Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits
Abstract
:1. Introduction
2. TPS Extraction
2.1. Hot Water Extraction
2.2. Ultrasonic-Assisted Extraction (UAE)
2.3. Microwave-Assisted Extraction (MAE)
2.4. Enzymolysis Extraction
2.5. Other Extraction Methods
3. Preliminary Physicochemical Properties of TPS
3.1. Monosaccharide Composition
3.2. Molecular Weight (Mw)
3.3. Solubility
3.4. Viscosity
3.5. Emulsifying and Stability
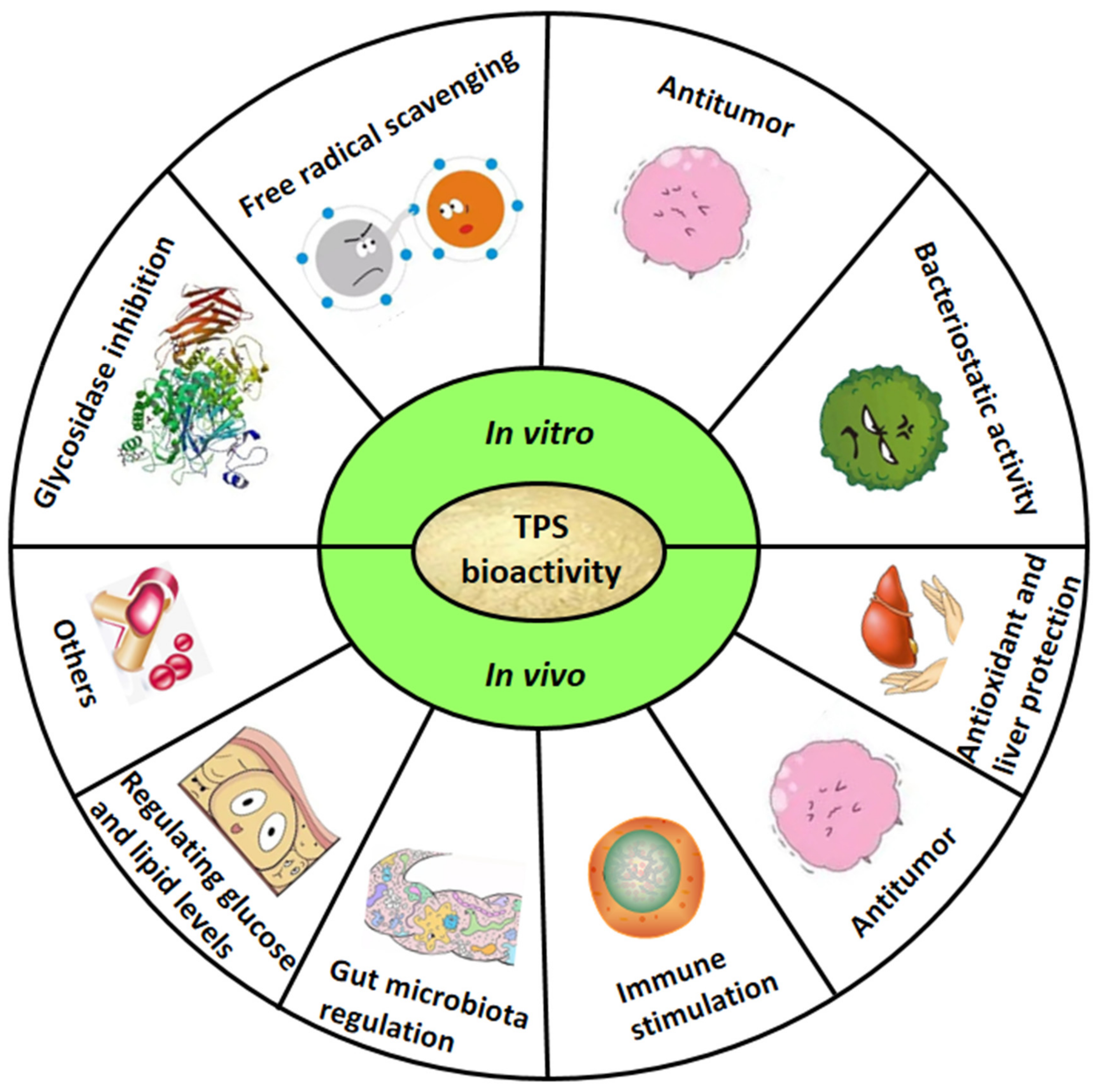
4. In Vitro Bioactivity of TPS
4.1. Glycosidase Inhibition
4.2. Free Radical Scavenging
4.3. Antitumor Activity
4.4. Bacteriostatic Activity
5. In Vivo Bioactivity of TPS
5.1. Antioxidant and Hepatoprotective Activity
5.2. Antitumor Activity
5.3. Immunostimulatory Activity
5.4. Gut Microbiota-Modulating Activity
5.5. Glucose and Lipid Metabolism-Regulating Activity
5.6. Others
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Zhou, Y.; Zhang, Q.; Zhang, K.; Peng, P.; Chen, L.; Xiao, B. Hydrothermal extraction, structural characterization, and inhibition HeLa cells proliferation of functional polysaccharides from Chinese tea Zhongcha 108. J. Funct. Foods 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Ye, X.; Tang, X.; Li, F.; Zhu, J.; Wu, M.; Wei, X.; Wang, Y. Green and Oolong Tea Extracts with Different Phytochemical Compositions Prevent Hypertension and Modulate the Intestinal Flora in a High-Salt Diet Fed Wistar Rats. Front. Nutr. 2022, 9, 892801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, M.; Zhou, H.; Cheng, L.; Wei, X.; Wang, Y. Liubao brick tea activates the PI3K-Akt signaling pathway to lower blood glucose, metabolic disorders and insulin resistance via altering the intestinal flora. Food Res. Int. 2021, 148, 110594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, C.; Zhou, H.; Wei, X.; Wang, Y. Comparative evaluation for phytochemical composition and regulation of blood glucose, hepatic oxidative stress and insulin resistance in mice and HepG2 models of four typical Chinese dark teas. J. Sci. Food Agric. 2021, 101, 6563–6577. [Google Scholar] [CrossRef]
- Ding, Y.; Pu, L.; Kan, J. Hypolipidemic effects of lipid-lowering granulated tea preparation from Monascus -fermented grains (adlay and barley bran) mixed with lotus leaves on Sprague–Dawley rats fed a high-fat diet. J. Funct. Foods 2017, 32, 80–89. [Google Scholar] [CrossRef]
- Orem, A.; Alasalvar, C.; Kural, B.V.; Yaman, S.; Orem, C.; Karadag, A.; Pelvan, E.; Zawistowski, J. Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. J. Funct. Foods 2017, 31, 311–319. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Peng, F.; Xiao, F.; Ren, D.; Xue, H.; Chen, T.; Mushtaq, G.; Kamal, M.A. Combination of selenium-enriched green tea polysaccharides and Huo-ji polysaccharides synergistically enhances antioxidant and immune activity in mice. J. Sci. Food Agric. 2015, 95, 3211–3217. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Zhang, J.; Li, F.; Wei, K.; Wei, X.; Wang, Y. Valorization of polysaccharides obtained from dark tea: Preparation, physicochemical, antioxidant, and hypoglycemic properties. Foods 2021, 10, 2276. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Xiao, J.; Wang, Y. Protective effects of tea polysaccharides and polyphenols on skin. J. Agric. Food Chem. 2009, 57, 7757–7762. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Jin, Z. Structure analysis of an acidic polysaccharide isolated from green tea. Nat. Prod. Res. 2009, 23, 678–687. [Google Scholar] [CrossRef]
- Xiao, J.; Huo, J.; Jiang, H.; Yang, F. Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromol. 2011, 49, 1143–1151. [Google Scholar] [CrossRef]
- Jin, F.; He, J.; Jia, L.-Y.; Tu, Y.-Y. Optimizing conditions for the extraction of polysaccharides of white tea. Biotechnol. Biotechnol. Equip. 2015, 29, 921–925. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhi, L.; Yang, Y.; Rui, Z.; Yin, J.; Jiang, Y.; Wan, H. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of water-soluble and alkali-soluble polysaccharide conjugates prepared from green tea. Carbohydr. Polym. 2010, 82, 28–33. [Google Scholar] [CrossRef]
- Cai, W.; Xie, L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090. [Google Scholar] [CrossRef]
- Li, Q.; Shi, J.; Li, J.; Liu, L.; Zhao, T.; McClements, D.J.; Fu, Y.; Wu, Z.; Duan, M.; Chen, X. Influence of thermal treatment on the physicochemical and functional properties of tea polysaccharide conjugates. LWT-Food Sci. Technol. 2021, 150, 111967. [Google Scholar] [CrossRef]
- Qin, H.; Huang, L.; Teng, J.; Wei, B.; Xia, N.; Ye, Y. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation. Food Chem. 2021, 353, 129419. [Google Scholar] [CrossRef]
- Wei, X.; Chen, M.; Xiao, J.; Ying, L.; Lan, Y.; Zhang, H.; Wang, Y. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr. Polym. 2010, 79, 418–422. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Wei, X.; Yang, Z.; Xiao, J.; Jin, Z. Sulfation of tea polysaccharides: Synthesis, characterization and hypoglycemic activity. Int. J. Biol. Macromol. 2010, 46, 270–274. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.; Hu, X.; Wei, X.; Wang, Y. Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycoconj. J. 2020, 37, 241–250. [Google Scholar] [CrossRef]
