Influence of Nanoparticles on the Dielectric Response of a Single Component Resin Based on Polyesterimide
Abstract
1. Introduction
2. Materials and Methods
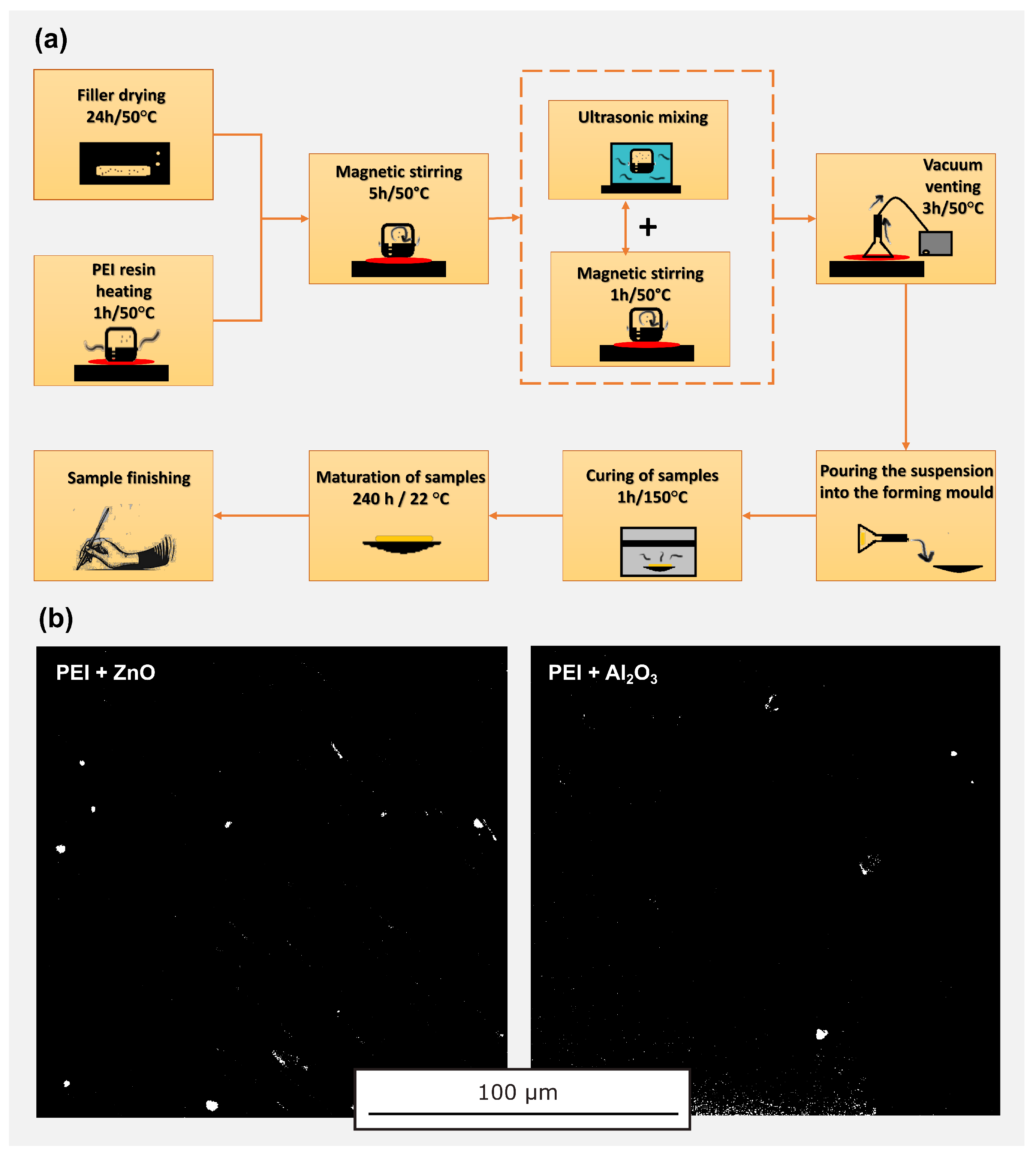
2.1. Base Materials and Sample Preparation
2.2. NMR Measurements
2.3. DMA Measurements
2.4. Dielectric Response Measurement
3. Results
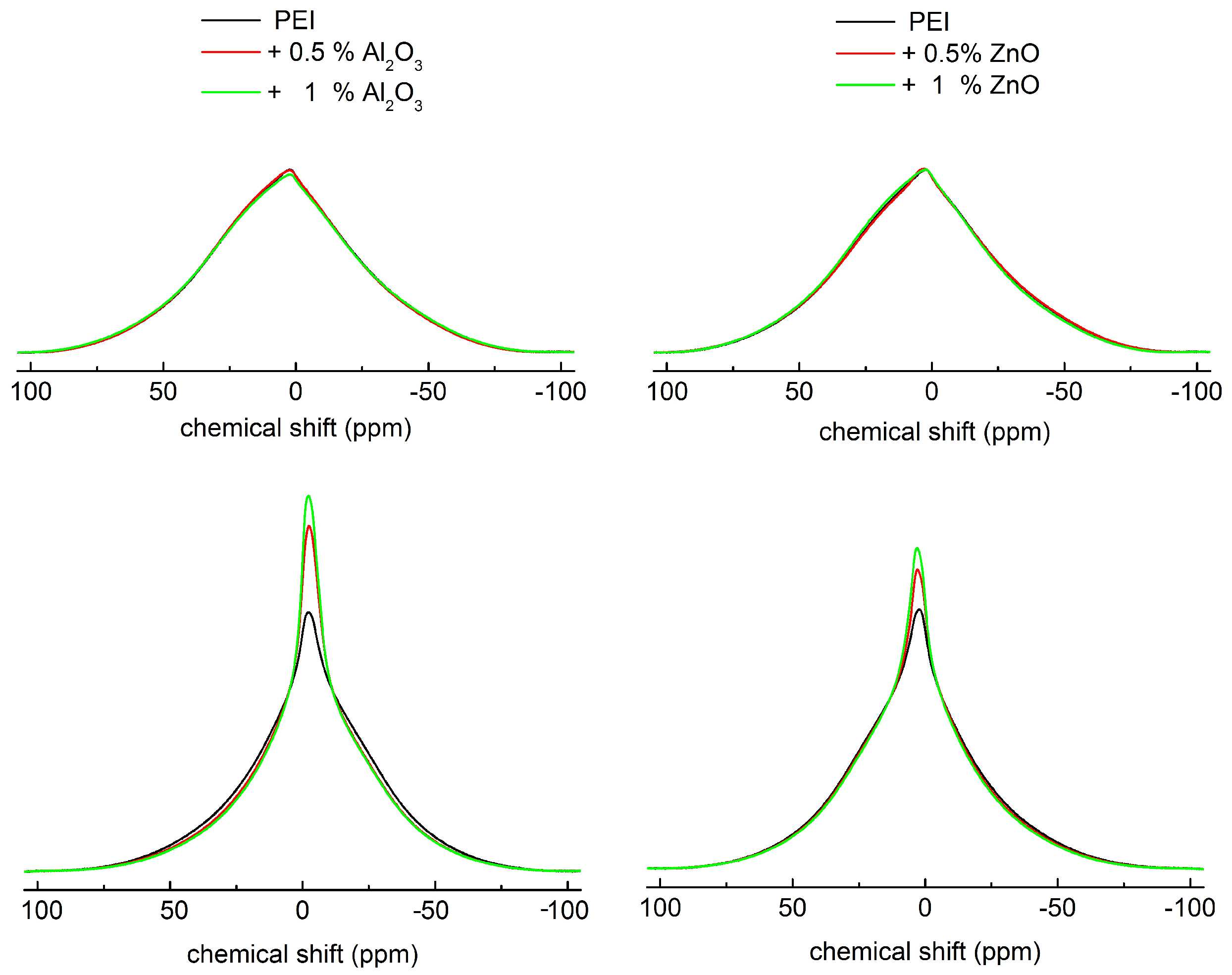
3.1. NMR Spectral Analyses of PEI Composites
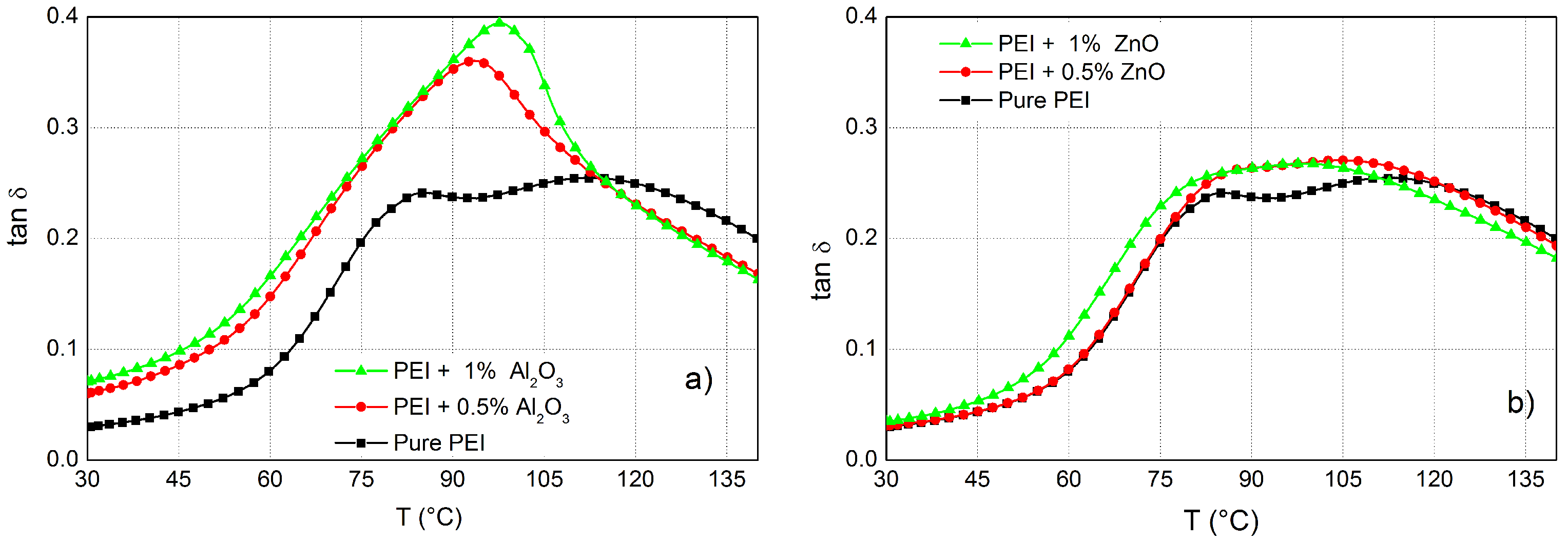
3.2. DMA Analysis of PEI Composites
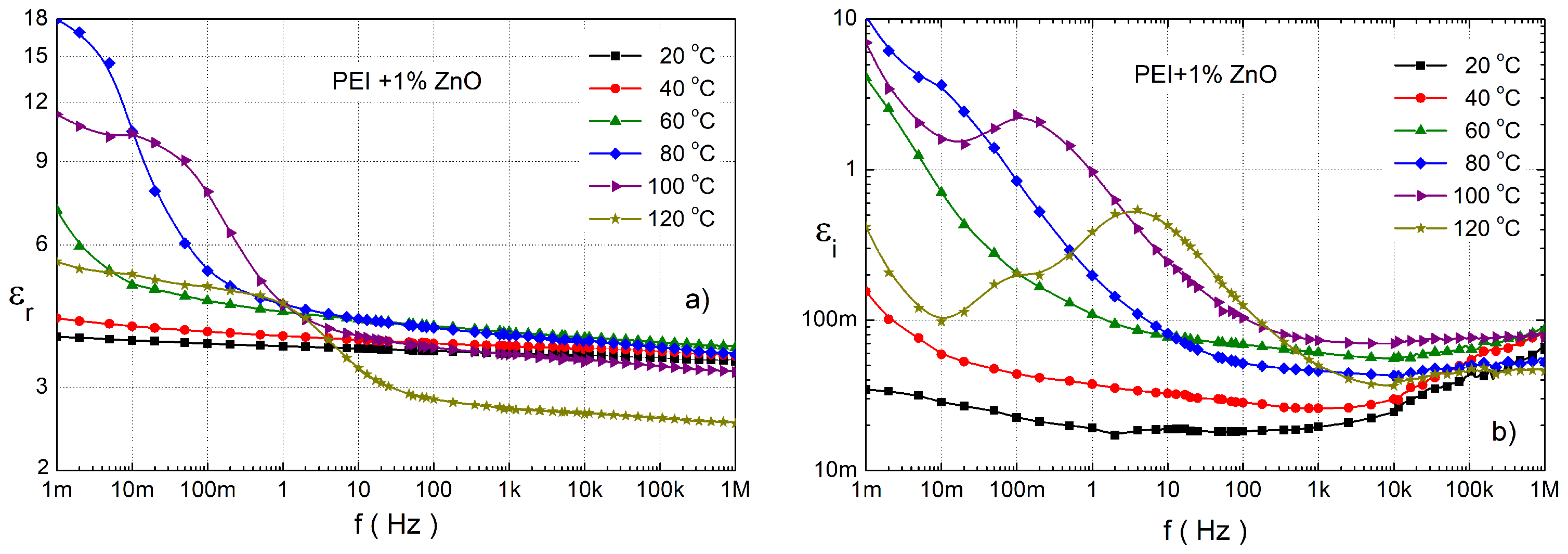
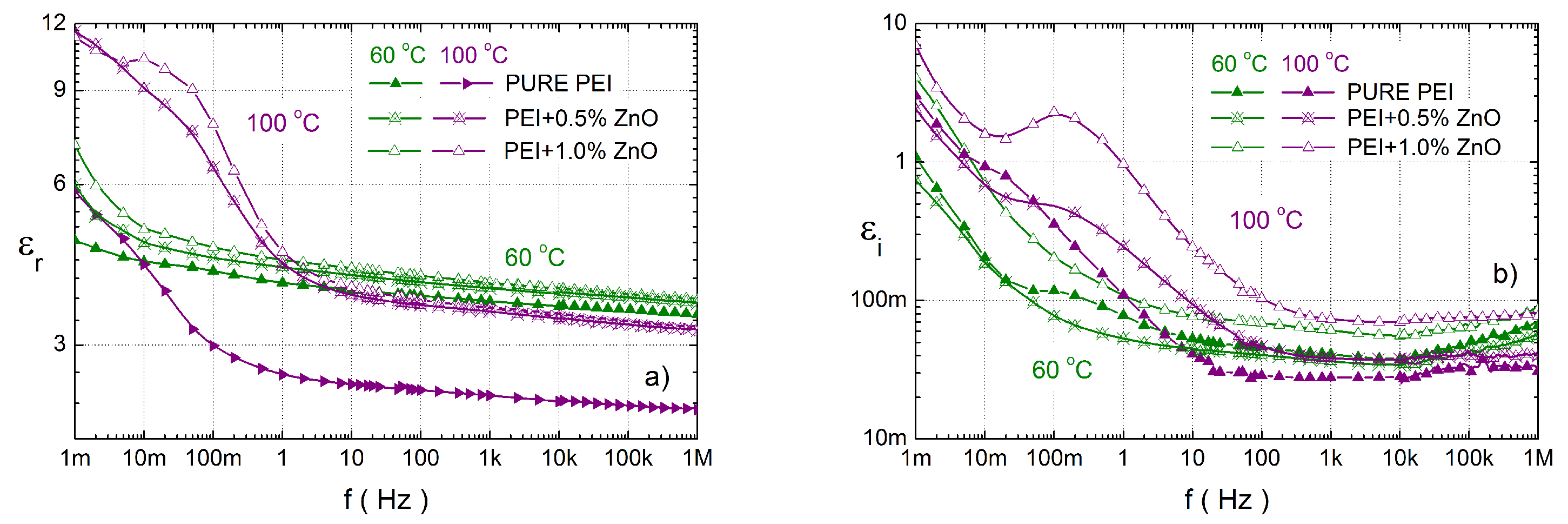
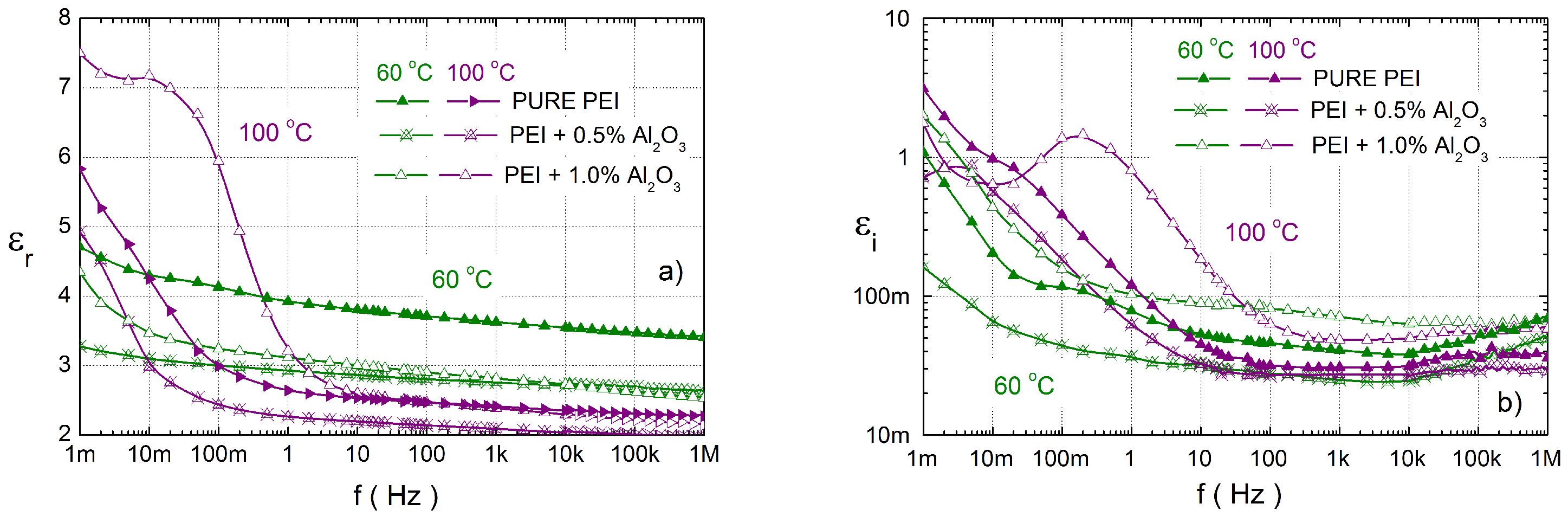
3.3. Dielectric Response Characterisation of PEI Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godovsky, D.Y. Device Applications of Polymer-Nanocomposites. In Biopolymers · PVA Hydrogels, a Snionic Polymerisation Nanocomposites. Advances in Polymer Science; Springer: Berlin, Germany, 2000; Volume 153, pp. 165–204. [Google Scholar] [CrossRef]
- Watson, B.W.; Meng, L.; Fetrow, C.; Qin, Y. Core/Shell Conjugated Polymer/Quantum Dot Composite Nanofibers through Orthogonal Non-Covalent Interactions. Polymers 2016, 8, 408. [Google Scholar] [CrossRef] [PubMed]
- Talbi, F.; David, E.; Malec, D.; Mary, D. Dielectric Properties of Polyesterimide/SiO2 Nanocomposites. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Richland, WA, USA, 20–23 October 2019; pp. 66–69. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Samadi Kafil, H.; Salehi, R. In situ synthesized chitosan–gelatin/ZnO nanocomposite scaffold with drug delivery properties: Higher antibacterial and lower cytotoxicity effects. J. Appl. Polym. Sci. 2019, 136, 47590. [Google Scholar] [CrossRef]
