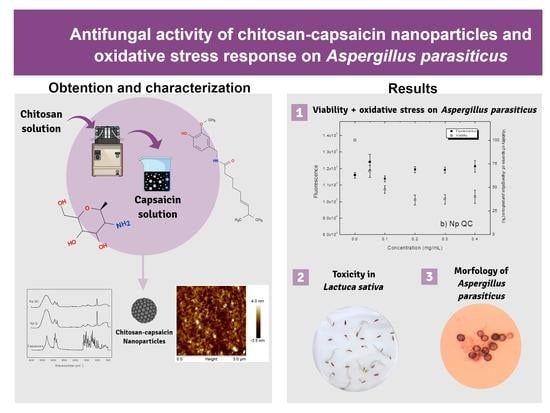
Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture and Propagation of the Fungus
2.3. Preparation of the Nanoparticles
2.4. Preparation of the Capsaicin Solution (Control)
2.5. Physical and Physicochemical Characterization of Nanoparticles
2.5.1. Form, Size, and Superficial Load
2.5.2. FT-IR and Nanoparticle Entrapment Capacity
2.6. Toxicological Analysis of the Nanoparticles
2.6.1. Acute Toxicity in Artemia salina Nauplii
2.6.2. Acute Phytotoxicity in Lettuce Seeds (Lactuca sativa)
2.7. Antifungal Activity of the Nanoparticles of A. parasiticus
2.7.1. Morphometric Analysis
2.7.2. Spore Viability
2.7.3. Quantification of Reactive Oxygen Species (ROS)
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Physical and Physicochemical Characterization of the Nanoparticles
3.1.1. Form, Size, and Zeta Potential
3.1.2. FT-IR and Nanoparticle Entrapment Capacity
3.2. Toxicity of Nanoparticles
3.2.1. Acute Toxicity in Artemia salina Nauplii
3.2.2. Acute Phytotoxicity in Lettuce Seeds (Lactuca sativa)
3.3. Antifungal Activity of the Nanoparticles of Chitosan (Np Q) and Chitosan-Capsaicin (Np QC) Nanoparticles on A. parasiticus
3.3.1. Morphometric Analysis
3.3.2. Spore Viability
3.3.3. Quantification of Reactive Oxygen Species (ROS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, T.-H.; Park, M.K.; Nakamura, H.; Ban, H.S. Capsaicin inhibits HIF-1α accumulation through suppression of mitochondrial respiration in lung cancer cells. Biomed. Pharmacother. 2022, 146, 112500. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, J.M.; Jiménez, M.; de la Fuente, J.C. Extraction kinetics of pre-pelletized Jalapeño peppers with supercritical CO2. J. Supercrit. Fluids 2003, 25, 33–44. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical carbon dioxide extraction of Capsicum peppers: Global yield and capsaicinoid content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Udeanu, D.; Coc, L.; Pop, A.; Cioates Negut, C.; Tanase, C.; Stan, R.; Meghea, A. Improved anti-obesity effect of herbal active and endogenous lipids co-loaded lipid nanocarriers: Preparation, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2019, 99, 12–24. [Google Scholar] [CrossRef]
- Tobolka, A.; Škorpilová, T.; Dvořáková, Z.; Cusimamani, E.F.; Rajchl, A. Determination of capsaicin in hot peppers (Capsicum spp.) by direct analysis in real time (DART) method. J. Food Compos. Anal. 2021, 103, 104074. [Google Scholar] [CrossRef]
- Moreno-Limón, S.; Salcedo-Martínez, S.; Cárdenas-Ávila, M.; Hernández-Piñero, J.; Núñez-González, M. Efecto antifúngico de capsaicina y extractos de chile piquín (Capsicum annuum l. var aviculare) sobre el crecimiento in vitro de Aspergillus flavus. Polibotánica 2012, 34, 191–204. [Google Scholar]
- Singh, T.; Chittenden, C. In-vitro antifungal activity of chilli extracts in combination with Lactobacillus casei against common sapstain fungi. Int. Biodeterior. Biodegrad. 2008, 62, 364–367. [Google Scholar] [CrossRef]
- Xing, F.; Cheng, G.; Yi, K. Study on the antimicrobial activities of the capsaicin microcapsules. J. Appl. Polym. Sci. 2006, 102, 1318–1321. [Google Scholar] [CrossRef]
