanit, P.; Ling-Chan, L.W.; Hui-Teng, N.; Shee-Ween, O.; Wan-En, O.; Yong-Jie, H. Thin fly ash/ladle furnace slag geopolymer: Effect of elevated temperature exposure on flexural properties and morphological characteristics. Ceram. Int. 2022, 48, 16562–16575. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Cárdenas, J.J.G.; Álvarez, G.E.S.; Rivera, V.B.A. Damage evaluation and deformation behavior of mine tailing-based Geopolymer under uniaxial cyclic compression. Ceram. Int. 2021, 47, 10773–10785. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Cárdenas, J.J.G.; Álvarez, G.E.S.; Rivera, V.A.; González, J. Fracture and failure processes of geopolymerized mine tailings under uniaxial compression. In Proceedings of the 54th US Rock Mechanics/Geomechanics Symposium, Golden, CO, USA, 28 June–1 July 2020; Volume 20, pp. 1–9. [Google Scholar]
- Williams, R.P.; Riessen, A.V. Determination of the reactive component of fly ashes for geopolymer production using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Kato, H. Selective growth of silver particles on the facets of synthetic diamond. Cryst. Eng. Comm. 2016, 18, 7430–7434. [Google Scholar] [CrossRef]
- Snellings, R.; Salze, A.; Scrivener, K.L. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89–98. [Google Scholar] [CrossRef]
- Haha, M.B.; De Weerdt, K.; Lothenbach, B. Quantification of the degree of reaction of fly ash. Cem. Concr. Res. 2010, 40, 1620–1629. [Google Scholar] [CrossRef]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Constr. Build. Mater. 2022, 314, 125574. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Cárdenas, J.J.G.; Álvarez, G.E.S.; Rivera, V.B.A. Specimen size effects on the mechanical behaviors and failure patterns of the mine tailings-based geopolymer under uniaxial compression. Constr. Build. Mater. 2021, 281, 122525. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef] [PubMed]