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Sun, Y.; Yang, S.; Yang, X. Characterisation of polysaccharides from green tea of Huangshan Maofeng with antioxidant and hepatoprotective effects. Food Chem. 2013, 141, 3415–3423. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Lu, X.; Wang, D.; Zhao, Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013, 58, 184–192. [Google Scholar] [CrossRef]
- Karadag, A.; Pelvan, E.; Dogan, K.; Celik, N.; Ozturk, D.; Akaln, K.; Alasalvar, C. Optimisation of green tea polysaccharides by ultrasound-assisted extraction and their in vitro antidiabetic activities. Qual. Assur. Saf. Crops Foods 2019, 11, 479–490. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Omondi Onyango, S. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 105355. [Google Scholar] [CrossRef]
- Tsubaki, S.; Iida, H.; Sakamoto, M.; Azuma, J. Microwave heating of tea residue yields polysaccharides, polyphenols, and plant biopolyester. J. Agric. Food Chem. 2008, 56, 11293–11299. [Google Scholar] [CrossRef]
- Baik, J.H.; Shin, K.S.; Park, Y.; Yu, K.W.; Suh, H.J.; Choi, H.S. Biotransformation of catechin and extraction of active polysaccharide from green tea leaves via simultaneous treatment with tannase and pectinase. J. Sci. Food Agric. 2015, 95, 2337–2344. [Google Scholar] [CrossRef]
- Chang, B.Y.; Kim, T.Y.; Kim, S.Y. Polysaccharides from pectinase digests of green tea enhances host immune defence through toll-like receptor 4. Food Agric. Immunol. 2018, 29, 870–885. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, F.; Liu, Y.; An, Y.; Chang, D.; Wang, J.; Xia, F.; Liu, N.; Chen, X.; Cao, Y. Comparison of water- and alkali-extracted polysaccharides from Fuzhuan brick tea and their immunomodulatory effects in vitro and in vivo. Food Funct. 2022, 13, 806–824. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, L.Y. Supercritical extraction technology in tea polysaccharide extracting application. Adv. Mater. Res. 2012, 347–353, 1683–1688. [Google Scholar] [CrossRef]
- Li, S.; Cao, X. Extraction of tea polysaccharides (TPS) using anionic reverse micellar system. Sep. Purif. Technol. 2014, 122, 306–314. [Google Scholar] [CrossRef]
- Shashidhar, G.M.; Giridhar, P.; Manohar, B. Functional polysaccharides from medicinal mushroom Cordyceps sinensis as a potent food supplement: Extraction, characterization and therapeutic potentials—A systematic review. RSC Adv. 2015, 5, 16050–16066. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, 469–474. [Google Scholar] [CrossRef]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Fan, M.; Sun, X.; Qian, Y.; Xu, Y.; Wang, D.; Cao, Y. Effects of metal ions in tea polysaccharides on their in vitro antioxidant activity and hypoglycemic activity. Int. J. Biol. Macromol. 2018, 113, 418–426. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Ye, H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Li, X.; Wang, L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276. [Google Scholar] [CrossRef]
- Dean, J.R.; Xiong, G. Extraction of organic pollutants from environmental matrices: Selection of extraction technique. TrAC Trends Anal. Chem. 2000, 19, 553–564. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Hu, T.; Wu, P.; Zhan, J.; Wang, W.; Shen, J.; Ho, C.T.; Li, S. Influencing factors on the physicochemical characteristics of tea polysaccharides. Molecules 2021, 26, 3457. [Google Scholar] [CrossRef]
- Wang, D.F.; Wang, C.H.; Jun, L.L.; Guiwen, Z.Z. Components and activity of polysaccharides from coarse tea. J. Agric. Food Chem. 2001, 49, 507–510. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Chen, L.; Yu, C.; Wang, H.; Wei, X.; Wang, Y. Comparison and structural characterization of polysaccharides from natural and artificial Se-enriched green tea. Int. J. Biol. Macromol. 2019, 130, 388–398. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liu, Y.; Chen, X.; Wei, X. Extraction, characterization and antioxidant activities of Se-enriched tea polysaccharides. Int. J. Biol. Macromol. 2015, 77, 76–84. [Google Scholar] [CrossRef]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77. [Google Scholar] [CrossRef]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Wang, M.; Chen, G.; Chen, D.; Ye, H.; Sun, Y.; Zeng, X.; Liu, Z. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. Int. J. Biol. Macromol. 2019, 140, 858–870. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Z.Y.; Li, T.Q.; Song, W.; Xiao, W.; Zheng, J.; Chen, H.; Chen, G.H.; Zou, H.Y. Anti-tumor activity and the mechanism of a green tea (Camellia sinensis) polysaccharide on prostate cancer. Int. J. Biol. Macromol. 2019, 122, 95–103. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.E.; Wang, N.; Liu, X.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; et al. Chemical Composition and Antioxidant Activities of Polysaccharides from Yingshan Cloud Mist Tea. Oxid. Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef] [Green Version]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Loey, A.; Hendrickx, M.E. The effect of high pressure homogenization on pectin: Importance of pectin source and pH. Food Hydrocoll. 2015, 43, 189–198. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zheng, Z.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Zhang, W.; Yuan, S.; Zhou, L.; Wang, L.; Ding, Q.; Wang, D.; Yang, W.; Cai, Z.; et al. Antioxidant capacity and cytotoxicity of sulfated polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 82, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, H.; Zhang, N.; Chen, S.; Zhang, Y. Extrusion treatment for improved physicochemical and antioxidant properties of highmolecular weight polysaccharides isolated from coarse tea. Food Res. Int. 2012, 136, 735–741. [Google Scholar]