- Ivankova, E.; Vaganov, G.; Didenko, A.; Popova, E.; Elokhovskiy, V.; Bugrov, A.; Svetlichnyi, V.; Kasatkin, I.; Yudin, V. Investigation of Polyetherimide Melt-Extruded Fibers Modified by Carbon Nanoparticles. Materials 2021, 14, 7251. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Yang, W.; He, F.; Yao, J. The Thermal Properties and Degradability of Chiral Polyester-Imides Based on Several l/d-Amino Acids. Polymers 2020, 12, 2053. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, C.; Wang, W.; Wen, X.; He, S.; Chen, W. Developing a novel environmental friendly polyester-imide impregnating resin. In Proceedings of the IEEE Electrical Insulation Conference (EIC), Seattle, WA, USA, 7–10 June 2015; pp. 551–554. [Google Scholar] [CrossRef]
- Fetouhi, L.; Martinez-Vega, J.; Petitgas, B. Electric conductivity, aging and chemical degradation of polyesterimide resins used in the impregnation of rotating machines. IEEE Trans. Electr. Insul. 2018, 25, 294–305. [Google Scholar] [CrossRef]
- Fetouhi, L.; Malec, D.; Manfe, P.; Martinez-Vega, J. Experimental Study on the Evolution of Dielectric Properties of Impregnating Varnishes with Thermal Aging. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Cancun, Mexico, 21–24 October 2018; pp. 618–621. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Huang, L.; Lv, X.; Tang, Y.; Ge, G.; Zhang, P.; Li, Y. Effect of Alumina Nanowires on the Thermal Conductivity and Electrical Performance of Epoxy Composites. Polymers 2020, 12, 2126. [Google Scholar] [CrossRef]
- Gao, J.; Long, Y.; Wu, K.; Li, J.; Yin, G. Nonmonotonic dielectric relaxation behavior of thermochromic epoxy composite. Mater. Lett. 2022, 3, 131678. [Google Scholar] [CrossRef]
- Min, D.; Cui, H.; Hai, Y.; Li, P.; Xing, Z.; Zhang, C.; Li, S. Interfacial regions and network dynamics in epoxy/POSS nanocomposites unravelling through their effects on the motion of molecular chains. Compos. Sci. Technol. 2020, 199, 108329. [Google Scholar] [CrossRef]