- Kar, D.; Bandyopadhyay, S.; Dimri, U.; Mondal, D.B.; Nanda, P.K.; Das, A.K.; Batabyal, S.; Dandapat, P.; Bandyopadhyay, S. Antibacterial effect of silver nanoparticles and capsaicin against MDR-ESBL producing Escherichia coli: An in vitro study. Asian Pac. J. Trop. Dis. 2016, 6, 807–810. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Valle-Gallego, A.; Stefani, R.; Menchicchi, B.; David, L.; Rochas, C.; Santander-Ortega, M.J.; Alonso, M.J. Chitosan-based nanocapsules: Physical characterization, stability in biological media and capsaicin encapsulation. Colloid Polym. Sci. 2012, 290, 1423–1434. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Mujtaba, M.; Ilk, S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Islek, C. Supplementing capsaicin with chitosan-based films enhanced the anti-quorum sensing, antimicrobial, antioxidant, transparency, elasticity and hydrophobicity. Int. J. Biol. Macromol. 2018, 115, 438–446. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Qi, W.; Han, F.; Shao, J.-Z.; Gao, J.-Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351. [Google Scholar]
- Matica, A.; Menghiu, G.; Ostafe, V. Toxicity of chitosan based products. New Front. Chem. 2017, 26, 65–74. [Google Scholar]
- Luque-Alcaraz, A.G.; Cortez-Rocha, M.O.; Velázquez-Contreras, C.A.; Acosta-Silva, A.L.; Santacruz-Ortega, H.d.C.; Burgos-Hernández, A.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Enhanced antifungal effect of chitosan/pepper tree (Schinus molle) essential oil bionanocomposites on the viability of Aspergillus parasiticus spores. J. Nanomater. 2016, 2016, 38. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.-T.; Hadinoto, K. A new solubility enhancement strategy of capsaicin in the form of high-payload submicron capsaicin-chitosan colloidal complex. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 62–71. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Jiang, X.; Yu, L.; Wang, C. Highly Dual Antifouling and Antibacterial Ultrafiltration Membranes Modified with Silane Coupling Agent and Capsaicin-Mimic Moieties. Polymers 2020, 12, 412. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-P.; Chen, J.-C.; Wu, C.-C.; Chen, C.-T.; Tang, N.-Y.; Ho, Y.-T.; Lo, C.; Lin, J.-P.; Chung, J.-G.; Lin, J.-G. Capsaicin-induced Apoptosis in Human Hepatoma HepG2 Cells. Anticancer Res. 2009, 29, 165–174. [Google Scholar]
- Gálvez-Iriqui, A.C.; García-Romo, J.S.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Burboa-Zazueta, M.G.; Luque-Alcaraz, A.G.; Calderón-Santoyo, M.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Phytotoxicity, cytotoxicity, and in vivo antifungal efficacy of chitosan nanobiocomposites on prokaryotic and eukaryotic cells. Environ. Sci. Pollut. Res. 2021, 28, 3051–3065. [Google Scholar] [CrossRef]
- Su, Y.; Wu, D.; Xia, H.; Zhang, C.; Shi, J.; Wilkinson, K.J.; Xie, B. Metallic nanoparticles induced antibiotic resistance genes attenuation of leachate culturable microbiota: The combined roles of growth inhibition, ion dissolution and oxidative stress. Environ. Int. 2019, 128, 407–416. [Google Scholar] [CrossRef]
- Wang, X.; Yang, F.; Zhao, J.; Xu, Y.; Mao, D.; Zhu, X.; Luo, Y.; Alvarez, P.J.J. Bacterial exposure to ZnO nanoparticles facilitates horizontal transfer of antibiotic resistance genes. NanoImpact 2018, 10, 61–67. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, J.; Wang, Y.; Verstraete, W.; Yuan, Z.; Guo, J. Insights of metallic nanoparticles and ions in accelerating the bacterial uptake of antibiotic resistance genes. J. Hazard. Mater. 2022, 421, 126728. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Afifi, F.U. Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. J. Microbiol. Methods 2007, 68, 19–25. [Google Scholar] [CrossRef]
- Muniyandi, P.; Palaninathan, V.; Veeranarayanan, S.; Ukai, T.; Maekawa, T.; Hanajiri, T.; Mohamed, M.S. ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering. Polymers 2020, 12, 451. [Google Scholar] [CrossRef] [Green Version]