- Zhao, Z.Y.; Huangfu, L.T.; Dong, L.L.; Liu, S.L. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind. Crop Prod. 2014, 58, 31–35. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Dong, F.; Liu, X.; Lv, Q.; Liu, F.; Chen, L.; Wang, T.; Wang, Z.; Zhang, Y. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr. Polym. 2016, 140, 461–471. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Liu, M.; Wang, Q.; Li, Y. Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. Int. J. Biol. Macromol. 2018, 112, 326–332. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wang, J.M.; Ouyang, J.M.; Kuang, L. Antioxidant Activities and Repair Effects on Oxidatively Damaged HK-2 Cells of Tea Polysaccharides with Different Molecular Weights. Oxid. Med. Cell. Longev. 2018, 2018, 5297539. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr. Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef]
- Huang, S.Q.; Ding, S.; Fan, L. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of selenium-containing polysaccharides isolated from selenium-enriched tea and its bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef]
- Kardos, N.; Luche, J.L. Sonochemistry of carbohydrate compounds. Carbohydr. Res. 2001, 332, 115–131. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Zhou, C.; Xu, T.; Chen, X.; Wu, Q.; Zhang, K.; Li, Y.; Li, D.; Chen, Y. Effects of ultra-high pressure treatment on structure and bioactivity of polysaccharides from large leaf yellow tea. Food Chem. 2022, 387, 132862. [Google Scholar] [CrossRef]
- Liu, C.M.; Liang, R.H.; Dai, T.T.; Ye, J.P.; Zeng, Z.C.; Luo, S.J.; Chen, J. Effect of dynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocoll. 2016, 57, 55–61. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, C.; Han, Z.; Chen, Z.; Wei, X.; Wang, Y. Comparative analysis of existence form for selenium and structural characteristics in artificial selenium-enriched and synthetic selenized green tea polysaccharides. Int. J. Biol. Macromol. 2020, 154, 1408–1418. [Google Scholar] [CrossRef]
- Ren, Y.; Xiao, W.; Rong, L.; Han, X.; Shen, M.; Liu, W.; Luo, Y.; Xie, J. The role of alkali in sweet potato starch-Mesona chinensis Benth polysaccharide gels: Gelation, rheological and structural properties. Int. J. Biol. Macromol. 2021, 170, 366–374. [Google Scholar] [CrossRef]
- Junker, F.; Michalski, K.; Guthausen, G.; Bunzel, M. Characterization of covalent, feruloylated polysaccharide gels by pulsed field gradient-stimulated echo (PFG-STE)-NMR. Carbohydr. Polym. 2021, 267, 118232. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Chen, Z.; Chen, H. Physicochemical characterization of the oolong tea polysaccharides with high molecular weight and their synergistic effects in combination with polyphenols on hepatocellular carcinoma. Biomed. Pharmacother. 2017, 90, 160–170. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Chen, Z.; Gao, X.; Wang, C.; Panichayupakaranant, P.; Chen, H. Ultrafiltration isolation, physicochemical characterization, and antidiabetic activities analysis of polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2020, 85, 4025–4032. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence stability of emulsions containing globular milk proteins. Adv. Colloid Interface Sci. 2006, 123–126, 259–293. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.C.; Marangoni, A.G. Advances in the application of food emulsifier α-gel phases: Saturated monoglycerides, polyglycerol fatty acid esters, and their derivatives. J. Colloid Interface Sci. 2016, 483, 394–403. [Google Scholar] [CrossRef]
- Desplanques, S.; Renou, F.; Grisel, M.; Malhiac, C. Impact of chemical composition of xanthan and acacia gums on the emulsification and stability of oil-in-water emulsions. Food Hydrocoll. 2012, 27, 401–410. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Liu, C.M.; Guo, X.J.; Liang, R.H.; Liu, W.; Chen, J. Alkylated pectin: Molecular characterization, conformational change and gel property. Food Hydrocoll. 2017, 69, 341–349. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Li, X.; Ma, Z. Effects of acetylation on the emulsifying properties of Artemisia sphaerocephala Krasch. polysaccharide. Carbohydr. Polym. 2016, 144, 531–540. [Google Scholar] [CrossRef]