- Helal, E.; David, E.; Frechette, M.; Demarquette, N.R. Thermoplastic elastomer nanocomposites with controlled nanoparticles dispersion for HV insulation systems: Correlation between rheological, thermal, electrical and dielectric properties. Eur. Polym. J. 2017, 94, 68–86. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, C.; Liang, G.; Gu, A.; Wang, W. Polyester-imide solventless impregnating resin and its nano-silica modified varnishes with excellent corona resistance and thermal stability. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 372–379. [Google Scholar] [CrossRef]
- Okuzumi, S.; Murakami, Y.; Nagao, M.; Sekiguchi, Y.; Reddy, C.C.; Murata, Y. DC Breakdown Strength and Conduction Current of MgO/LDPE Composite Influenced by Filler Size. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena, Quebec, QC, Canada, 26–29 October 2008; pp. 722–725. [Google Scholar] [CrossRef]
- Zazoum, B.; Frechette, M.; David, E. LDPE/TiO2 nanocomposites: Effect of poss on structure and dielectric properties. IEEE Trans. Dielectr. Electr. Insul. 2016, 3, 2505–2507. [Google Scholar] [CrossRef]
- Katayama, J.; Ohki, Y.; Fuse, N.; Kozako, M.; Tanaka, T. Effects of nanofiller materials on the dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 157–165. [Google Scholar] [CrossRef]
- Kavanagh, D.F.; Gyftakis, K.N.; McCulloch, M.D. Thermal Degradation Phenomena of Polymer Film on Magnet Wire for Electromagnetic Coils. IEEE Trans. Ind. Appl. 2021, 199, 458–467. [Google Scholar] [CrossRef]
- Ramprasad, R.; Shi, N.; Tang, C. Modeling the physics and chemistry of interfaces in nanodielectrics. In Dielectric Polymer Nanocomposites; Nelson, J.K., Ed.; Springer: Boston, MA, USA, 2010; pp. 133–161. [Google Scholar] [CrossRef]
