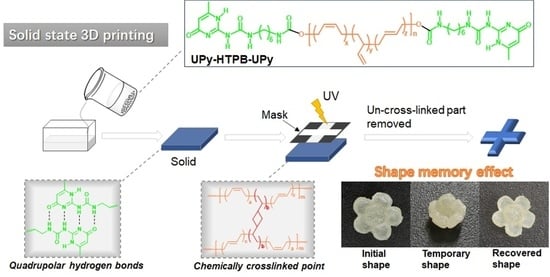
Room-Temperature Solid-State UV Cross-Linkable Vitrimer-like Polymers for Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Experimental
2.1. Materials and Synthesis
2.2. Characterization
- (i)
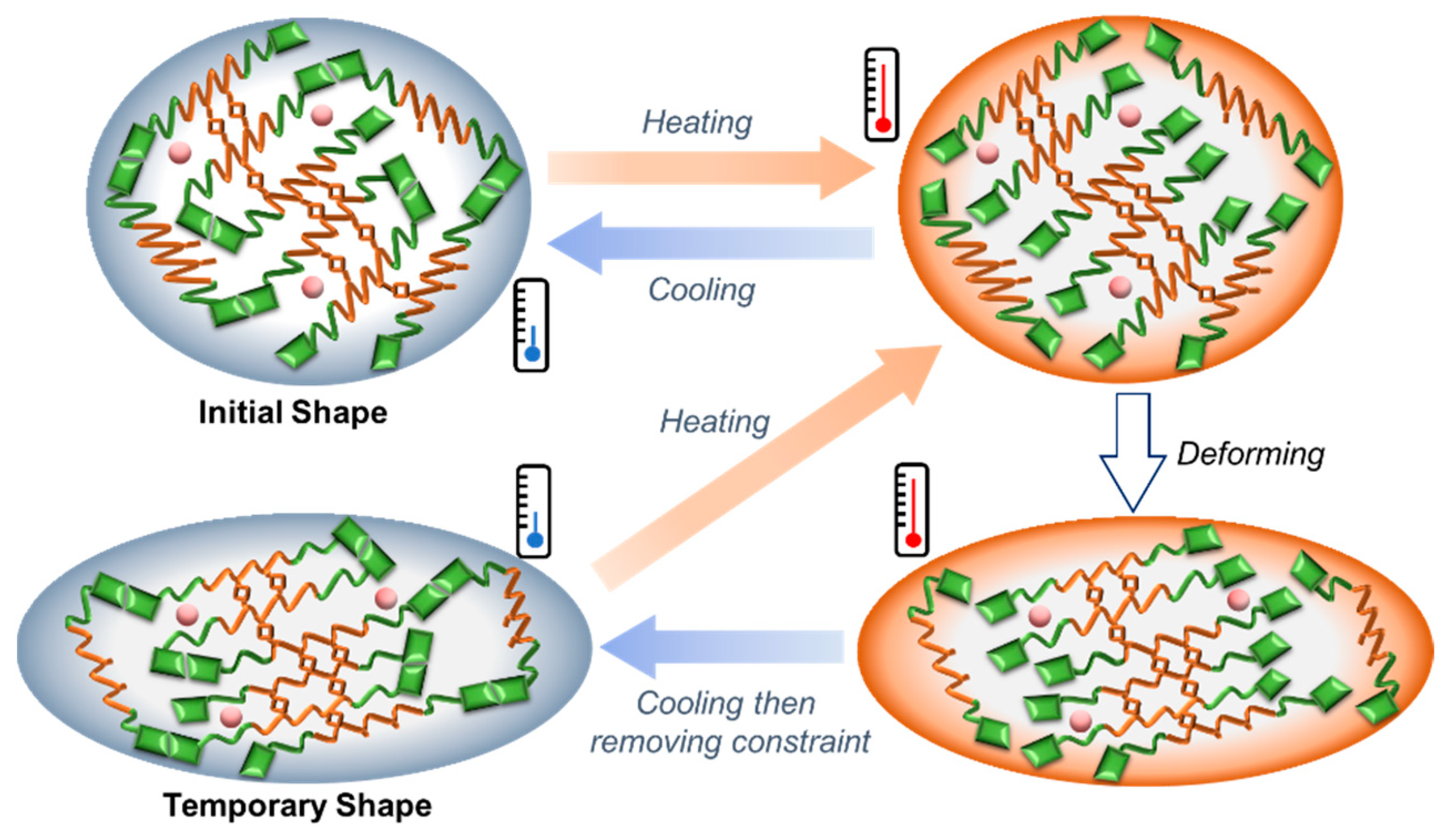
- The original strain is ε1 (0 in the first cycle). Heat the sample to 90 °C (the temperature that quadruple hydrogen bonding breaks) and hold for two minutes. After loading, the corresponding strain is ε1.