- Han, L.; Hu, B.; Ma, R.; Gao, Z.; Nishinari, K.; Phillips, G.O.; Yang, J.; Fang, Y. Effect of arabinogalactan protein complex content on emulsification performance of gum arabic. Carbohydr. Polym. 2019, 224, 115170. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.; Meng, H.; Li, W.; Zhang, Y. Characteristics of the emulsion stabilized by polysaccharide conjugates alkali-extracted from green tea residue and its protective effect on catechins. Ind. Crop Prod. 2019, 140, 111611. [Google Scholar] [CrossRef]
- Li, Q.; Shi, J.; Du, X.; McClements, D.J.; Chen, X.; Duan, M.; Liu, L.; Li, J.; Shao, Y.; Cheng, Y. Polysaccharide conjugates from Chin brick tea (Camellia sinensis) improve the physicochemical stability and bioaccessibility of β-carotene in oil-in-water nanoemulsions. Food Chem. 2021, 357, 129714. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Han, Y.; Li, Q.; Wu, L.; Zhang, J.; Zhong, X.; Xie, J.; Shao, S.; Zhang, Y.; et al. Emulsifying Properties of Polysaccharide Conjugates Prepared from Chin-Brick Tea. J. Agric. Food Chem. 2019, 67, 10165–10173. [Google Scholar] [CrossRef]
- Shi, F.; Tian, X.; McClements, D.J.; Chang, Y.; Shen, J.; Xue, C. Influence of molecular weight of an anionic marine polysaccharide (sulfated fucan) on the stability and digestibility of multilayer emulsions: Establishment of structure-function relationships. Food Hydrocoll. 2021, 113, 106418. [Google Scholar] [CrossRef]
- Benjamin, O.; Silcock, P.; Leus, M.; Everett, D.W. Multilayer emulsions as delivery systems for controlled release of volatile compounds using pH and salt triggers. Food Hydrocoll. 2012, 27, 109–118. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Omid, M.; Kalbasi-Ashtari, A. Prediction of the physicochemical properties of spray-dried black mulberry (Morus nigra) juice using artificial neural networks. Food Bioprocess Technol. 2013, 6, 585–590. [Google Scholar] [CrossRef]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional significance and structure–activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two alpha-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef]
- Yang, E.; Cho, J.Y.; Sohn, U.D.; Kim, I.K. Calcium sensitization induced by sodium fluoride in permeabilized rat mesenteric arteries. Korean J. Physiol. Pharmacol. 2010, 14, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.; Huo, J.; Zhaoa, X.; Zheng, J.; Wei, X. Effect of different drying methods on chemical composition and bioactivity of tea polysaccharides. Int. J. Biol. Macromol. 2013, 62, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhang, N.N.; Zhang, H.Y.; Liu, Y.; Lu, Y.M.; Xia, T.; Chen, Y. Characterization, antioxidant and hypoglycemic activities of an acid-extracted tea polysaccharide. Int. J. Polym. Anal. Charact. 2022, 27, 195–204. [Google Scholar] [CrossRef]
- Fan, M.; Zhu, J.; Qian, Y.; Yue, W.; Xu, Y.; Zhang, D.; Yang, Y.; Gao, X.; He, H.; Wang, D. Effect of purity of tea polysaccharides on its antioxidant and hypoglycemic activities. J. Food Biochem. 2020, 44, 13277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Xu, R.; Ye, H.; Sun, Y.; Tu, Y.; Zeng, X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). Food Chem. Toxicol. 2012, 50, 2473–2480. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant activities of different fractions of polysaccharide conjugates from green tea (Camellia Sinensis). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Tian, L.; Yan, Z.; Chao, G.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Xie, B. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J. Agric. Food Chem. 2004, 52, 3333–3336. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, X.; Huang, G. Preparation, structure, and properties of tea polysaccharide. Chem. Biol. Drug Des. 2022, 99, 75–82. [Google Scholar] [CrossRef]
- Liu, M.; Gong, Z.; Liu, H.; Wang, J.; Wang, D.; Yang, Y.; Zhong, S. Structural characterization and anti-tumor activity in vitro of a water-soluble polysaccharide from dark brick tea. Int. J. Biol. Macromol. 2022, 205, 615–625. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhang, D.; Zhang, Y.; Wen, Y.; Li, L.; Zheng, L. Tumoricidal effects of a selenium (Se)-polysaccharide from Ziyang green tea on human osteosarcoma U-2 OS cells. Carbohydr. Polym. 2013, 98, 1186–1190. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Hong, T.; Qi, W.; Zhang, K.; Geng, F.; Nie, S. Lysosome-mediated mitochondrial apoptosis induced by tea polysaccharides promotes colon cancer cell death. Food Funct. 2021, 12, 10524–10537. [Google Scholar] [CrossRef]
- Marco, M.L. Defining how microorganisms benefit human health. Microb. Biotechnol. 2021, 14, 35–40. [Google Scholar] [CrossRef]
- Nunes, S.C.; Serpa, J. Recycling the Interspecific Relations with Epithelial Cells: Bacteria and Cancer Metabolic Symbiosis. Adv. Exp. Med. Biol. 2020, 1219, 77–91. [Google Scholar]
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. 2), 5–14. [Google Scholar] [CrossRef] [Green Version]
- Parlet, C.P.; Brown, M.M.; Horswill, A.R. Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol. 2019, 27, 497–507. [Google Scholar]
- Lee, J.H.; Shim, J.S.; Lee, J.S.; Kim, J.K.; Yang, I.S.; Chung, M.S.; Kim, K.H. Inhibition of pathogenic bacterial adhesion by acidic polysaccharide from green tea (Camellia sinensis). J. Agric. Food Chem. 2006, 54, 8717–8723. [Google Scholar] [CrossRef]