- Arikan, O.; Uydur, C.H.C.; Kumru, C.F. Prediction of dielectric parameters of an aged MV cable: A comparison of curve fitting, decision tree and artificial neural network methods. Electr. Power Syst. Res. 2022, 208, 107892. [Google Scholar] [CrossRef]
- Havran, P.; Cimbala, R.; Kurimsky, J.; Dolnik, B.; Kolcunova, I.; Medved, D.; Kiraly, J.; Kohan, V.; Sarpataky, L. Dielectric Properties of Electrical Insulating Liquids for High Voltage Electric Devices in a Time-Varying Electric Field. Energies 2022, 15, 391. [Google Scholar] [CrossRef]
- Fetouhi, L.; Petitgas, B.; Dantras, E.; Martinez-Vega, J. Mechanical, dielectric, and physicochemical properties of impregnating resin based on unsaturated polyesterimides. Eur. Phys. J. Appl. Phys. 2017, 80, 10901. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, J.M. Dielectric Properties of Epoxy Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 12–23. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Polymeric nanoparticles: Recent development in synthesis and application. Express Polym. Lett. 2016, 10, 895–913. [Google Scholar] [CrossRef]
- Kudelcik, J.; Hardon, S.; Hockicko, P.; Kudelcikova, M.; Hornak, J.; Prosr, P.; Trnka, P. Study of the Complex Permittivity of a Polyurethane Matrix Modified by Nanoparticles. IEEE Access 2021, 9, 49547–49556. [Google Scholar] [CrossRef]
- Michal, O.; Mentlik, V.; Hornak, J. Impact of ultrasonic mixing on the electrical properties of PEI/SiO2 nanocomposites. AIP Conf. Proc. 2021, 2411, 050010. [Google Scholar] [CrossRef]
- Gornicka, B.; Prociow, E. Polyester and Polyesterimide Compounds with Nanofillers for Impregnating of Electrical Motors. Acta Phys. Pol. 2009, 115, 842–845. Available online: http://przyrbwn.icm.edu.pl/APP/PDF/115/a115z420.pdf (accessed on 5 May 2020). [CrossRef]
- Gornicka, B.; Gorecki, L.; Gryzlo, K.; Kaczmarek, D.; Wojcieszak, D. Evaluation of Polyesterimide Nanocomposites Using Methods of Thermal Analysis. IOP Conf. Ser. Mater. Sci. Eng. 2016, 113, 012012. [Google Scholar] [CrossRef]
