Cooperative Effect of ZIF-67-Derived Hollow NiCo-LDH and MoS2 on Enhancing the Flame Retardancy of Thermoplastic Polyurethane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 3D Hollow NiCo-LDH
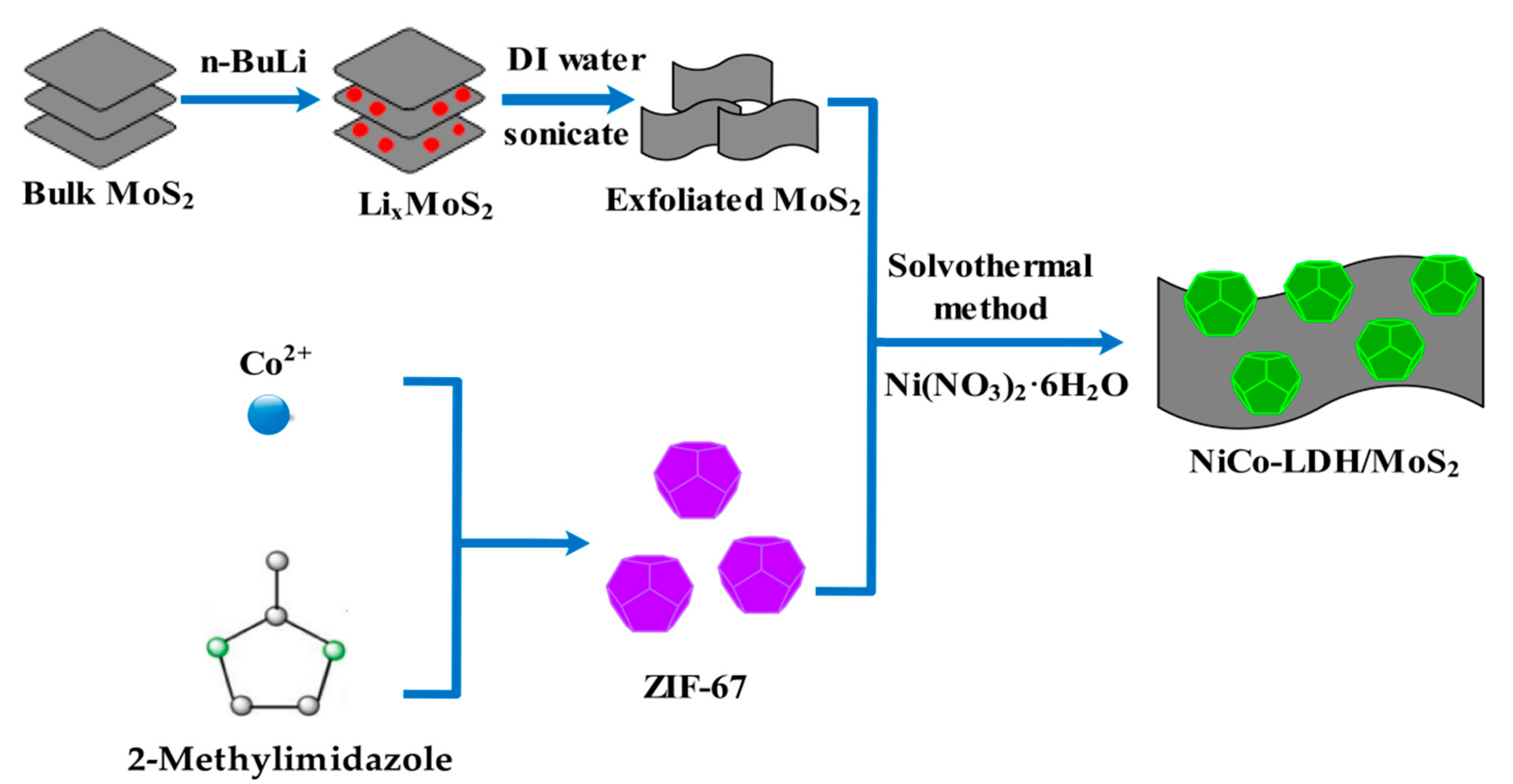
2.3. Synthesis of 3D Hollow NiCo-LDH/MoS2 hybrid material