- Luque-Alcaraz, A.G.; Lizardi, J.; Goycoolea, F.M.; Valdez, M.A.; Acosta, A.L.; Iloki-Assanga, S.B.; Higuera-Ciapara, I.; Argüelles-Monal, W. Characterization and Antiproliferative Activity of Nobiletin-Loaded Chitosan Nanoparticles. J. Nanomater. 2012, 2012, 100. [Google Scholar] [CrossRef]
- Luque-Alcaraz, A.G.; Lizardi-Mendoza, J.; Goycoolea, F.M.; Higuera-Ciapara, I.; Argüelles-Monal, W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 2016, 6, 59250–59256. [Google Scholar] [CrossRef]
- Cruz-Ramírez, S.-G.; Lopez-Saiz, C.-M.; Rosas-Burgos, E.-C.; Cinco-Moroyoqui, F.-J.; Velázquez, C.; Hernández, J.; Burgos-Hernández, A. Antimutagenic, antiproliferative, and antioxidant effect of extracts obtained from octopus (Paraoctopus limaculatus). Food Sci. Technol. 2015, 35, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Frías-Escalante, M.; Burgos-Hernández, A.; Plascencia-Jatomea, M.; Aldana-Madrid, M.; Cortez-Rocha, M. Antifungal, acute toxicity and mutagenicity activity of extracts from Datura stramonium, Jacquinia macrocarpa and Krameria erecta on Fusarium verticillioides. Afr. J. Biotechnol. 2015, 14, 2251–2257. [Google Scholar]
- Chávez-Magdaleno, M.; Luque-Alcaraz, A.; Gutiérrez-Martínez, P.; Cortez-Rocha, M.; Burgos-Hernández, A.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Effect of Chitosan-Pepper Tree (Schinus molle) Essential Oil Biocomposites on the Growth Kinetics, Viability and Membrane Integrity of Colletotrichum Gloeosporioides. Rev. Mex. Ing. Química 2018, 17, 29–45. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Bouman, B.A.; Donnelly, J.P.; Verweij, P.E. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 2001, 39, 3402–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kulterer, M.R.; Reischl, M.; Reichel, V.E.; Hribernik, S.; Wu, M.; Köstler, S.; Kargl, R.; Ribitsch, V. Nanoprecipitation of cellulose acetate using solvent/nonsolvent mixtures as dispersive media. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 23–29. [Google Scholar] [CrossRef]
- Lugo-Medina, E.; García-Gutiérrez, C.; Ruelas-Ayala, R.D. Nanotecnología y Nanoencapsulación de Plaguicidas. Ra Ximhai 2010, 6, 63–67. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and Antimicrobial Properties of Peppermint Oil Nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–806. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of monodisperse chitosan nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar] [CrossRef]
- MubarakAli, D.; LewisOscar, F.; Gopinath, V.; Alharbi, N.S.; Alharbi, S.A.; Thajuddin, N. An inhibitory action of chitosan nanoparticles against pathogenic bacteria and fungi and their potential applications as biocompatible antioxidants. Microb. Pathog. 2018, 114, 323–327. [Google Scholar] [CrossRef]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Bagur-González, M.G.; Estepa-Molina, C.; Martín-Peinado, F.; Morales-Ruano, S. Toxicity assessment using Lactuca sativa L. bioassay of the metal (loid) s As, Cu, Mn, Pb and Zn in soluble-in-water saturated soil extracts from an abandoned mining site. J. Soils Sediments 2011, 11, 281–289. [Google Scholar] [CrossRef]
- Rodríguez Romero, A.J.; Robles Salazar, C.A.; Ruíz Picos, R.A.; López López, E.; Sedeño Díaz, J.E.; Rodríguezdorantes, A. Índices de germinación y elongación radical de Lactuca sativa en el biomonitoreo de la calidad del agua del río Chalma. Rev. Int. Contam. Ambient. 2014, 30, 307–316. [Google Scholar]
