Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Creation of Alginate-Based Nanofibers
2.2.1. Preparation of Alginate and PVA (Alg-PVA) Electrospinning Solution
2.2.2. Preparation of Alginate and Agarose (Alg-Ag) Electrospinning Solution
2.2.3. Preparation of Cipro-Loaded Alginate-Based Electrospinning Solution
2.3. Electrospinning Alginate-Based Nanofibers
2.4. Analysis of Nanofibers
2.5. Release Studies of Drug-Loaded Alginate-Based Nanofibers
3. Results
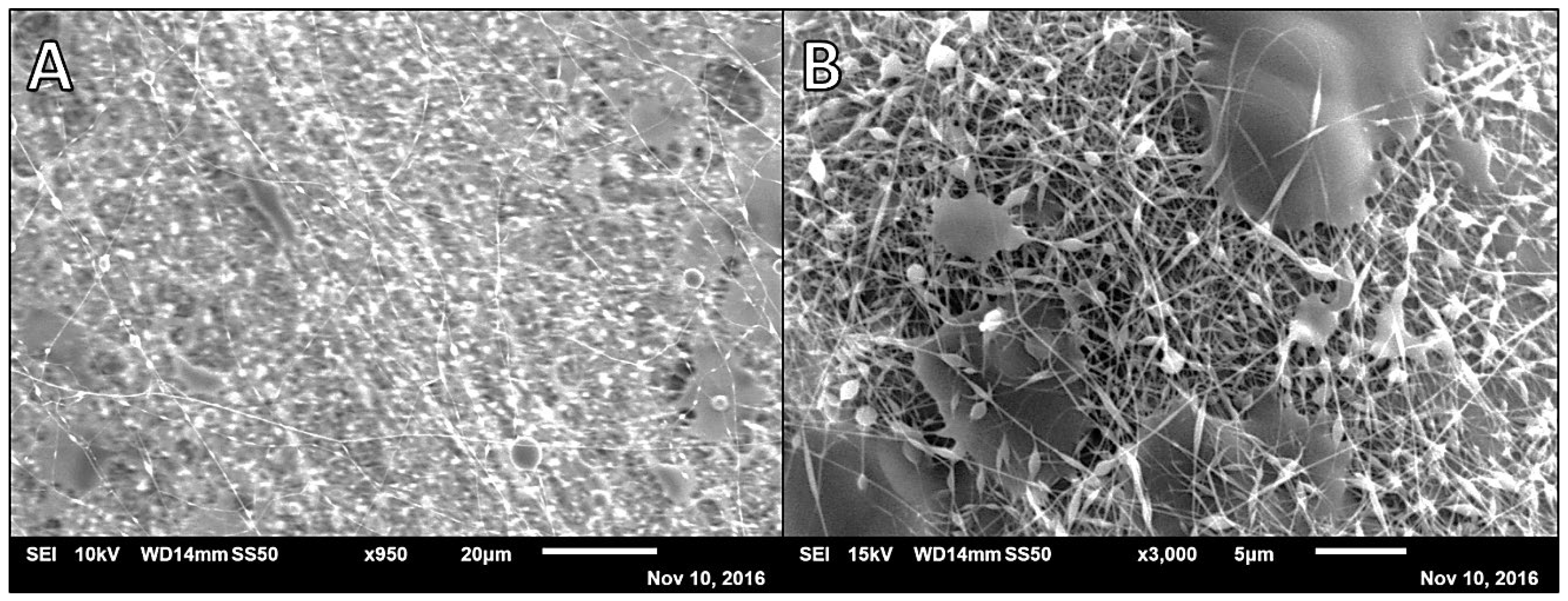
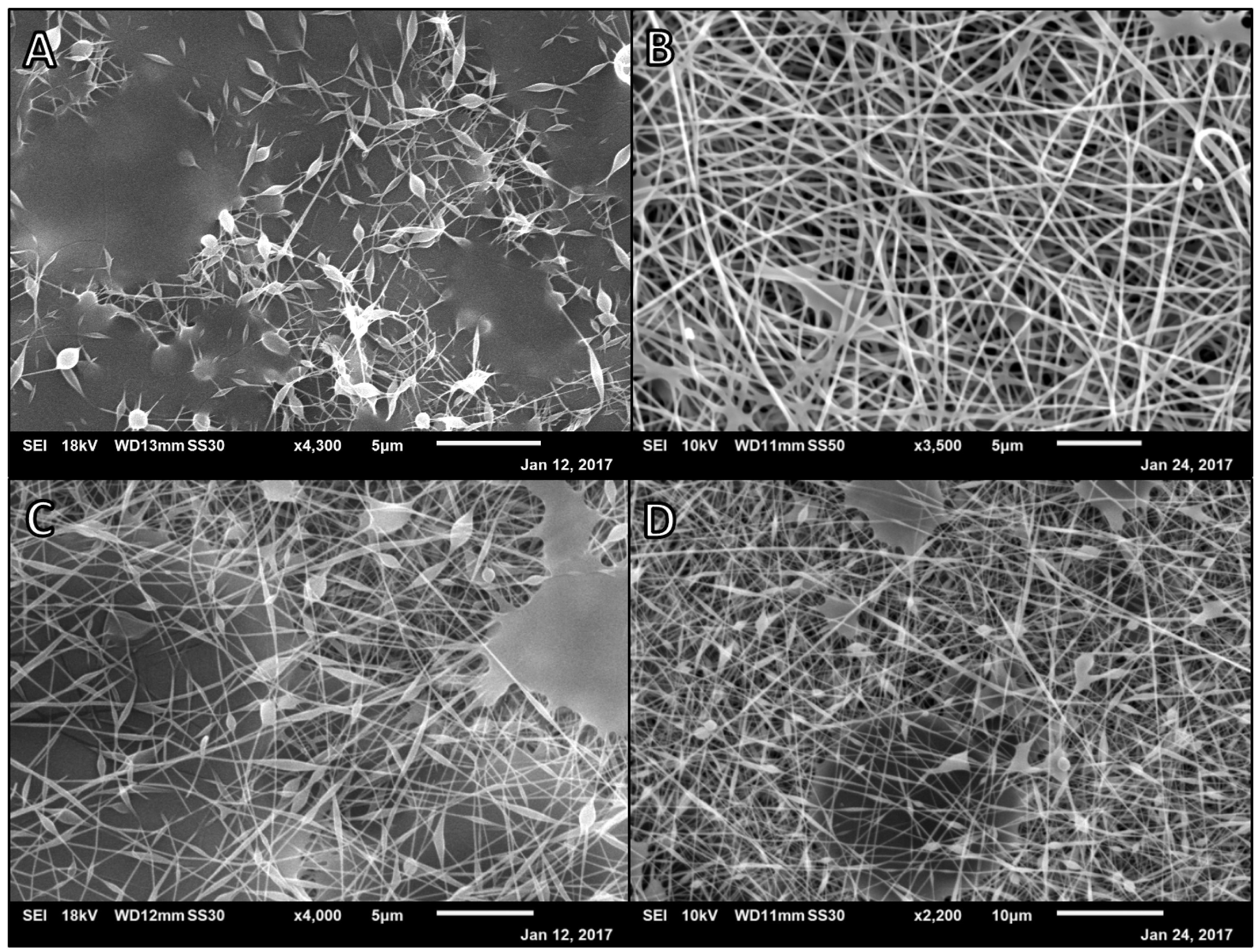
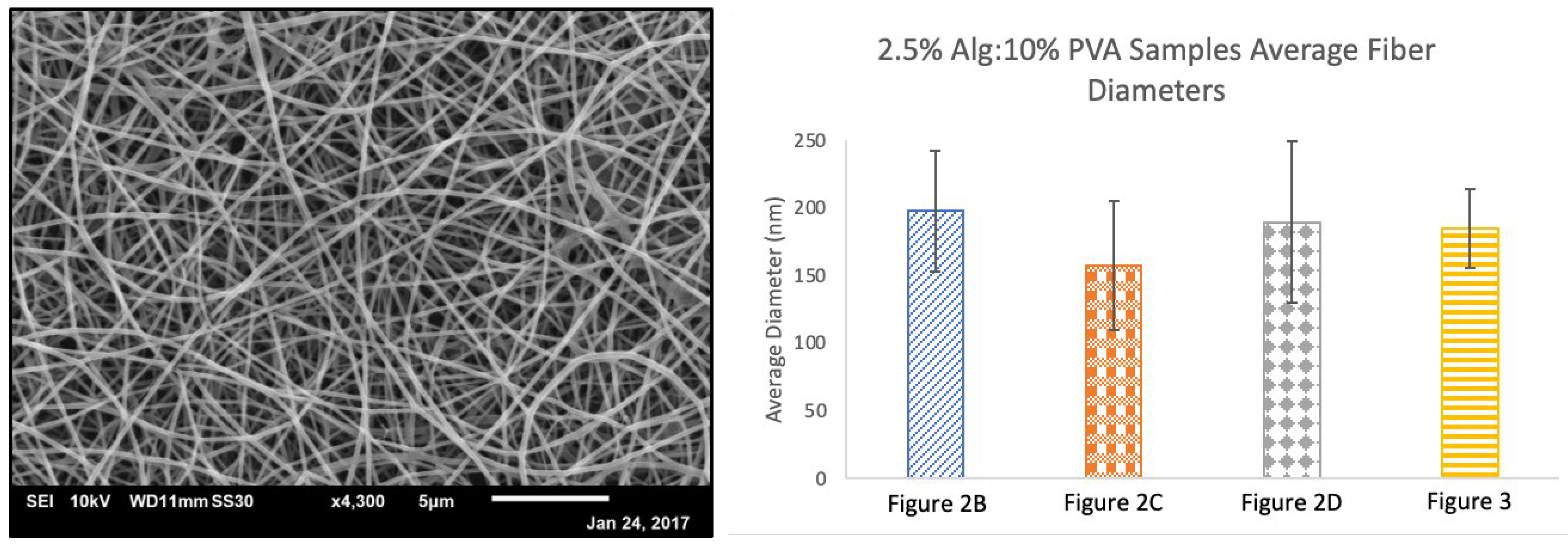
3.1. Alg-PVA Nanofibers
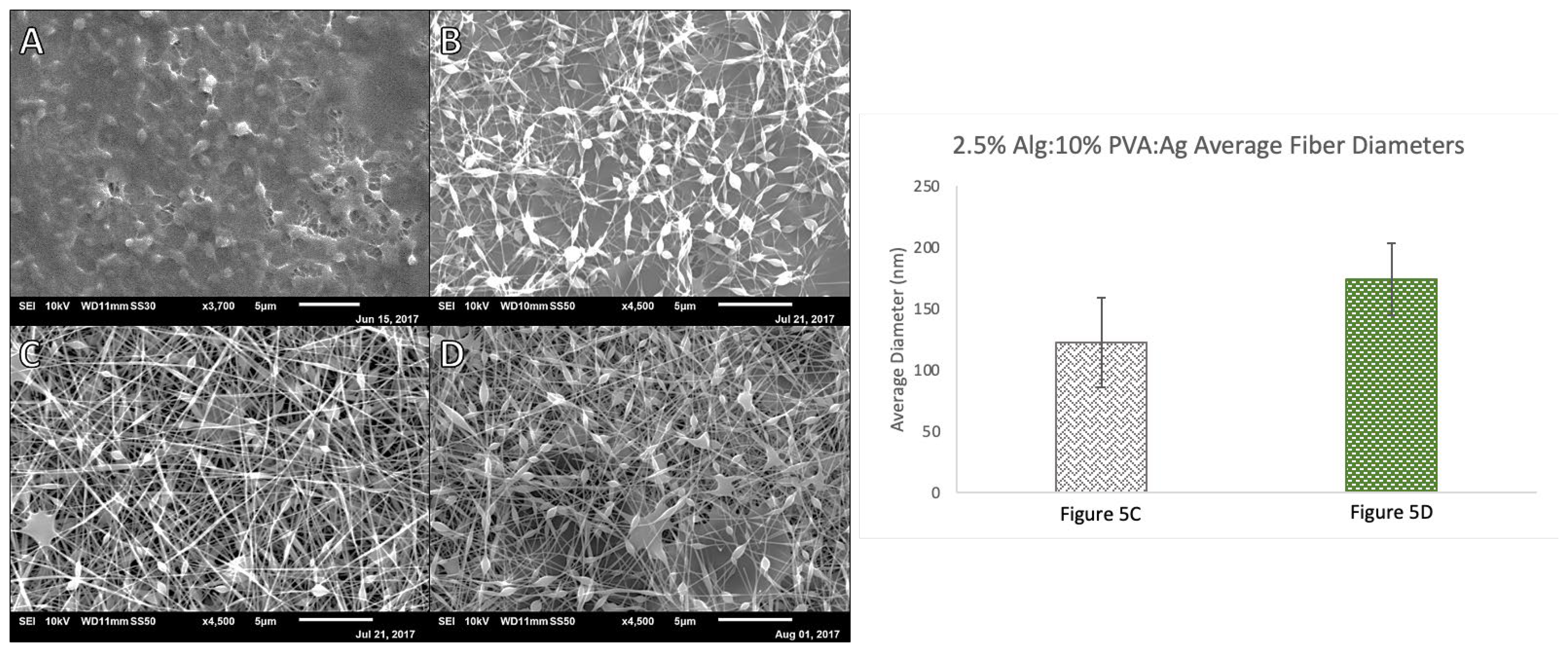
3.2. Alg-Ag and Alginate, PVA, and Agarose (Alg-PVA-Ag) Nanofibers
3.3. Cipro-Loaded Alg-PVA Nanofibers