- Ren, D.; Hu, Y.; Luo, Y.; Yang, X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015, 6, 3342–3350. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.; Sun, Y.; Yang, X. Protective effects of Ziyang tea polysaccharides on CCl4-induced oxidative liver damage in mice. Food Chem. 2014, 143, 371–378. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Urbanska, K.; Arnaut, L.G.; Pereira, M.M.; Abreu, A.R.; Simoes, S.; Stochel, G. Biodistribution and photodynamic efficacy of a water-soluble, stable, halogenated bacteriochlorin against melanoma. ChemMedChem 2011, 6, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, B.; Gu, Y. Pharmacological evaluation of tea polysaccharides with antioxidant activity in gastric cancer mice. Carbohydr. Polym. 2012, 90, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Cts, A.; Lmds, A.; Ydra, B.; Nd, A.; Smmp, A.; Gls, A.; Pajg, A.; Mi, A. Polysaccharides from green and black teas and their protective effect against murine sepsis. Food Res. Int. 2013, 53, 780–785. [Google Scholar]
- Monobe, M.; Ema, K.; Tokuda, Y.; Maeda-Yamamoto, M. Enhancement of the phagocytic activity of macrophage-like cells with a crude polysaccharide derived from green tea (Camellia sinensis) extract. J. Agric. Chem. Soc. Jpn. 2010, 74, 1306–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.Z.; Jin, G.M.; Wang, L.K.; Yang, J.F. Effect of tea polysaccharides on immune functions and antioxdative activity in broilers. J. Tea Sci. 2005, 25, 61–64. [Google Scholar]
- Yu, Z.; Shi, Y.T.; Ni, D.J. Effect of enzymatic modification green tea polysaccharide on immune function of the immunosuppressant mice. J. Tea Sci. 2010, 30 (Suppl. 1), 567–572. [Google Scholar]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The impacts of natural polysaccharides on intestinal microbiota and immune responses—A review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, W.; Zhang, X.; Yang, J. Characterization of acidic tea polysaccharides from yellow leaves of Wuyi Rock Tea and their hypoglycemic activity via intestinal flora regulation in rats. Foods 2022, 11, 617. [Google Scholar] [CrossRef]
- Bai, Y.; Zeng, Z.; Xie, Z.; Chen, G.; Chen, D.; Sun, Y.; Zeng, X.; Liu, Z. Effects of polysaccharides from Fuzhuan brick tea on immune function and gut microbiota of cyclophosphamide-treated mice. J. Nutr. Biochem. 2022, 101, 108947. [Google Scholar] [CrossRef]
- Chen, D.; Ding, Y.; Ye, H.; Sun, Y.; Zeng, X. Effect of long-term consumption of tea (Camellia sinensis L.) flower polysaccharides on maintaining intestinal health in BALB/c mice. J. Food Sci. 2020, 85, 1948–1955. [Google Scholar] [CrossRef]
- Wei, X.; Cai, X.; Xiong, S.; Wang, Y. Hypoglycemic effect of oral crude tea flower polysaccharides on alloxan modeling Sprague-Dawley rats and the possible mechanism. CyTA-J. Food 2012, 10, 325–332. [Google Scholar] [CrossRef]
- Deng, Y.T.; Lin-Shiau, S.Y.; Shyur, L.F.; Lin, J.K. Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food Funct. 2015, 6, 1539–1546. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Wu, T.; Guo, Y.; Liu, R.; Wang, K.; Zhang, M. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2469–2478. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Chen, Y.; Liu, R.; Zhang, M. Oolong tea polysaccharide and polyphenols prevent obesity development in Sprague-Dawley rats. Food Nutr. Res. 2018, 62, 10. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yang, J.; Li, M.; Yang, X.; Wang, P.; Xu, J. Protective effects of Chinese Fenggang zinc selenium tea on metabolic syndrome in high-sucrose-high-fat diet-induced obese rats. Sci. Rep. 2018, 8, 3528. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Q.; Zhang, T.; Chen, X.; Cheng, Y. Antibacterial activity and mechanism of green tea polysaccharide conjugates against Escherichia coli. Ind. Crop Prod. 2020, 152, 112464. [Google Scholar] [CrossRef]
- Sun, H. Evaluation of antioxidant activity of polysaccharides isolated from Camellia sinensis (tea) in exhausting training mice. J. Med. Plant Res. 2011, 5, 791–795. [Google Scholar]
- Wang, Y.; Zhao, Y.; Andrae-Marobela, K.; Okatch, H.; Xiao, J. Tea polysaccharides as food antioxidants: An old woman’s tale? Food Chem. 2013, 138, 1923–1927. [Google Scholar] [CrossRef]
- Williams, S.N.; Shih, H.; Guenette, D.K.; Brackney, W.; Denison, M.S.; Pickwell, G.V.; Quattrochi, L.C. Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem. Biol. Interact. 2000, 128, 211–229. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Yang, W.; Ren, D.; Zhao, Y.; Liu, L.; Yang, X. Fuzhuan Brick Tea Polysaccharide Improved Ulcerative Colitis in Association with Gut Microbiota-Derived Tryptophan Metabolism. J. Agric. Food Chem. 2021, 69, 8448–8459. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, Z.H.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. An insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol. Nutr. Food Res. 2021, 65, 2000461. [Google Scholar] [CrossRef]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel alpha-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef]