- Cota-Arriola, O.; Cortez-Rocha, M.O.; Rosas-Burgos, E.C.; Burgos-Hernández, A.; López-Franco, Y.L.; Plascencia-Jatomea, M. Antifungal effect of chitosan on the growth of Aspergillus parasiticus and production of aflatoxin B1. Polym. Int. 2011, 60, 937–944. [Google Scholar] [CrossRef]
- Wang, K.; Dang, W.; Xie, J.; Zhu, R.; Sun, M.; Jia, F.; Zhao, Y.; An, X.; Qiu, S.; Li, X. Antimicrobial peptide protonectin disturbs the membrane integrity and induces ROS production in yeast cells. Biochim. Biophys. Acta 2015, 1848, 2365–2373. [Google Scholar] [CrossRef] [Green Version]
- Masood, A.; Dogra, J.V.V.; Jha, A.K. The influence of colouring and pungent agents of red Chilli (Capsicum annum) on growth and aflatoxin production by Aspergillus flavus. Lett. Appl. Microbiol. 1994, 18, 184–186. [Google Scholar] [CrossRef]
- López-Meneses, A.K.; Plascencia-Jatomea, M.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Luque-Alcaraz, A.G.; Cortez-Rocha, M.O. Antifungal and antimycotoxigenic activity of essential oils from Eucalyptus globulus, Thymus capitatus and Schinus molle. Food Sci. Technol. 2015, 35, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Esin, S.; Gazzarri, M.; Batoni, G.; Chiellini, F. Preparation, physical–chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int. J. Biol. Macromol. 2014, 67, 124–131. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kaur, N.D.; Shukla, G.; Mehta, S. Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int. J. Biol. Macromol. 2018, 115, 18–28. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016, 154, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Silva, S.; Vicente, S.; Neto, C.; Castro, P.; Veiga, M.; Madureira, R.; Tavaria, F.; Pintado, M. Chitosan nanoparticles as alternative anti-staphylococci agents: Bactericidal, antibiofilm and antiadhesive effects. Mater. Sci. Eng. C 2017, 79, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yien, L.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar]
- Ayala, A.; Colina, M.; Molina, J.; Vargas, J.; Rincón, D.; Medina, J.; Rosales, L.; Cárdenas, H. Evaluación de la actividad antifúngica del quitosano contra el hongo Mycosphaerella fijiensis Morelet que produce la sigatoka negra que ataca el plátano. Rev. Iberoam. Polim. 2014, 15, 312–338. [Google Scholar]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free. Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Shi, Z.; Neoh, K.; Kang, E. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef]
- Assadi, Z.; Emtiazi, G.; Zarrabi, A. Novel synergistic activities of tetracycline copper oxide nanoparticles integrated into chitosan micro particles for delivery against multiple drug resistant strains: Generation of reactive oxygen species (ROS) and cell death. J. Drug Deliv. Sci. Technol. 2018, 44, 65–70. [Google Scholar] [CrossRef]
- Anbu, A.S.; Velmurugan, P.; Lee, J.-H.; Oh, B.-T.; Venkatachalam, P. Biomolecule-loaded chitosan nanoparticles induce apoptosis and molecular changes in cancer cell line (SiHa). Int. J. Biol. Macromol. 2016, 88, 18–26. [Google Scholar] [CrossRef]
- Scott, B.; Eaton, C.J. Role of reactive oxygen species in fungal cellular differentiations. Curr. Opin. Microbiol. 2008, 11, 488–493. [Google Scholar] [CrossRef]
- Hamdi, H.; Salem, I.B.; Othmène, Y.B.; Annabi, E.; Abid-Essefi, S. The involvement of ROS generation on Epoxiconazole-induced toxicity in HCT116 cells. Pestic. Biochem. Physiol. 2018, 148, 62–67. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, B.; Li, J.; Zhang, B.; Wang, H.; Li, M. Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free. Radic. Biol. Med. 2016, 99, 572–583. [Google Scholar] [CrossRef]