- Silva de Vargas, A.; Dal Molin, D.C.C.; Masuero, Â.B.; Vilela, A.C.F.; Castro-Gomes, J.; De Gutierrez, R.M. Strength development of alkali-activated fly ash produced with combined NaOH and Ca(OH)2 activators. Cem. Concr. Compos. 2014, 53, 341–349. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Tchakouté, H.K.; Ranjbar, N.; Elimbi, A.; Tchadjie, L.N.; Njopwouo, D. Gel composition and strength properties of alkali-activated oyster shell-volcanic ash: Effect of synthesis conditions. J. Am. Ceram. Soc. 2016, 99, 3159–3166. [Google Scholar] [CrossRef]
- Perera-Mercado, Y.A.; Betancourt-Galindo, R.; Saucedo-Salazar, E.M.; Puente-Urbina, B.A.; Medellín-Banda, D.I.; Neira-Velázquez, M.G.; Gutierrez-Villarreal, M.H.; García-Rodríguez, S.P. Production of micrometer-sized composite polymer-magnetic spheres using as precursor metallurgical wastes. Polym. Polym. Compos. 2014, 22, 387–392. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Nguyen, T.A.H.; Chan, T.-S.; Lu, Y.-R.; Huang, L. Biochar mediated uranium immobilization in magnetite rich Cu tailings subject to organic matter amendment and native plant colonization. J. Hazard. Mater 2022, 427, 127860. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Z.; Yao, Z.; Shuai, Q.; Jiang, Z.; Peng, X.; Li, Y.; An, R.; Jiang, X.; Li, H. Preparation of non-sintered lightweight aggregates through co-mechanochemical treatment of oil-contaminated drill cutting, circulation fluidized bed combustion fly ash, and quicklime. Environ. Sci. Pollut. Res. Int. 2020, 27, 20904–20911. [Google Scholar] [CrossRef]
- Demir, F.; Derun, E.M. Modelling and optimization of gold mine tailings based geopolymer by using response surface method and its application in Pb2+ removal. J. Clean. Prod. 2019, 237, 117766. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Rózycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR, and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Wei-Ken, P.; Ramli, M.; Chee-Ban, C. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar]