- Tanaka, T.; Imai, T. Advanced Nanodielectrics (Fundamental and Applications); Pan Stanford Publishing Pte Ltd.: Singapore, 2017; pp. 141–178. ISBN 978-9814745024. [Google Scholar]
- Yegorov, A.; Bogdanovskaya, M.; Ivanov, V.; Kosova, O.; Tcarkova, K.; Retivov, V.; Zhdanovich, O. Nanocomposite Polyimide Materials. Characterizations of Some Composite Materials; Saleh, H.E.M., Koller, M., Eds.; IntechOpen: London, UK, 2019; Chapter 6. [Google Scholar] [CrossRef]
- Kudelcik, J.; Hardon, S.; Trnka, P.; Michal, O.; Hornak, J. Dielectric Responses of Polyurethane/Zinc Oxide Blends for Dry-Type Cast Cold-Curing Resin Transformers. Polymers 2021, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M. Polyurethane/Zinc Oxide (PU/ZnO) Composite—Synthesis, Protective Property and Application. Polymers 2020, 12, 1535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Bai, H.; Ming, W. ZnO Nanoparticles Coated with Amphiphilic Polyurethane for Transparent Polyurethane Nanocomposites with Enhanced Mechanical and UV Shielding Performance. ACS Appl. Nano Mater. 2020, 3, 59–67. [Google Scholar] [CrossRef]
- Park, J.J. Electrical Properties of Epoxy Composites with Micro-sized Fillers. Trans. Electr. Electron. Mater. 2018, 19, 475–480. [Google Scholar] [CrossRef]
- Tang, Y.; Ge, G.; Li, Y.; Huang, L. Effect of Al2O3 with Different Nanostructures on the Insulating Properties of Epoxy-Based Composites. Materials 2020, 13, 4235. [Google Scholar] [CrossRef]
- Eker, Y.R.; Özcan, M.; Ozcan, A.; Kirkici, H. The inuence of Al2O3 and TiO2 additives on the electrical resistivity of epoxy resin based composites at low temperature. Macromol. Mater. Eng. 2019, 304, 1800670. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Yang, H.; Zhou, K.; Ning, X. Electrical properties of epoxy/ZnO nano-composite. J. Mater. Sci. Mater. Electron. 2018, 29, 12765–12770. [Google Scholar] [CrossRef]