- Chen, H.; Min, Z.; Xie, B. Components and antioxidant activity of polysaccharide conjugate from green tea. Food Chem. 2005, 90, 17–21. [Google Scholar] [CrossRef]
- Chen, G.; Chen, R.; Chen, D.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Tea polysaccharides as potential therapeutic options for metabolic diseases. J. Agric. Food Chem. 2019, 67, 5350–5360. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef]
- Mao, Y.; Wei, B.; Teng, J.; Xia, N.; Zhao, M.; Huang, L.; Ye, Y. Polysaccharides from Chinese Liupao dark tea and their protective effect against hyperlipidemia. Int. J. Food Sci. Technol. 2018, 53, 599–607. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Duan, X.; Tang, T.; Ke, Y.; Zhang, L.; Li, C.; Liu, A.; Su, Z.; Hu, B. Antioxidant and anticoagulant activities of mycelia polysaccharides from Catathelasma ventricosum after sulfated modification. Ind. Crop Prod. 2018, 112, 53–60. [Google Scholar] [CrossRef]
- Feng, Y.; Li, W.; Wu, X.; Liang, H.; Ma, S. Rapid and efficient microwave-assisted sulfate modification of lentinan and its antioxidant and antiproliferative activities in vitro. Carbohydr. Polym. 2010, 82, 605–612. [Google Scholar] [CrossRef]
- Bowe, W.; Patel, N.B.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis: From anecdote to translational medicine. Benef. Microbes 2014, 5, 185–199. [Google Scholar] [CrossRef]
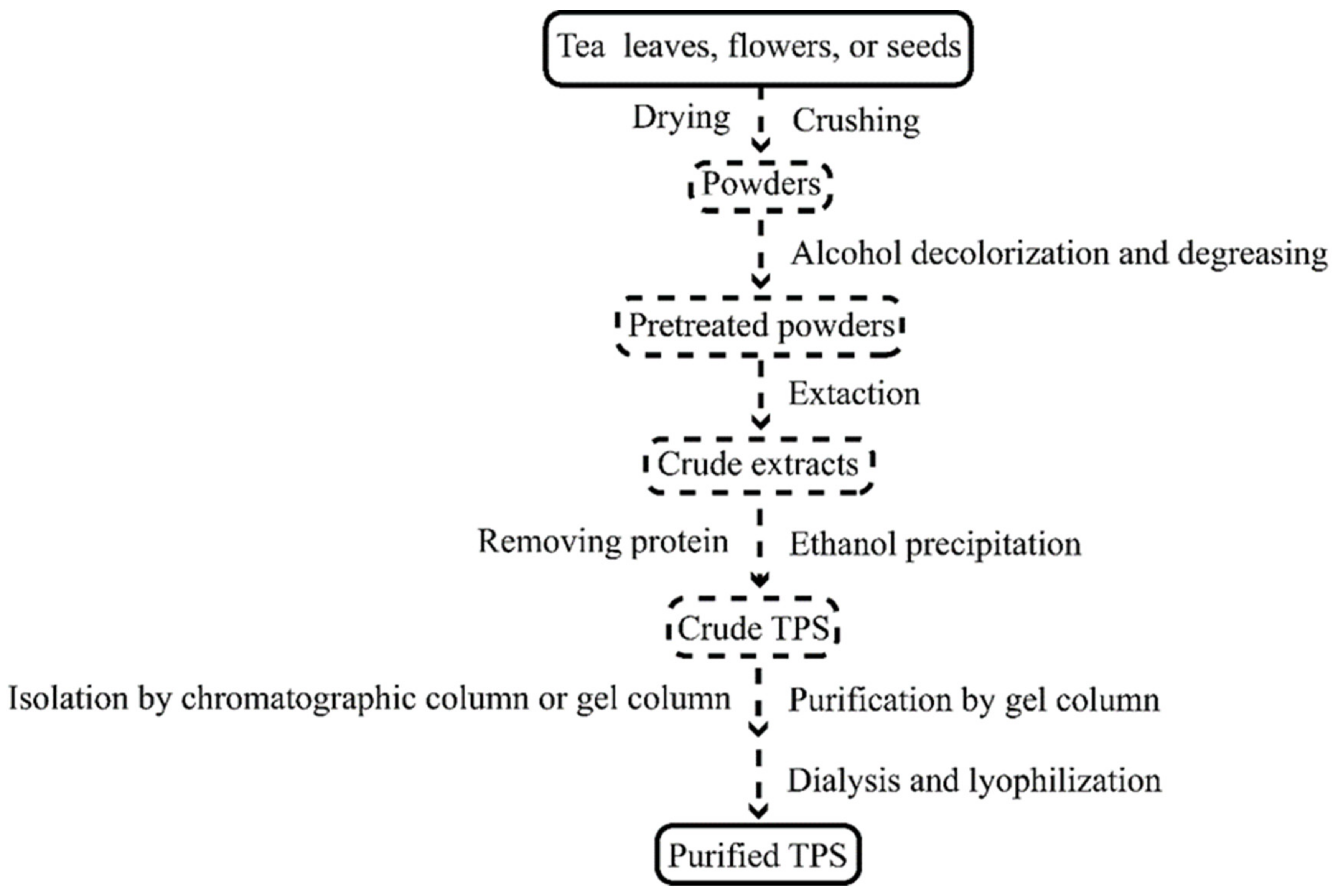

| Extraction Method | TPS Origin | Extraction Step | Ref |
|---|---|---|---|
| Hot water extraction | Green tea leaves and flowers | Pre-extraction with 95% ethanol at 40 °C for 2 h, repeated three times; a water bath extraction at 60 °C for 2 h, repeated 3 times | [14] |
| Fuan Baicha and Pingyang Tezaocha | Extraction at 80 °C for 1.5 h, repeated two times | [15] | |
| Fuzhuan tea | 2 h extraction time, 1:20 solid–liquid ratio, and 95 °C extraction temperature; repeated three times | [10] | |
| White tea | 8 min extraction time, 54.1 °C extraction temperature, 12.48 L/g material–water ratio; repeated four times | [16] | |
| Green tea | Heating in a water bath at 90 °C for 2 h with continuous stirring | [17] | |
| Green tea | Pre-extraction with absolute ethanol for 24 h and extraction with deionized water at 60 °C for 90 min | [18] | |
| Chin brick tea | 80% ethanol pretreatment and continuous stirring with distilled water (1:20, w/v) at 90 °C for 2 h | [19] | |
| Liupao tea | 80% ethanol pretreatment for 24 h and extraction with deionized water at 70 °C for 2 h; repeated three times | [20] | |
| Tea flowers | Extraction at 90 °C for 1 h (2 times) | [21] | |
| Green tea | 80% ethanol pretreatment at 70 °C for 1.5 h, extraction with ethanol at 40 °C for 3 h | [22] | |
| Green tea | Pretreatment with two times volume of 95% ethanol at 50 °C for 4 h, 1:8 solid–liquid ratio, and extraction with stirring at 50 °C for 120 min | [23] | |
| Green tea | Pretreatment with 95% alcohol (1:5, w/v) for 2 h, extraction in hot water (1:10, w/v) at 80 °C; repeated 3 times for 1 h each time | [24] | |
| Green tea | 95% ethanol (1:6, w/v) pretreatment at 60 °C for 4 h and extraction with distilled water (1:10, w/v) at 80 °C for 4 h; repeated 3 times | [25] | |
| Keemun black tea | Pretreatment with 95% ethanol (1:6, w/v) at 80 °C for 2 h and immersed in distilled water (1:10, w/v) at 80 °C for 4 h; repeated four times | [26] | |
| Ultrasonic-assisted extraction | Low-grade green tea | 80 °C extraction temperature, 60 min extraction time, 400 W ultrasonic power, and 22 mL:g liquid–solid ratio | [27] |
| Coarse tea | Pretreatment in an ultrasonic bath (50 °C, 200 W) for 30 min followed by extraction in a water bath for 90 min; repeated three times | [23] | |
| Green tea flowers | Ultrasonic power (25 °C, 100, 150, 200, 250, and 300 W) extraction for 5 min; repeated 2 times | [21] | |
| Yellow tea | 95% ethanol pretreatment for 6 h, 90 °C water bath extraction for 55 min (repeated twice), and sonication (20 kHz, 500 W) for 55 min | [21] | |
| Microwave-assisted extraction | Green, black, and oolong teas | 1:20 solid/liquid ratio, 200–230 °C extraction temperature, and 2 min extraction time | [28] |
| Green tea flowers | Extraction at controlled microwave power for 5 min followed by extraction with distilled water for 5 min at the same microwave power | [21] | |
| Green tea | Extraction in a 600 W microwave apparatus for 30 min, followed by stirring in a water bath for 90 min; repeated three times | [29] | |
| Enzymolysis extraction | Green tea | Extraction at 100 °C for 3 h and aqueous extraction with pectinase and tannase at 35 °C for 2 h | [30] |
| Green tea | Extraction with complex enzymes (cellulase:pectinase:glucanase = 1:1:2) at 50 °C for 30 min, boiling at 90 °C for 10 min, and then extraction in a water bath at 50 °C for 80 min | [29] | |
| Green tea leaves and flowers | 95% ethanol pretreatment at 40 °C for 2 h (repeated 3 times), treatment with 0.5% (m/v) pentosan complex enzyme solution (45 °C, pH 5.5) for 2 h, and extraction in 45 °C water bath for 2 h | [14] | |
| Green tea | Heating in a water bath at 90 °C for 2–4 h, repeated twice; incubating with 0.5% pectinase (260,001 PGU/mL, v/w) at 40 °C for 30 min; and heating at 90 °C for 1 h to inactivate the enzyme | [31] | |