- Kunecki, P.; Panek, R.; Wdowin, M.; Franus, W. Synthesis of faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash. Int. J. Miner. Process 2017, 166, 69–78. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites? A re-examination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Li, H.; Wu, Q.; Zhang, C.; Yin, Z.; Hua, S. Insights to the sulfate resistance and microstructures of alkali-activated metakaolin/slag pastes. Appl. Clay Sci. 2021, 202, 105968. [Google Scholar] [CrossRef]
- Canfield, G.M.; Eichler, J.; Griffith, K.; Hearn, J.D. The role of calcium in blended fly ash geopolymers. J. Mater Sci. 2014, 49, 5922–5933. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F.P. Alkali binding in cement pastes: Part I. The C-S-H phase. Cem. Concr. Res. 1999, 29, 1893–1903. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F.P. Alkali sorption by C-S-H and C-A-S-H gels: Part II. Role of alumina. Cem. Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Huang, R.; Lin, W.-T.; Wang, H.-N. Mechanical and cementitious characteristics of ground granulated blast furnace slag and basic oxygen furnace slag blended mortar. Mater. Des. 2014, 60, 267–273. [Google Scholar] [CrossRef]

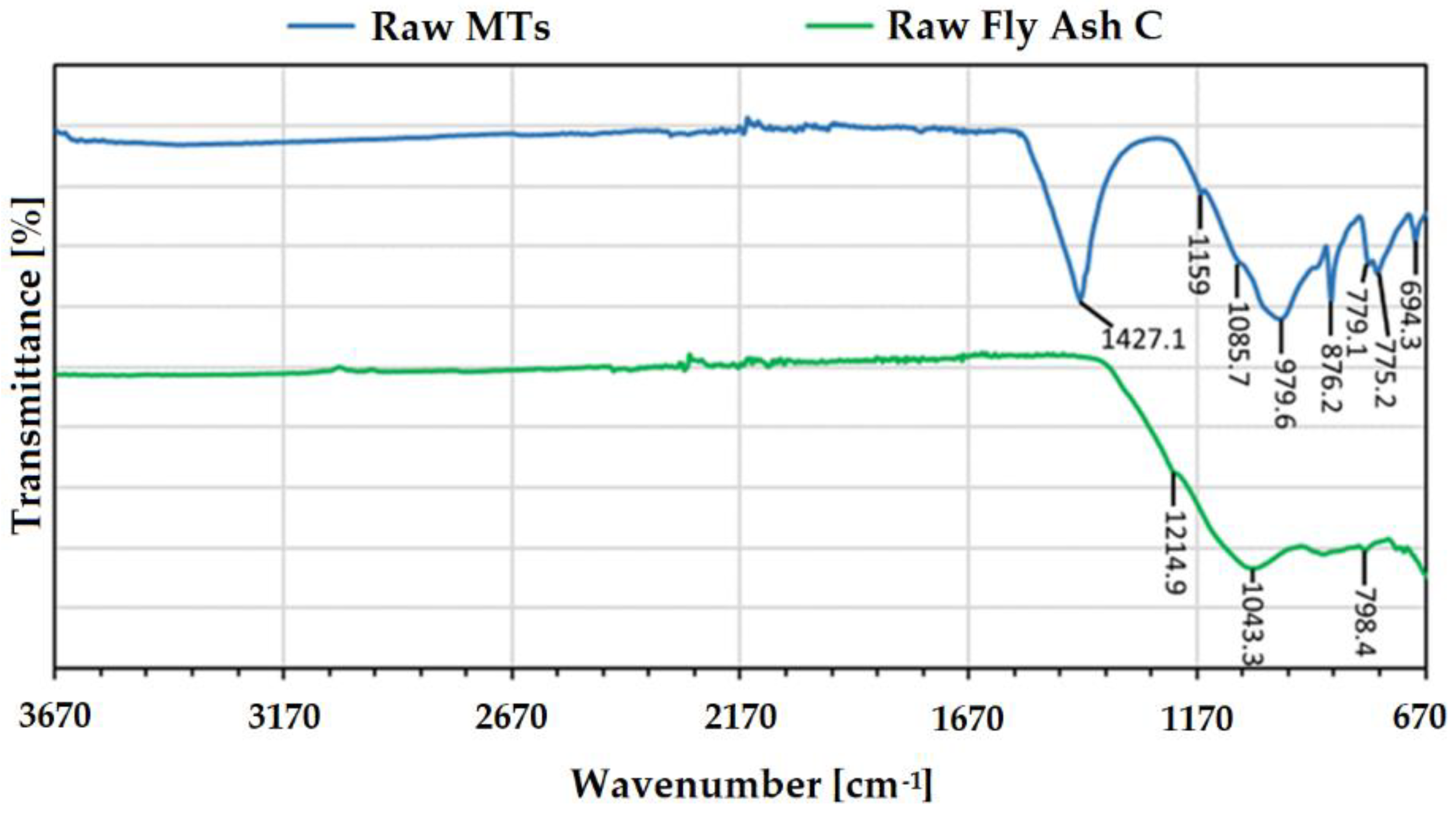
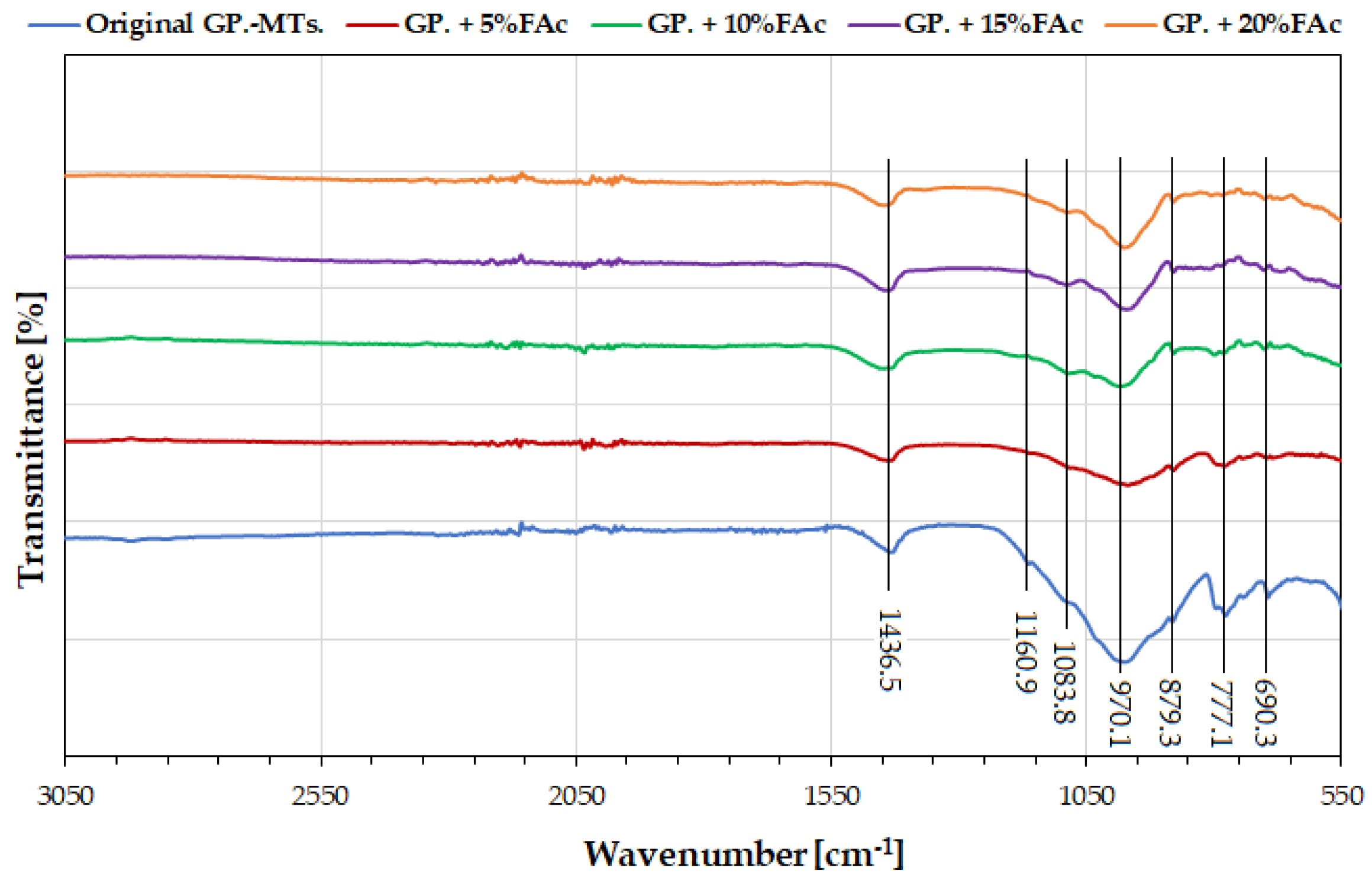


| Phase | Chemical Formula | PDF Code |
|---|---|---|
| Albite | Na(AlSi3O8) | 04-017-1022 |
| Anhydrite | (CaSO4) | 01-074-2421 |
| C-A-S-H | Ca3Al(Al3SiO10)(OH)2 | 00-001-1079 |
| Gehlenite | Ca2Al2SiO7 | 01-089-6887 |
| Grossular | Ca₃Al₂(SiO₄)₃ | 04-013-2106 |
| Magnetite | Fe2O3 | 01-075-0449 |
| Muscovite | KAl2(FOH)2 or (KF)2(Al2O3)3(-SiO2)6 | 00-001-1098 |
| N-A-S-H | Na17.6(Al16Si56O144)(H2O)38.4 | 04-017-1022 |
| Quartz | SiO2 | 01-077-8621 |
| Synthetic Diamond | C | 01-079-6061 |
| Phase | Raw MTs Wt.% | Original GP.-MTs Wt.% | Raw FAc Wt.% | FAc-GP. Wt.% | GP. + 5% FAc Wt.% | GP. + 10% FAc Wt.% | GP. + 15% FAc Wt.% | GP. + 20% FAc Wt.% |
|---|---|---|---|---|---|---|---|---|