- (ii)
- Cool the sample to room temperature. Five minutes later, unload. The resulted strain is ε2.
- (iii)
- Heat to 90 °C for recovery. The final strain is recorded to be ε3.
3. Results and Analysis
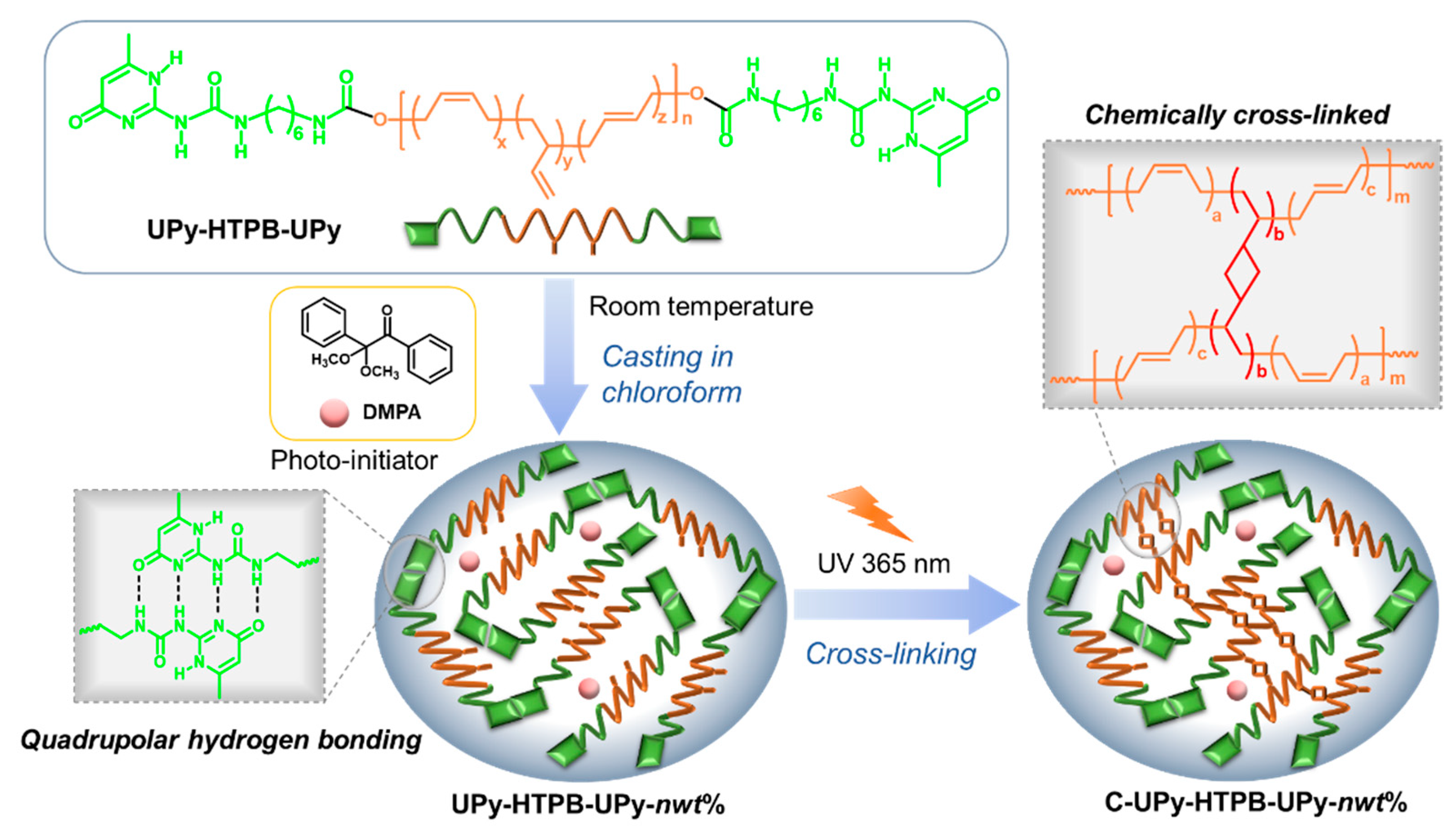
3.1. Synthesis of C-UPy-HTPB-UPy
3.1.1. Quadrupolar Hydrogen Bond
3.1.2. UV Cross-Linking
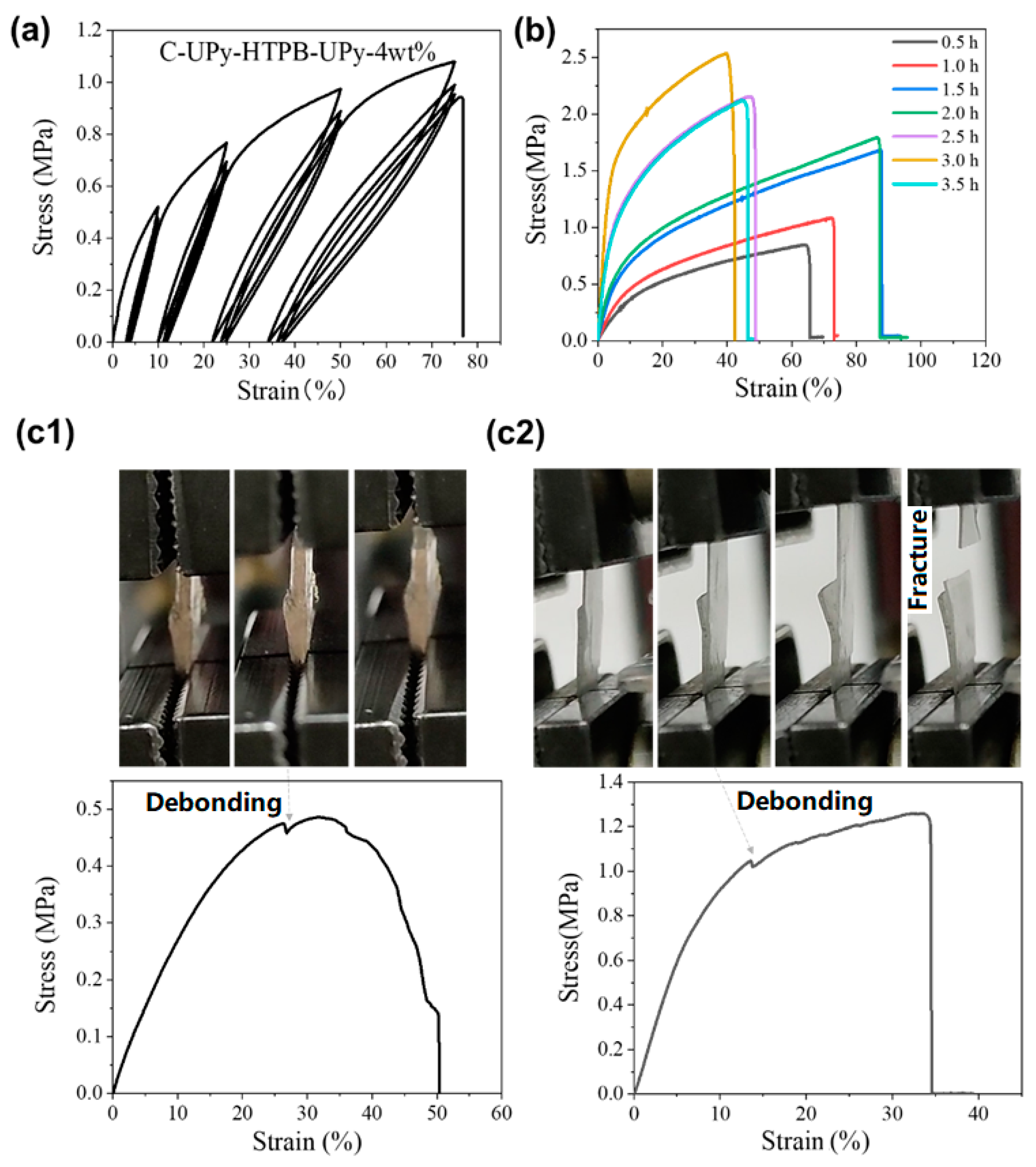
3.2. Mechanical Properties
3.2.1. Effect of Photo-Initiator Content and Curing Time
3.2.2. Bonding
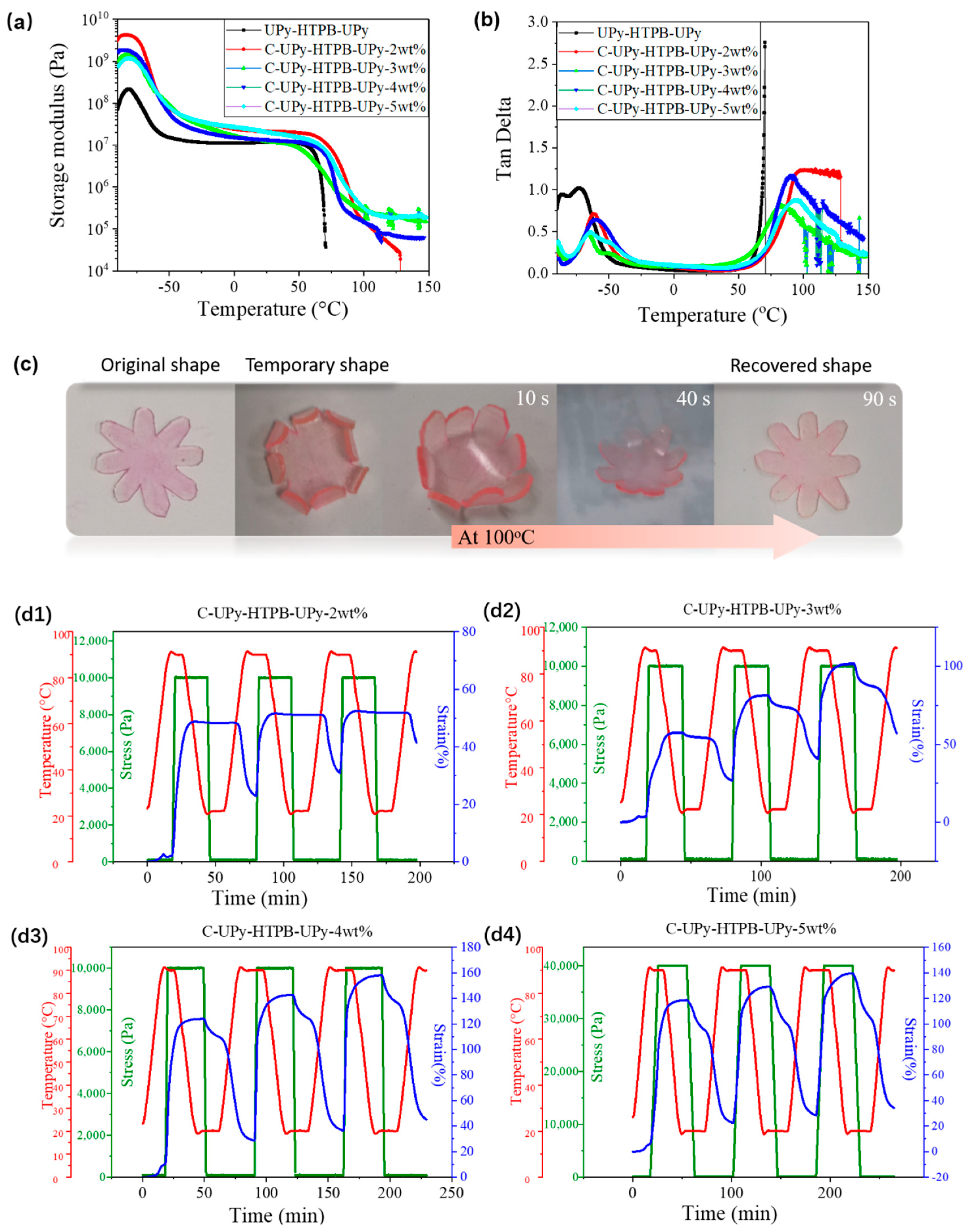
3.3. DMA and Shape Memory Behavior of C-UPy-HTPB-UPy
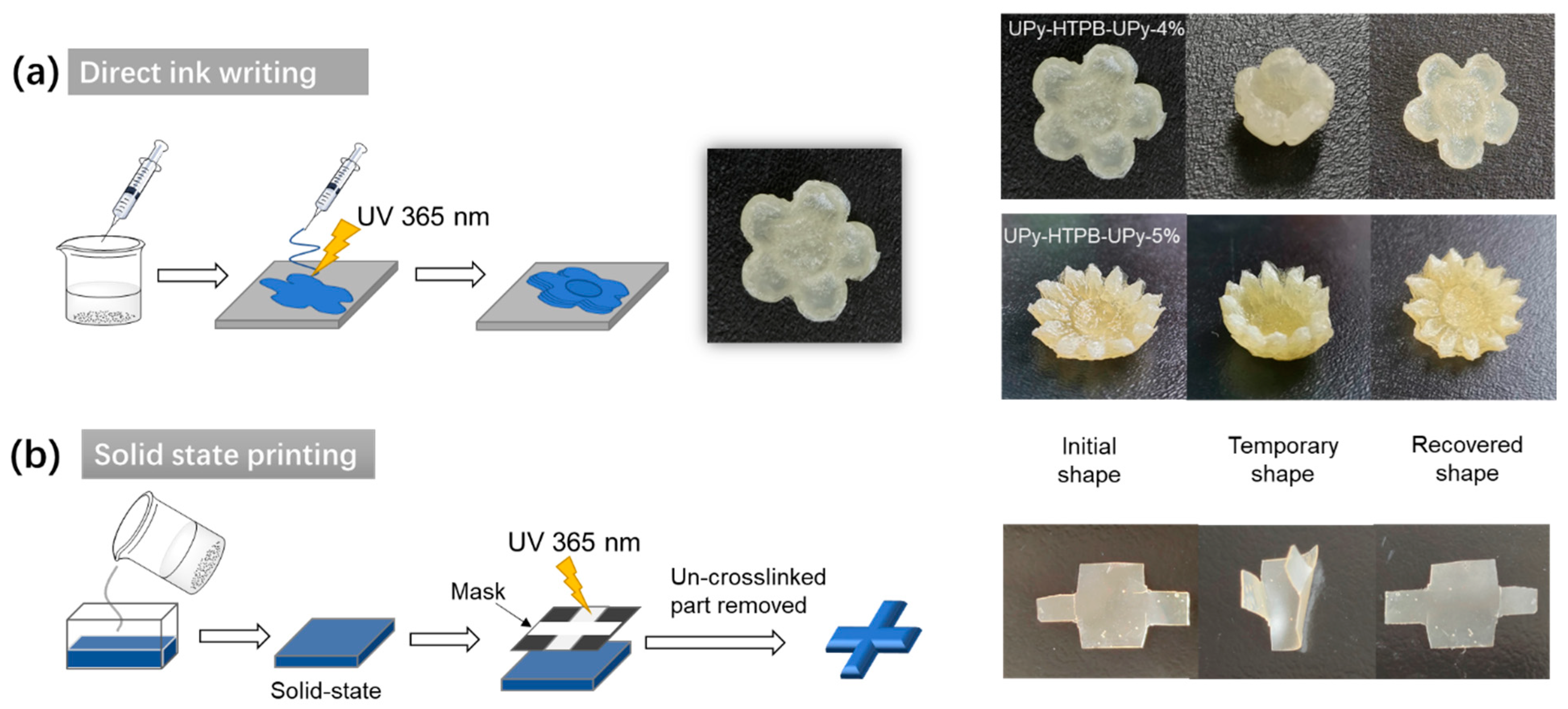
4. Potential Applications in Additive Manufacturing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kuang, X.; Roach, D.J.; Wu, J.; Hamel, C.M.; Ding, Z.; Wang, T.; Dunn, M.L.; Qi, H.J. Advances in 4D Printing Materials and Applications. Adv. Funct. Mater. 2018, 29, 1805290. [Google Scholar] [CrossRef]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, C.; Luo, H.; Huang, W.; Yang, Z.; Chen, X.; He, J. Method for 3D Printing of Polymer in Condensed State. CN111070673A, 24 December 2019. [Google Scholar]