| Hydro/solvothermal extraction | Chinese tea Zhongcha 108 | Extraction at 120 °C for 1 h | [1] |
| Alkali-assisted extraction | Fuzhuan brick tea | Extraction with 0.1 M NaOH solution (pH = 10.0) at 60 °C, repeated 3 times | [32] |
| Supercritical fluid extraction | Green tea | 380 μm particle size, 20% absolute ethanol, 35 MPa extraction pressure, 45 °C extraction temperature, and 2 h extraction time | [33] |
| Anionic reverse micelle extraction | Green tea | pH = 4.6, 0.06 M guanidine hydrochloride, 7% methanol, and 0.05 M NaCl; forward extraction | [34] |
| TPS Origin | Monosaccharide Composition and Molar Ratio | Ref |
|---|---|---|
| Green tea | WE, Rha: Ara: Gal: Glc: Xyl: Man: Fru: GalA = 4.11: 9.96: 28.05: 29.22: 3.46: 4.62: 4.14: 16.43, respectively; UAE, 2.27: 9.22: 27.54: 36.05: 5.38: 4.75: 6.72: 8.07, respectively; MAE, 4.03: 11.84: 27.06: 31.09: 3.64: 6.17: 6.84: 9.33, respectively; EE, 5.40: 8.86: 12.32: 44.24: 3.15: 4.38: 11.78: 9.87, respectively | [21] |
| Green tea | Ara: Xyl: Fuc: Glc: Gal = 6.49: 2.60: 6.53: 43.27: 41.11, respectively; | [46] |
| Natural and artificial selenium-enriched green teas | ASe-TPS2, Rha: Ara: Glc: Xyl: GalA = 1.93: 7.05: 1.00: 1.05: 26.12, respectively; NSe-TPS2, Ara: Gal: GluA: GalA = 0.59: 1.00: 0.49: 1.24, respectively | [47] |
| Selenium-enriched green tea | Se-TPS1, Fuc: Rha: Ara: Gal: Glc: GlcA: GalA = 0.07: 0.21: 0.58: 1.00: 0.47: 0.17: 1.75, respectively; Se-TPS2, Fuc: Rha: Ara: Gal: Glc: GlcA: GalA = 0.07: 0.28: 0.59: 1.00: 0.10: 0.49: 1.24, respectively; Se-TPS3, Fuc: Rha: Ara: Gal: Glc: GlcA: GalA = 0.07: 0.38: 0.72: 1.00: 0.30: 0.19: 0.88, respectively | [48] |
| Fuzhuan brick tea | FBTPS, Rib: Man: Ara: Rha: Gal: Glc: GlcA: GalA = 1.69: 3.66: 11.83: 12.11: 19.15: 21.97: 1.41: 28.17, respectively | [52] |
| Fuzhuan brick tea | FBTPS-3, Man: Rha: GalA: Gal: Ara = 8.7: 15.5: 42.2: 19.7: 13.9, respectively | [53] |
| Green tea | GTP consisting only of Glc | [54] |
| Yingshan Cloud Mist green tea | GTPS, Rha: Ara: Xyl: Man: Glc: and Gal = 11.4: 26.1: 1.9: 3.0: 30.7: 26.8, respectively | [55] |
| Selenium-enriched green tea | SeTPS-1, Glc: Gal = 80.1: 2.3; SeTPS-2, Glc: Gal = 80.1: 2.3, respectively | [56] |
| Yellow tea | YTPS-N, Man: Rib: Rha: GlcA: GalA: Glc: Gal: Ara = 1.65: 1: 10.95: 1.06: 2.03: 5.49: 3.50: 4.02; YTPS-U, 1.72: 1: 11.05: 1.09: 2.13: 5.36: 3.62: 4.17, respectively | [26] |
| Large-leaf yellow tea | LYTP, Ara: Gal: GalA: Rha: Glc: GlcA: Man | [57] |
| Bioactivity | TPS Origin | Regulatory Mechanism | Ref |
|---|---|---|---|
| Antioxidant and hepatoprotective activity | Ziyang green tea | Ameliorating high-fructose diet-induced pancreatic β-cell damage and inhibiting hepatic steatosis and oxidative damage | [112] |
| Ziyang green tea | Mediating antioxidant and free radical scavenging, thereby effectively preventing liver damage | [113] | |
| Green tea | Promoting superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activity in blood, liver, and heart | [114] | |
| Huangshan Maofeng | Inhibiting lipid peroxidation while promoting the body’s antioxidant activity to protect the liver | [23] | |
| Longjing 43 tea flower | Inhibiting the elevation of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, reducing the formation of malondialdehyde (MDA), and simultaneously enhancing the activities of SOD and GPx to reduce liver damage | [98] | |
| Keemun black tea | Improving the enzymatic and non-enzymatic antioxidant defense system to protect the liver, thereby effectively alleviating the production of free radicals in the body and inhibiting lipid peroxidation in liver tissue | [24] | |
| Antitumor activity | Dark brick tea | Inhibiting cancer cell proliferation and migration and inducing cancer cell apoptosis | [103] |
| Ziyang green tea | Inhibiting the proliferation of human osteosarcoma cells (U-2 OS) | [104] | |
| Green tea | Targeting lysosomes and activated caspase-9/-3 via the lysosome-mitochondrial pathway to induce apoptosis in colon cancer cells (CT26) | [105] | |
| Tea flowers | Inhibiting the proliferative activity of human gastric cancer cells (BGC-823) | [98] | |
| Green tea | Increasing the levels of SOD, CAT, and GPx while inhibiting lipid peroxidation and pro-inflammatory cytokine levels from attenuating oxidative damage and inflammatory responses | [115] | |
| Ziyang green tea | Inhibiting the proliferation of osteosarcoma cells in vitro and the growth of tumor volume and tumor weight in vivo | [104] | |
| Green and black teas | Inhibiting pulmonary neutrophil recruitment and oxidative tissue damage, resulting in higher anti-inflammatory effects and resistance to murine sepsis | [116] | |
| Oolong tea | Inhibiting tumor growth, reducing liver toxicity and nephrotoxicity, stimulating the body’s antioxidant activity and immune function, and finally achieving an anti-liver cancer effect | [75] | |
| Green tea | Increasing the Bcl2-associated X protein (Bax)/B-cell lymphoma-2 (Bcl-2) ratio, elevating caspase-3 protein expression, and decreasing miR-93 expression in prostate cancer cells | [54] | |