- Soulintzis, A.; Kontos, G.; Karahaliou, P.; Psarras, G.C.; Georga, S.N.; Krontiras, C.A. Dielectric relaxation processes in epoxy resin—ZnO composites. J. Polym. Sci. B Polym. Phys. 2009, 47, 445–454. [Google Scholar] [CrossRef]
- Kudelcik, J.; Jahoda, E.; Hornak, J.; Michal, O.; Trnka, P. Partial discharges and dielectric parameters of epoxy resin filled with nanoparticles. In Proceedings of the 19th International Scientific Conference on Electric Power Engineering (EPE), Brno, Czech Republic, 16–18 May 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Shi, H.; Gao, N.; Jin, H.; Zhang, G.; Peng, Z. Investigation of the effects of nano-filler on dielectric properties of epoxy based composites. In Proceedings of the IEEE 9th International Conference on the Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009; pp. 804–807. [Google Scholar] [CrossRef]
- Klampar, M.; Liedermann, K. Dielectric relaxation spectroscopy of epoxy resins with TiO2, Al2O3, WO3 and SiO2 nanofillers. In Proceedings of the IEEE International Symposium on Electrical Insulation, San Juan, PR, USA, 10–13 June 2012; pp. 637–640. [Google Scholar] [CrossRef]
- Hornak, J.; Trnka, P.; Kadlec, P.; Michal, O.; Mentlík, V.; Šutta, P.; Csanyi, G.M.; Tamus, Z.A. Magnesium Oxide Nanoparticles: Dielectric Properties, Surface Functionalization and Improvement of Epoxy-Based Composites Insulating Properties. Nanomaterials 2018, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- ELAN-Protect_UP_343. Available online: https://products.elantas.com/beckinsulation/productReport/ELAN-protect%C2%AE_UP_343.pdf?language=en&country=&download=productReport&productid=00000300&brandid=66958 (accessed on 5 May 2020).