- Salvekar, A.V.; Nasir, F.H.B.A.; Chen, Y.H.; Maiti, S.; Ranjan, V.D.; Chen, H.M.; Wang, H.; Huang, W.M. Rapid Volumetric Additive Manufacturing in Solid State: A Demonstration to Produce Water-Content-Dependent Cooling/Heating/Water-Responsive Shape Memory Hydrogels. 3d Print. Addit. Manuf. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Huang, W.M.; Zhao, Y.; Wang, C.C.; Ding, Z.; Purnawali, H.; Tang, C.; Zhang, J.L. Thermo/chemo-responsive shape memory effect in polymers: A sketch of working mechanisms, fundamentals and optimization. J. Poly. Res. 2012, 19, 9952. [Google Scholar] [CrossRef]
- Wang, T.X.; Chen, H.M.; Salvekar, A.V.; Lim, J.; Chen, Y.; Xiao, R.; Huang, W.M. Vitrimer-Like Shape Memory Polymers: Characterization and Applications in Reshaping and Manufacturing. Polymers 2020, 12, 2330. [Google Scholar] [CrossRef]
- Lei, L.; Han, L.; Ma, H.; Zhang, R.; Li, X.; Zhang, S.; Li, C.; Bai, H.; Li, Y. Well-Tailored Dynamic Liquid Crystal Networks with Anionically Polymerized Styrene-Butadiene Rubbers toward Modulating Shape Memory and Self-Healing Capacity. Macromolecules 2021, 54, 2691–2702. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.; Yoo, J.; Choi, S.-H.; Char, K. Self-Healable Antifreeze Hydrogel Based on Dense Quadruple Hydrogen Bonding. Macromolecules 2021, 54, 6389–6399. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wei, W.; Yang, F.; Wu, M.; Wu, Q.; Xie, T.; Chen, Y. Enhanced Mechanochemiluminescence from End-Functionalized Polyurethanes with Multiple Hydrogen Bonds. Macromolecules 2021, 54, 1557–1563. [Google Scholar] [CrossRef]
- Jiang, Z.-C.; Xiao, Y.-Y.; Kang, Y.; Pan, M.; Li, B.-J.; Zhang, S. Shape Memory Polymers Based on Supramolecular Interactions. ACS Appl. Mater. Interfaces 2017, 9, 20276–20293. [Google Scholar] [CrossRef]
- Lamers, B.A.G.; van der Tol, J.J.B.; Vonk, K.M.; de Waal, B.F.M.; Palmans, A.R.A.; Meijer, E.W.; Vantomme, G. Consequences of Molecular Architecture on the Supramolecular Assembly of Discrete Block Co-oligomers. Macromolecules 2020, 53, 10289–10298. [Google Scholar] [CrossRef]
- Scavuzzo, J.; Tomita, S.; Cheng, S.; Liu, H.; Gao, M.; Kennedy, J.P.; Sakurai, S.; Cheng, S.Z.D.; Jia, L. Supramolecular Elastomers: Self-Assembling Star–Blocks of Soft Polyisobutylene and Hard Oligo(β-alanine) Segments. Macromolecules 2015, 48, 1077–1086. [Google Scholar] [CrossRef]