| Immunostimulatory activity | Green tea | Activating the TLR7 receptor and enhancing the macrophage activity | [117] |
| Green tea | Improving the serum IgG level, thymus index, macrophage activity, and lymphocyte transformation rate in broilers, as well as increasing the serum antioxidant enzyme activity | [118] | |
| Selenium-enriched green tea | Enhancing the regulatory mechanism involved in free radical scavenging, synergistically improving immune function, and reducing oxidative stress | [9] | |
| Green tea | Enhancing the body’s cellular immunity and humoral immunity | [119] | |
| Fuzhuan brick tea | In vitro: promoting the in vitro proliferation activity and phagocytic capability of macrophages and enhancing the activity of acid phosphatase; in vivo: promoting the release of tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and nitric oxide (NO) and then inhibiting decreases in thymus/spleen index and colon rupture | [30] | |
| Selenium-enriched green tea | Improving the spleen and thymus index, promoting the lymphocyte proliferation and NK cell activity in the spleen, promoting the CD4 T cell proliferation, and reducing oxidative stress | [9] | |
| Gut microbiota modulating activity | Fuzhuan brick tea | Reducing the disease activity index (DAI) in mice with enteritis, alleviating the colonic tissue damage and inflammation, and simultaneously promoting the proliferation of beneficial gut microbiota and the increase in short-chain fatty acids (SCFAs) | [120] |
| Wuyi rock tea | Improving gut microbiota composition and microbial structural dysbiosis in type 2 diabetic rats | [121] | |
| Fuzhuan brick tea | Promoting the secretion and mRNA expression of mucin 2, occludin, and zonula occludens 1 (ZO-1); altering gut microbiota composition; and stimulating the proliferation of beneficial bacteria and production of SCFAs | [30] | |
| Fuzhuan brick tea | Increasing the phylogenetic diversity of the gut microbiota, suppressing the increase in the relative abundance of pathogenic bacteria, and altering key OUTs associated with metabolic syndrome | [8] | |
| Fuzhuan brick tea | Altering the gut morphology and ZO-1 expression, increasing the relative abundance of Muribaculaceae, and decreasing the relative abundance of Lachnospiraceae, Helicobacteraceae, and Clostridaceae | [122] | |
| Tea flowers | Protecting the intestinal barrier function and promoting the increase in the number of beneficial microorganisms and their metabolites, thereby maintaining intestinal health and improving adaptive intestinal immunity | [123] | |
| Glucose and lipid metabolism-regulating activity | Pu-erh tea | Inhibiting intestinal alpha-glucosidase activity | [36] |
| Green, oolong, and black teas | Enhancing in vitro free radical scavenging activity and α-glucosidase inhibition in skeletal muscle cells | [76] | |
| Tea flowers | Protecting cell membranes from peroxidative damage and reducing oxidative stress | [124] | |
| Pu-erh tea | Inhibiting the intestinal alpha-glucosidase activity | [125] | |
| Green tea | Adjusting body weight, reducing serum triglyceride (TG) and leptin (LT) levels, inhibiting fatty acid absorption, improving anti-inflammatory activity, and treating obesity | [126] | |
| Black tea | Inhibiting the formation and accumulation of fat, promoting the decomposition of fat, and promoting the expression of essential genes involved in fat metabolism | [127] | |
| Oolong tea | Decreasing serum LT levels in obese rats, improving blood lipids and antioxidant levels, and affecting lipid metabolism pathways | [128] | |
| Fenggang zinc selenium tea | Improving oxidative stress, inhibiting lipid peroxidation, and enhancing liver protection | [129] | |
| Anticoagulant activity | Green tea | Inhibiting the intrinsic and common coagulation pathways of fibrinogen-to-fibrin conversion without inhibiting the extrinsic pathway | [16] |
| Bacteriostatic activity | Green tea | Destroying the cell wall of Escherichia coli and increasing the permeability of the cell membrane and the content of intracellular ROS | [130] |
| Anti-fatigue activity | Ziyang green tea | Preventing lipid peroxidation by modification of GPx activity | [22] |
| Skincare activity | Green tea | Promoting skin’s moisturization and enhancing fibroblast proliferation capability | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Sun, Y.; Qian, L. Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits. Polymers 2022, 14, 2775. https://doi.org/10.3390/polym14142775
Wang Q, Yang X, Zhu C, Liu G, Sun Y, Qian L. Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits. Polymers. 2022; 14(14):2775. https://doi.org/10.3390/polym14142775
Chicago/Turabian StyleWang, Qian, Xiaoyan Yang, Changwei Zhu, Guodong Liu, Yujun Sun, and Lisheng Qian. 2022. "Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits" Polymers 14, no. 14: 2775. https://doi.org/10.3390/polym14142775
APA StyleWang, Q., Yang, X., Zhu, C., Liu, G., Sun, Y., & Qian, L. (2022). Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits. Polymers, 14(14), 2775. https://doi.org/10.3390/polym14142775