| Muscovite | 9.3 | 6.5 | - | - | 4.8 | 2.4 | 6.0 | 5.1 |
| Quartz | 51.1 | 38.9 | 5.4 | 1.6 | 29.3 | 25.4 | 27.6 | 24.6 |
| Gehlenite | - | - | 13.1 | 1.3 | - | - | - | - |
| Albite | 12.5 | 9.2 | - | - | 5.2 | 6.6 | 5.7 | 5.4 |
| Grossular | - | - | 9.6 | 1.7 | - | - | - | - |
| Anhydrite | - | - | 0.8 | - | - | - | - | - |
| Magnetite | 0.7 | - | - | - | - | - | - | - |
| Zeolite | - | 1.2 | - | - | - | 0.7 | - | 0.7 |
| Calcium Aluminum Silicate Hydrate (C-A-S-H) | 0.8 | 0.7 | - | - | - | - | 1 | 0.5 |
| Sodium Aluminum Silicate Hydrate (N-A-S-H) | 1.9 | - | - | - | - | - | - | - |
| % of Amorphous | 23.2 | 43.1 | 70.9 | 95.3 | 59.6 | 63.9 | 58.6 | 62.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera-Mercado, Y.; Hedayat, A.; Tunstall, L.; Clements, C.; Hylton, J.; Figueroa, L.; Zhang, N.; Bolaños Sosa, H.G.; Tupa, N.; Yanqui Morales, I.; et al. Effect of the Class C Fly Ash on Low-Reactive Gold Mine Tailing Geopolymers. Polymers 2022, 14, 2809. https://doi.org/10.3390/polym14142809
Perera-Mercado Y, Hedayat A, Tunstall L, Clements C, Hylton J, Figueroa L, Zhang N, Bolaños Sosa HG, Tupa N, Yanqui Morales I, et al. Effect of the Class C Fly Ash on Low-Reactive Gold Mine Tailing Geopolymers. Polymers. 2022; 14(14):2809. https://doi.org/10.3390/polym14142809
Chicago/Turabian StylePerera-Mercado, Yibran, Ahmadreza Hedayat, Lori Tunstall, Cara Clements, Julia Hylton, Linda Figueroa, Nan Zhang, Héctor Gelber Bolaños Sosa, Néstor Tupa, Isaac Yanqui Morales, and et al. 2022. "Effect of the Class C Fly Ash on Low-Reactive Gold Mine Tailing Geopolymers" Polymers 14, no. 14: 2809. https://doi.org/10.3390/polym14142809
APA StylePerera-Mercado, Y., Hedayat, A., Tunstall, L., Clements, C., Hylton, J., Figueroa, L., Zhang, N., Bolaños Sosa, H. G., Tupa, N., Yanqui Morales, I., & Canahua Loza, R. S. (2022). Effect of the Class C Fly Ash on Low-Reactive Gold Mine Tailing Geopolymers. Polymers, 14(14), 2809. https://doi.org/10.3390/polym14142809