- NanoAmor, Amorphous Products—Zinc Oxide Nanopowder. Available online: https://www.nanoamor.com/inc/sdetail/19983 (accessed on 5 May 2020).
- NanoAmor, Amorphous Products—Aluminum Oxide (Al2O3) Nanopowder. Available online: https://www.nanoamor.com/inc/sdetail/23066 (accessed on 5 May 2020).
- Patel, S.; Sengupta, R.; Puntambekar, U.; Shingne, N. Effect of different types of silica particles on dielectric and mechanical properties of epoxy nanocomposites. Mater. Today Proc. 2021, 44, 1848–1852. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, G.; Yang, Y.; Wu, X.; Lei, Y. Effects of nanoparticles on reducing partial discharge induced degradation of polyimide/Al2O3 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 594–602. [Google Scholar] [CrossRef]
- Alam, T.M.; Allers, J.P.; Jones, B.H. Heterogeneous Polymer Dynamics Explored Using Static 1H NMR Spectra. Int. J. Mol. Sci. 2020, 21, 5176. [Google Scholar] [CrossRef]
- Uthaman, A.; Xian, G.; Thomas, S.; Wang, Y.; Zheng, Q.; Liu, X. Durability of an Epoxy Resin and Its Carbon Fiber- Reinforced Polymer Composite upon Immersion in Water, Acidic, and Alkaline Solutions. Polymers 2020, 12, 614. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Zhang, Z.; Sprenger, S. Epoxy Resin Filled with High Volume Content Nano-SiO2 Particles. J. Nanosci. Nanotechnol. 2009, 9, 1412–1417. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Prasanna Sanka, R.V.S.; Binder, W.H.; Park, C.; Jung, J.; Parthasarthy, V.; Rana, S.; Yun, G.J. Catalyst free self-healable vitrimer/graphene oxide nanocomposites. Compos. Part B Eng. 2019, 184, 107647. [Google Scholar] [CrossRef]
- Barabanova, A.I.; Afanas’ev, E.S.; Molchanov, V.S.; Askadskii, A.A.; Philippova, O.E. Unmodified Silica Nanoparticles Enhance Mechanical Properties and Welding Ability of Epoxy Thermosets with Tunable Vitrimer Matrix. Polymers 2021, 13, 3040. [Google Scholar] [CrossRef]
- Jena, K.K.; Chattopadhyay, D.K.; Raju, K.V.S.N. Synthesis and characterization of hyperbranched polyurethane–urea coatings. Eur. Polym. J. 2007, 45, 1825–1837. [Google Scholar] [CrossRef]
- Raihan, R.; Rabbi, F.; Vadlamudi, V.; Reifsnider, K. Composite materials damage modeling based on dielectric properties. Mater. Sci. Appl. 2015, 6, 1033–1053. [Google Scholar] [CrossRef][Green Version]
- Jonscher, A.K. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, R57–R70. [Google Scholar] [CrossRef]
- Tomara, G.N.; Kerasidou, A.P.; Patsidis, A.C.; Karahaliou, P.K.; Psarras, G.C.; Georga, S.N.; Krontiras, C.A. Dielectric response and energy storage efficiency of low content TiO2-polymer matrix nanocomposites. Compos. Part A Appl. Sci. Manuf. 2015, 71, 204–211. [Google Scholar] [CrossRef]
- Kontos, G.A.; Soulintzis, A.L.; Karahaliou, P.K.; Psarras, G.C.; Georga, S.N.; Krontiras, C.A.; Pisanias, M.N. Electrical relaxation dynamics in TiO2-polymer matrix composites. Express Polym. Lett. 2007, 1, 781–789. [Google Scholar] [CrossRef]
- Jahoda, E.; Kúdelčík, J.; Hornak, J.; Trnka, P. The influence of nanoparticles in the epoxy resin on dielectric parameters and partial discharges. In Proceedings of the 2018 ELEKTRO, Mikulov, Czech Republic, 21–23 May 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Ahmad, Y. Polymer dielectric materials. In Dielectric Material; Silaghi, M.A., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Zhao, W.; Fan, Y.; Chen, H. Dielectric properties and corona resistance of Si-B/epoxy nano-composites. J. Mater. Sci. Mater. Electron. 2019, 30, 16298–16307. [Google Scholar] [CrossRef]