- Gasperini, A.; Wang, G.-J.N.; Molina-Lopez, F.; Wu, H.-C.; Lopez, J.; Xu, J.; Luo, S.; Zhou, D.; Xue, G.; Tok, J.B.H.; et al. Characterization of Hydrogen Bonding Formation and Breaking in Semiconducting Polymers under Mechanical Strain. Macromolecules 2019, 52, 2476–2486. [Google Scholar] [CrossRef]
- Cao, K.; Liu, G. Low-Molecular-Weight, High-Mechanical-Strength, and Solution-Processable Telechelic Poly(ether imide) End-Capped with Ureidopyrimidinone. Macromolecules 2017, 50, 2016–2023. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, L.R.; Li, S.; Sturgess, C.; Wildman, R.; Jones, J.R.; Hayes, W. 3D Printing of Biocompatible Supramolecular Polymers and their Composites. ACS Appl. Mater. Interfaces 2016, 8, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Folmer, B.J.B.; Sijbesma, R.P.; Versteegen, R.M.; van der Rijt, J.A.J.; Meijer, E.W. Supramolecular Polymer Materials: Chain Extension of Telechelic Polymers Using a Reactive Hydrogen-Bonding Synthon. Adv. Mater. 2000, 12, 874–878. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Xie, H.; Xu, Z.Y.; Li, L.; Wang, Y.Z. 4D Printing of Shape Memory Aliphatic Copolyester via UV-assisted FDM Strategy for Medical Protective Devices. Chem. Eng. J. 2020, 396, 125242. [Google Scholar] [CrossRef]
- Zhou, Q.; Jie, S.; Li, B.-G. Facile synthesis of novel HTPBs and EHTPBs with high cis-1,4 content and extremely low glass transition temperature. Polymer 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, W.; Zhang, X.; Chen, J.; Yuan, J.; Deng, J. Chemical Modification of Hydroxyl-Terminated Polybutadiene and Its Application in Composite Propellants. Ind. Eng. Chem. Res. 2021, 60, 3819–3829. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, Q.; Jie, S.; Li, B.-G. High cis-1,4 Hydroxyl-Terminated Polybutadiene-Based Polyurethanes with Extremely Low Glass Transition Temperature and Excellent Mechanical Properties. Ind. Eng. Chem. Res. 2016, 55, 1582–1589. [Google Scholar] [CrossRef]
- Bai, J.; Li, H.; Shi, Z.; Yin, J. An Eco-Friendly Scheme for the Cross-Linked Polybutadiene Elastomer via Thiol–Ene and Diels–Alder Click Chemistry. Macromolecules 2015, 48, 3539–3546. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wen, Y.; Zeng, L.; Wang, X.; Chen, H.; Huang, W.M.; Bai, Y.; Yu, W.; Zhao, K.; Hu, P. Room-Temperature Solid-State UV Cross-Linkable Vitrimer-like Polymers for Additive Manufacturing. Polymers 2022, 14, 2203. https://doi.org/10.3390/polym14112203
Chen J, Wen Y, Zeng L, Wang X, Chen H, Huang WM, Bai Y, Yu W, Zhao K, Hu P. Room-Temperature Solid-State UV Cross-Linkable Vitrimer-like Polymers for Additive Manufacturing. Polymers. 2022; 14(11):2203. https://doi.org/10.3390/polym14112203
Chicago/Turabian StyleChen, Jian, Ya Wen, Lingyi Zeng, Xinchun Wang, Hongmei Chen, Wei Min Huang, Yuefeng Bai, Wenhao Yu, Keqing Zhao, and Ping Hu. 2022. "Room-Temperature Solid-State UV Cross-Linkable Vitrimer-like Polymers for Additive Manufacturing" Polymers 14, no. 11: 2203. https://doi.org/10.3390/polym14112203
APA StyleChen, J., Wen, Y., Zeng, L., Wang, X., Chen, H., Huang, W. M., Bai, Y., Yu, W., Zhao, K., & Hu, P. (2022). Room-Temperature Solid-State UV Cross-Linkable Vitrimer-like Polymers for Additive Manufacturing. Polymers, 14(11), 2203. https://doi.org/10.3390/polym14112203