3.4. Release Studies on Drug-Loaded Alg-PVA Nanofibers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bjorklund, A.; Lindvall, O. Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 2000, 3, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.O.; Novikov, L.N. Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation. J. Biomed. Mater. Res. Part A 2006, 77, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khuller, G.; Garg, S. Alginate-based oral drug delivery system for tuberculosis: Pharmacokinetics and therapeutic effects. J. Antimicrob. Chemother. 2003, 51, 931–938. [Google Scholar]
- Jeong, S.I.; Jeon, O.; Krebs, M.D.; Hill, M.C.; Alsberg, E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur. Cells Mater. 2012, 24, 331. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [Green Version]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Fu, R.; Li, C.; Yu, C.; Xie, H.; Shi, S.; Li, Z.; Wang, Q.; Lu, L. A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. 2016, 23, 818–829. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly (lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.; Yarin, A.; Zussman, E. Biohybrid nanosystems with polymer nanofibers and nanotubes. Appl. Microbiol. Biotechnol. 2006, 71, 387–393. [Google Scholar] [CrossRef]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly (vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef]
- Jia, H.; Zhu, G.; Vugrinovich, B.; Kataphinan, W.; Reneker, D.H.; Wang, P. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol. Prog. 2002, 18, 1027–1032. [Google Scholar] [CrossRef]
- Bhattarai, N.; Zhang, M. Controlled synthesis and structural stability of alginate-based nanofibers. Nanotechnology 2007, 18, 455601. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Hausman, D.S.; Mooney, D.J. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer 1999, 40, 3575–3584. [Google Scholar] [CrossRef]