- Sun, X.W.; Yang, Y. Introduction. In ZnO Nanostructures and Their Applications; Sun, X.W., Yang, Y., Eds.; Taylor& Francis Group: New York, NY, USA, 2012; pp. 1–14. [Google Scholar]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Velayutham, T.S.; Majid, W.H.A.; Gan, W.C.; Zak, A.K.; Gan, S.N. Theoretical and experimental approach on dielectric properties of ZnO nanoparticles and polyurethane/ZnO nanocomposites. J. Appl. Phys. 2012, 112, 054106. [Google Scholar] [CrossRef]
- Domun, N.; Paton, K.R.; Hadavinia, H.; Sainsbury, T.; Zhang, T.; Mohamud, H. Enhancement of fracture toughness of epoxy nanocomposites by combining nanotubes and nanosheets as fillers. Materials 2017, 10, 1179. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Malhaupt, R. Process, understanding and challenges in the field of nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, G.N.; Liu, J.W.; Peng, J.; Zhu, G.Y.; Gao, G. Investigation of temperature effects on voltage endurance for polyimide/Al2O3 nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1824–1834. [Google Scholar] [CrossRef]
- Rittigstein, P.; Torkelson, J.M. Polymer–nanoparticle interfacial interactions in polymer nanocomposites: Confinement effects on glass transition temperature and suppression of physical aging. J. Polym. Sci. B Polym. Phys. 2006, 44, 293–2943. [Google Scholar] [CrossRef]

| Property | Value |
|---|---|
| Shelf life (23 °C) | 6 months |
| Viscosity at 23 °C (mPa·s) | 7500 |
| Density at 23 °C (kg/m) | 1186 |
| Gel time at 120 °C (min) | 10 ± 2 |
| Curing time at 150 °C (min) | 60 |
| Water absorption (following ISO 62) | |
| at 23 °C/24 h (mg) | 3.4 |
| Parameter | ZnO | AlO |
|---|---|---|
| Diameter (nm) | 20 | 20 |
| Purity (%) | 99+ | 99.97 |
| Specific surface area (m/g) | ≥40 | 180 |
| Bulk density (g/cm) | 0.1–0.2 | 3.95 |
| Morphology of particles | spherical | spherical |
| Parameter | (60/100 C) | (60/100 C) | ||||
|---|---|---|---|---|---|---|
| Unit | - | - | (10 S/m) | (s) | (mHz) | - |
| Pure PEI | 3.73 / 2.5 | 0.047/ 0.033 | 0.19 | 11.91 | 13 | 0.28 |
| PEI + 0.5% ZnO | 3.97/3.59 | 0.066/0.079 | 0.25 | 1.45 | 109 | 0.22 |
| PEI + 1% ZnO | 4.08/3.69 | 0.071/0.127 | 0.37 | 1.11 | 145 | 0.14 |
| PEI + 0.5% AlO | 2.15/2.82 | 0.029 | 0.13 | 13 | 12 | 0 |
| PEI + 1% AlO | 2.93/2.5 | 0.077 | 0.15 | 0.76 | 200 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardoň, Š.; Kúdelčík, J.; Baran, A.; Michal, O.; Trnka, P.; Hornak, J. Influence of Nanoparticles on the Dielectric Response of a Single Component Resin Based on Polyesterimide. Polymers 2022, 14, 2202. https://doi.org/10.3390/polym14112202
Hardoň Š, Kúdelčík J, Baran A, Michal O, Trnka P, Hornak J. Influence of Nanoparticles on the Dielectric Response of a Single Component Resin Based on Polyesterimide. Polymers. 2022; 14(11):2202. https://doi.org/10.3390/polym14112202
Chicago/Turabian StyleHardoň, Štefan, Jozef Kúdelčík, Anton Baran, Ondrej Michal, Pavel Trnka, and Jaroslav Hornak. 2022. "Influence of Nanoparticles on the Dielectric Response of a Single Component Resin Based on Polyesterimide" Polymers 14, no. 11: 2202. https://doi.org/10.3390/polym14112202
APA StyleHardoň, Š., Kúdelčík, J., Baran, A., Michal, O., Trnka, P., & Hornak, J. (2022). Influence of Nanoparticles on the Dielectric Response of a Single Component Resin Based on Polyesterimide. Polymers, 14(11), 2202. https://doi.org/10.3390/polym14112202