| Treatment | Chitosan Concentration (mg/mL) | Capsaicin Concentration (mg/mL) | |
|---|---|---|---|
| Chitosan nanoparticles | |||
| Np Q-A | 0.4 | - | |
| Np Q-B | 0.3 | - | |
| Np Q-C | 0.2 | - | |
| Np Q-D | 0.1 | - | |
| Np Q-E | 0.05 | - | |
| Chitosan–capsaicine nanoparticles | |||
| Np QC-A | 0.4 | 0.75 | |
| Np QC-B | 0.3 | 0.50 | |
| Np QC-C | 0.2 | 0.25 | |
| Np QC-D | 0.1 | 0.125 | |
| Np QC-E | 0.05 | 0.06 | |
| Capsaicin control (capsaicin in solution) | |||
| Cap control-A | - | 0.75 | |
| Cap control-B | - | 0.50 | |
| Cap control-C | - | 0.25 | |
| Cap control-D | - | 0.125 | |
| Cap control-E | - | 0.06 | |
| Control | |||
| Control | 0 | 0 |
| Diameter (nm) | Zeta Potential (mV) | |
|---|---|---|
| Np Q | 44.8 ± 20.6 ᵃ | +25.6 ± 0.71 ᵃ |
| Np QC | 111.1 ± 14.1 ᵇ | +26.8 ± 6.14 ᵃ |
| Treatment | IER (%) a | IGN (%) b | RSG (%) c | RRE (%) d | GI e |
|---|---|---|---|---|---|
| Chitosan nanoparticles | |||||
| Np Q-A (0.4 mg/mL) | −1 | −1 | 0 | 0 | 0 |
| Np Q-B (0.3 mg/mL) | −1 | −1 | 0 | 0 | 0 |
| Np Q-C (0.2 mg/mL) | −1 | −0.88 | 12.00 | 0 | 0 |
| Chitosan/Capsaicine nanoparticles | |||||
| Np QC-A (0.4 mg/mL) | −1 | −1 | 0 | 0 | 0 |
| Np QC-B (0.3 mg/mL) | −1 | −1 | 0 | 0 | 0 |
| Np QC- C (0.2 mg/mL) | −1 | −0.88 | 12.00 | 0 | 0 |
| Capsaicine in solution | |||||
| Cap control-A (0.4 mg/mL) | −1 | −1 | 0 | 0 | 0 |
| Cap control- B (0.3 mg/mL) | −1 | −0.76 | 24.00 | 0 | 0 |
| Cap control-C (0.2 mg/mL) | −0.5084 | −0.32 | 68.00 | 49.15 | 33.44 |
| Control (H20) | 0 | 0 | 100 | 100 | 100 |
| Treatment | Diameter (µm) | Treatment | Diameter (µm) | Cap Control (Capsaicin Solution) | Diameter (µm) |
|---|---|---|---|---|---|
| Np Q-A | 5.30 ± 0.5793 a | Np QC-A | 5.34 ± 0.5481 a | C-A | 5.07 ± 0.8492 a |
| Np Q-B | 5.18 ± 0.7545 a | Np QC-B | 5.50 ± 0.6439 a | C-B | 5.39 ± 0.7043 a |
| Np Q-C | 5.83 ± 0.6640 a | Np QC-C | 6.14 ± 0.8612 a | C-C | 5.55 ± 0.8425 a |
| Np Q-D | 6.24 ± 0.7329 a | Np QC-D | 5.23 ± 0.6117 a | C-D | 5.41 ± 0.7497 a |
| Np Q-E | 5.85 ± 0.9189 a | Np QC-E | 5.94 ± 1.0180 a | C-E | 5.51 ± 0.8526 a |
| Control | 5.35 ± 0.8363 a | Control | 5.35 ± 0.8363 a | Control | 5.35 ± 0.8363 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Núñez-Mexía, S.A.; Cortez-Rocha, M.O.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Rosas-Durazo, A.d.J.; Parra-Vergara, N.V.; Plascencia-Jatomea, M. Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers 2022, 14, 2774. https://doi.org/10.3390/polym14142774
Hernández-Téllez CN, Luque-Alcaraz AG, Núñez-Mexía SA, Cortez-Rocha MO, Lizardi-Mendoza J, Rosas-Burgos EC, Rosas-Durazo AdJ, Parra-Vergara NV, Plascencia-Jatomea M. Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers. 2022; 14(14):2774. https://doi.org/10.3390/polym14142774
Chicago/Turabian StyleHernández-Téllez, Cynthia Nazareth, Ana Guadalupe Luque-Alcaraz, Sahily Alejandra Núñez-Mexía, Mario Onofre Cortez-Rocha, Jaime Lizardi-Mendoza, Ema Carina Rosas-Burgos, Aarón de Jesús Rosas-Durazo, Norma Violeta Parra-Vergara, and Maribel Plascencia-Jatomea. 2022. "Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus" Polymers 14, no. 14: 2774. https://doi.org/10.3390/polym14142774
APA StyleHernández-Téllez, C. N., Luque-Alcaraz, A. G., Núñez-Mexía, S. A., Cortez-Rocha, M. O., Lizardi-Mendoza, J., Rosas-Burgos, E. C., Rosas-Durazo, A. d. J., Parra-Vergara, N. V., & Plascencia-Jatomea, M. (2022). Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers, 14(14), 2774. https://doi.org/10.3390/polym14142774