- Xie, J.; Hsieh, Y.-L. Ultra-high surface fibrous membranes from electrospinning of natural proteins: Casein and lipase enzyme. J. Mater. Sci. 2003, 38, 2125–2133. [Google Scholar] [CrossRef]
- Arthanari, S.; Mani, G.; Jang, J.H.; Choi, J.O.; Cho, Y.H.; Lee, J.H.; Cha, S.E.; Oh, H.S.; Kwon, D.H.; Jang, H.T. Preparation and characterization of gatifloxacin-loaded alginate/poly (vinyl alcohol) electrospun nanofibers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 847–852. [Google Scholar] [CrossRef]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of lutein-loaded PVA/sodium alginate nanofibers and investigation of its release behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef] [Green Version]
- Abidian, M.R.; Martin, D.C. Multifunctional Nanobiomaterials for Neural Interfaces. Adv. Funct. Mater. 2009, 19, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Watthanaphanit, A.; Supaphol, P.; Tamura, H.; Furuike, T.; Tokura, S.; Rujiravanit, R. Novel Chitosan-Spotted Alginate Fibers from Wet-Spinning of Alginate Solutions Containing Emulsified Chitosan-Citrate Complex and their Characterization. Biomacromolecules 2009, 10, 320–327. [Google Scholar] [CrossRef]
- Haghi, A.K.; Akbari, M. Trends in electrospinning of natural nanofibers. Phys. Status Solidi 2007, 204, 1830–1834. [Google Scholar] [CrossRef]
- Pham, Q.P.; Upma Sharma, P.D.; Antonios, G.; Mikoc, P.D. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. Part A 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; John, M.J.; Sadiku, E.R.; Sefadi, J.S. Electrospun alginate nanofibers toward various applications: A review. Materials 2020, 13, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Kyzioł, A.; Michna, J.; Moreno, I.; Gamez, E.; Irusta, S. Preparation and characterization of electrospun alginate nanofibers loaded with ciprofloxacin hydrochloride. Eur. Polym. J. 2017, 96, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Teng, S.-H.; Wang, P.; Kim, H.-E. Blend fibers of chitosan–agarose by electrospinning. Mater. Lett. 2009, 63, 2510–2512. [Google Scholar] [CrossRef]
- Latiyan, S.; Kumar, T.S.; Doble, M. Fabrication and evaluation of multifunctional agarose based electrospun scaffolds for cutaneous wound repairs. J. Tissue Eng. Regen. Med. 2022, 16, 653–664. [Google Scholar] [CrossRef]
- Forget, A.; Arya, N.; Randriantsilefisoa, R.; Miessmer, F.; Buck, M.; Ahmadi, V.; Jonas, D.; Blencowe, A.; Shastri, V.P. Nonwoven carboxylated agarose-based fiber meshes with antimicrobial properties. Biomacromolecules 2016, 17, 4021–4026. [Google Scholar] [CrossRef]
- Kataria, K.; Sharma, A.; Garg, T.; Goyal, A.K.; Rath, G. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv. Lett. 2014, 4, 79–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent advances in poly(α-L-glutamic acid)-based nanomaterials for drug delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]

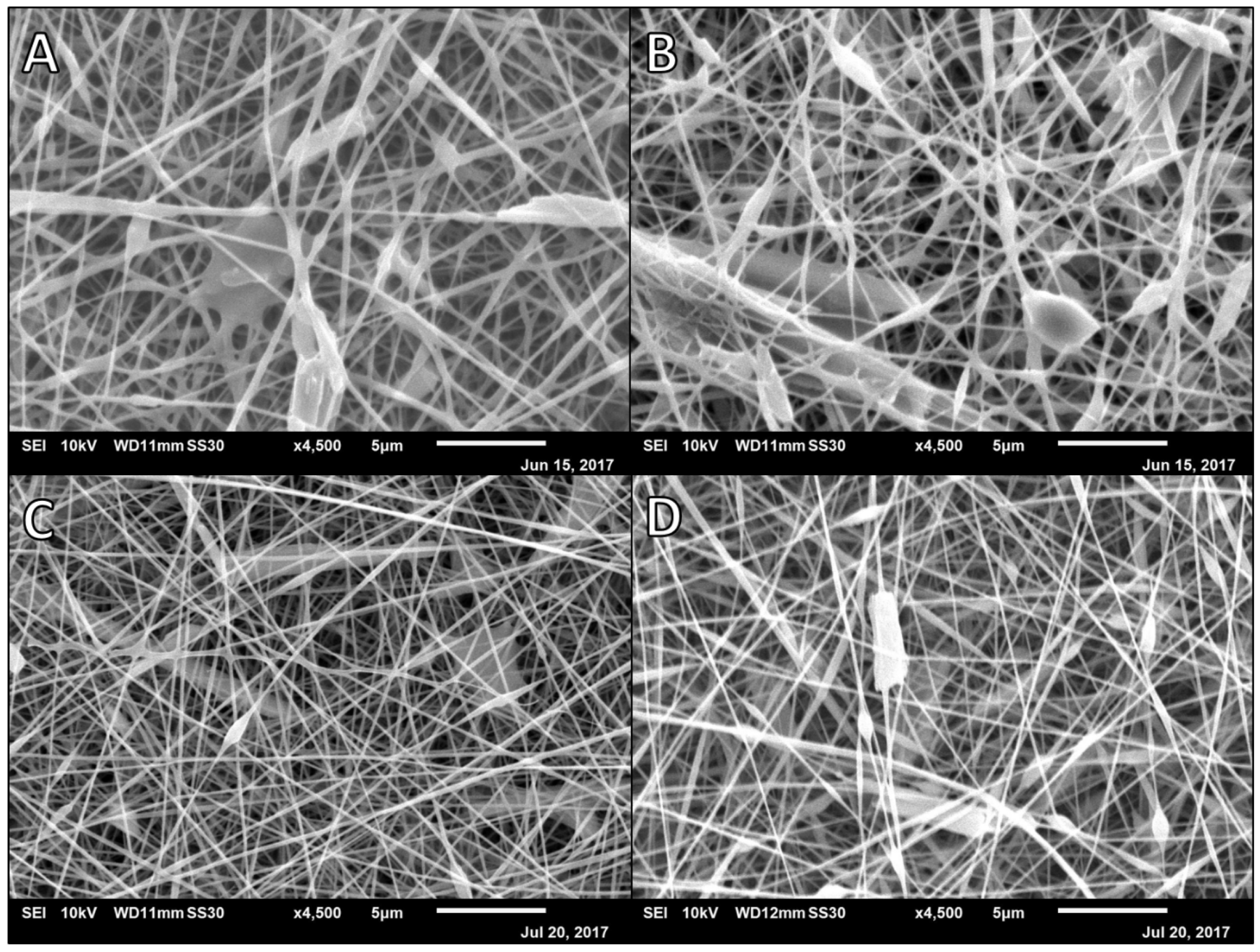

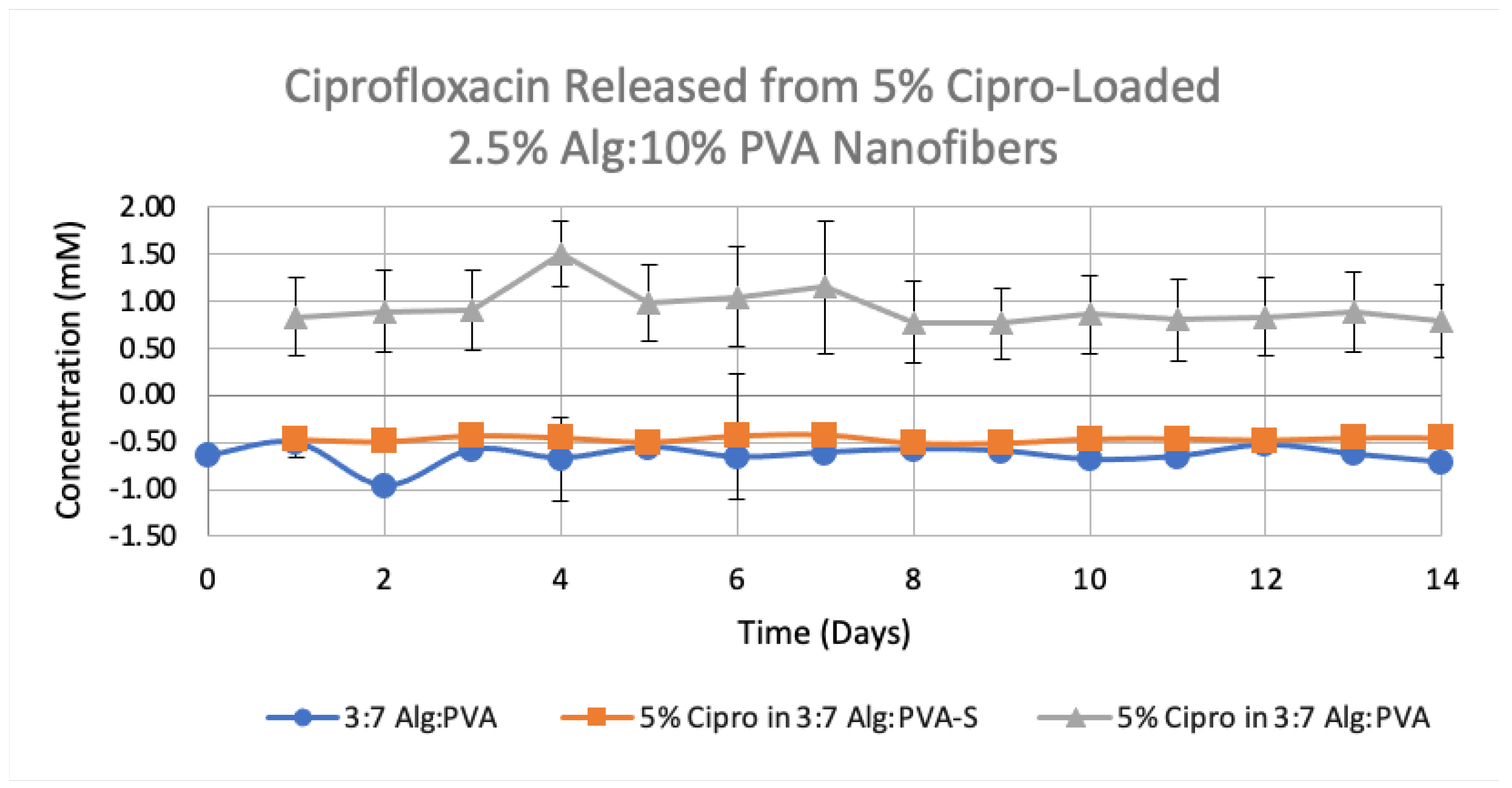
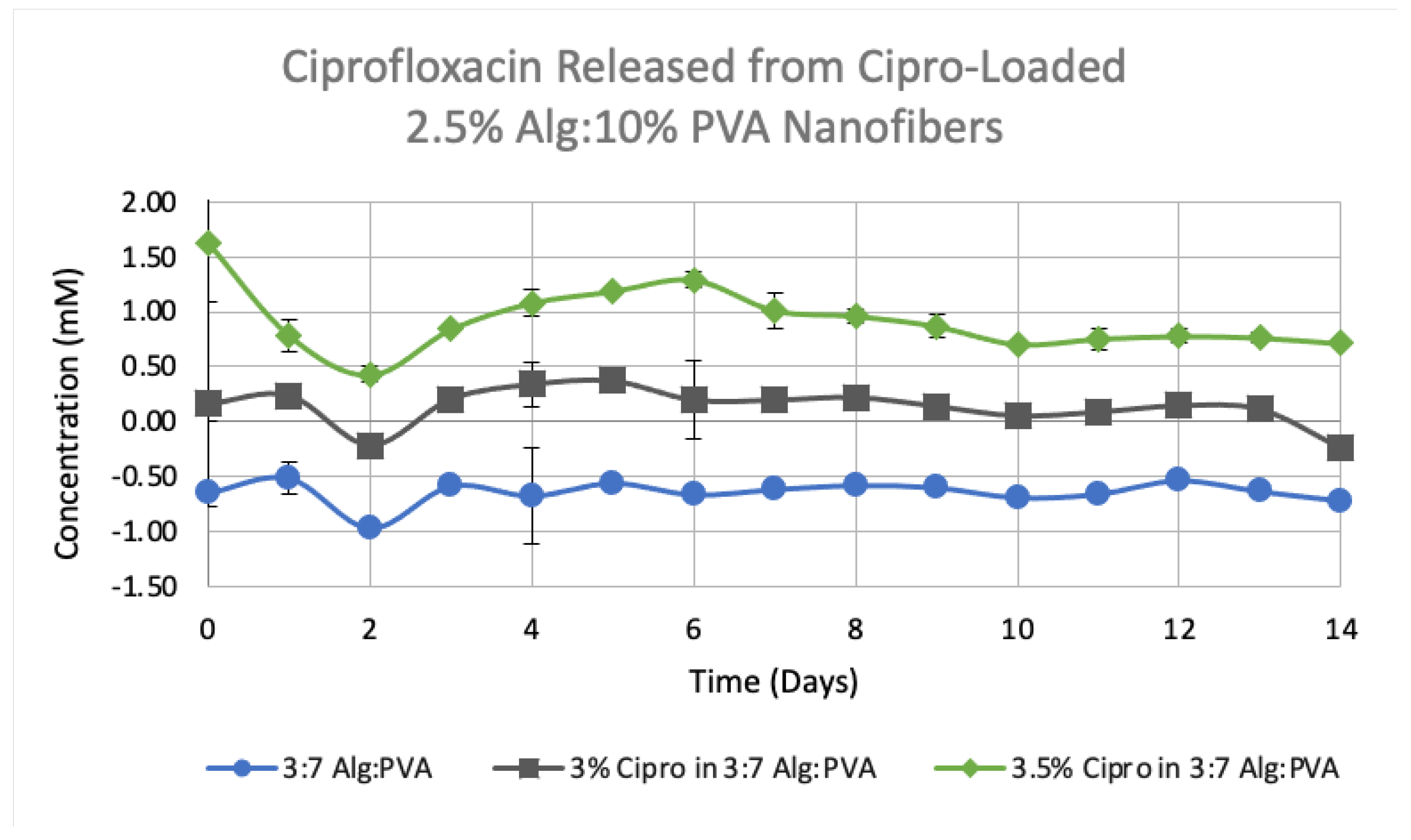
| Polymer Ratio | Polymer Formulation | Cipro Percentage (w/v) | Rate (μL/min) | Height (mm) | Voltage (kV) | Volume Dispersed (mL) | Average Cipro Release 1 (mM) |
|---|---|---|---|---|---|---|---|
| 3:7 | 2.5% Alg:10% PVA | 0 | 9.8 | 100 | 16 | 1 | 0 |
| 3:7 | 2.5% Alg:10% PVA | 3.0 | 9.8 | 100 | 16 | 1 | 0.134 |
| 3:7 | 2.5% Alg:10% PVA | 3.5 | 9.8 | 100 | 16 | 1 | 0.920 |
| 3:7 | 2.5% Alg:10% PVA | 5 | 9.8 | 100 | 16 | 1 | 0.924 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penton, K.E.; Kinler, Z.; Davis, A.; Spiva, J.A.; Hamilton, S.K. Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog. Polymers 2022, 14, 2773. https://doi.org/10.3390/polym14142773
Penton KE, Kinler Z, Davis A, Spiva JA, Hamilton SK. Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog. Polymers. 2022; 14(14):2773. https://doi.org/10.3390/polym14142773
Chicago/Turabian StylePenton, Kathryn E., Zachary Kinler, Amber Davis, Joshua A. Spiva, and Sharon K. Hamilton. 2022. "Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog" Polymers 14, no. 14: 2773. https://doi.org/10.3390/polym14142773
APA StylePenton, K. E., Kinler, Z., Davis, A., Spiva, J. A., & Hamilton, S. K. (2022). Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog. Polymers, 14(14), 2773. https://doi.org/10.3390/polym14142773