2.4. Synthesis of TPU Composites
2.5. Characterization
3. Results and Discussions
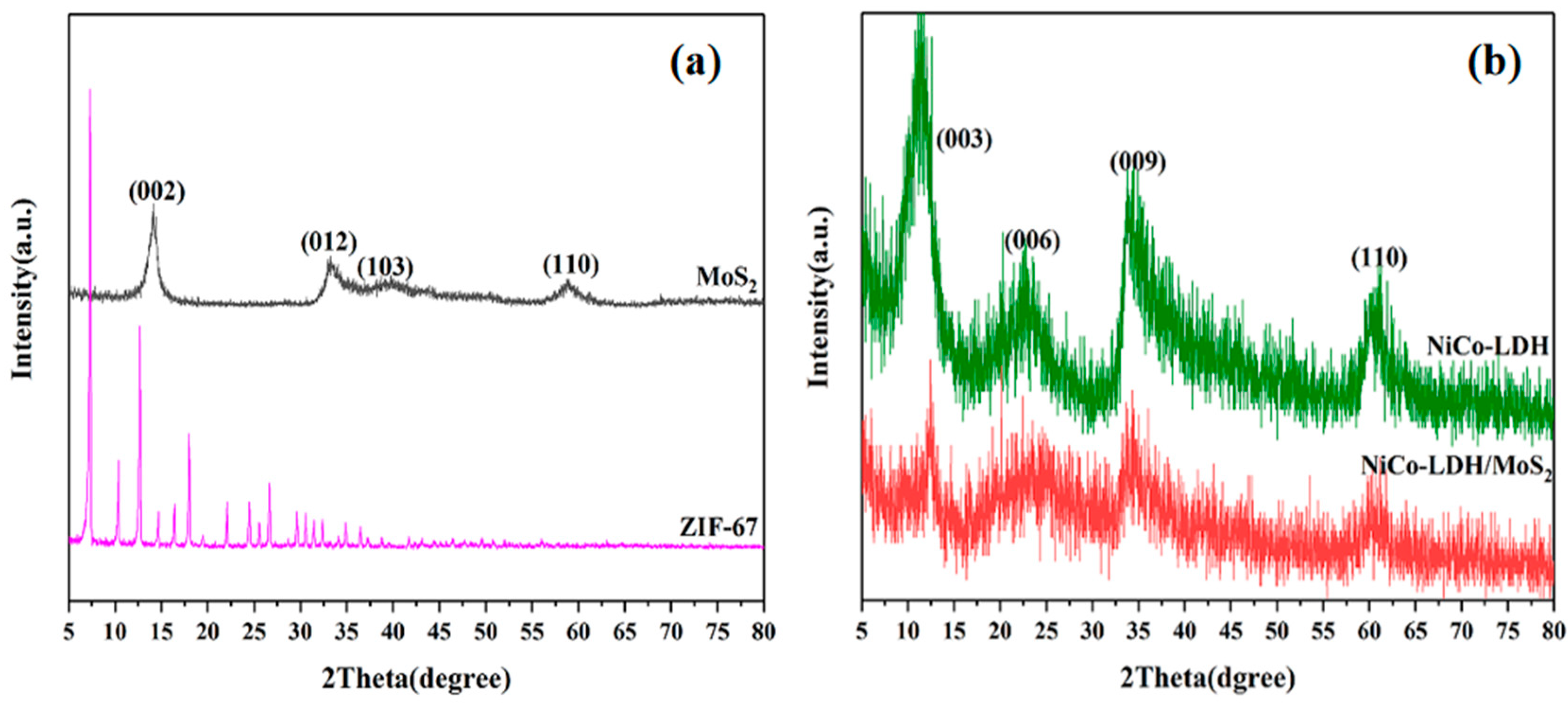
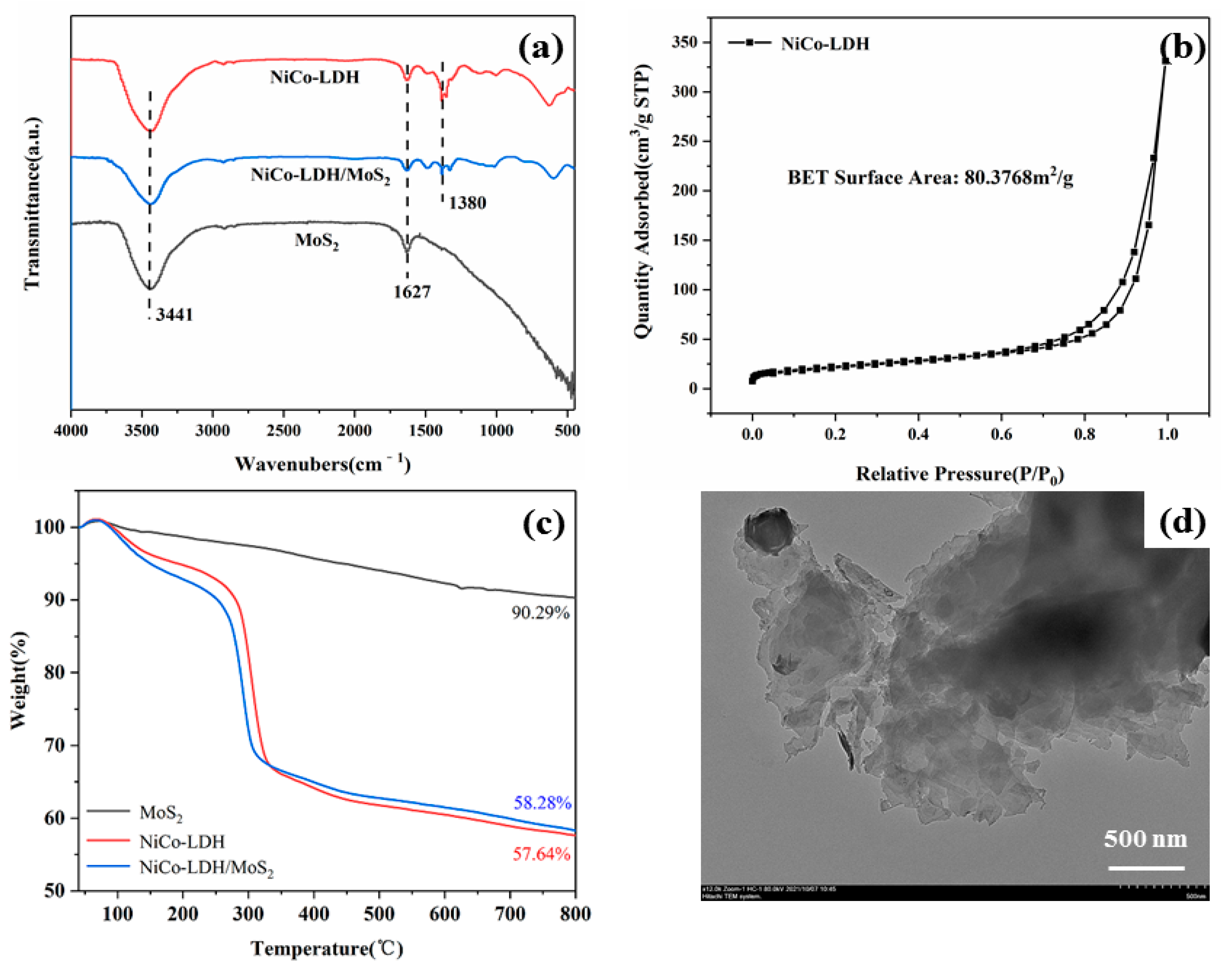
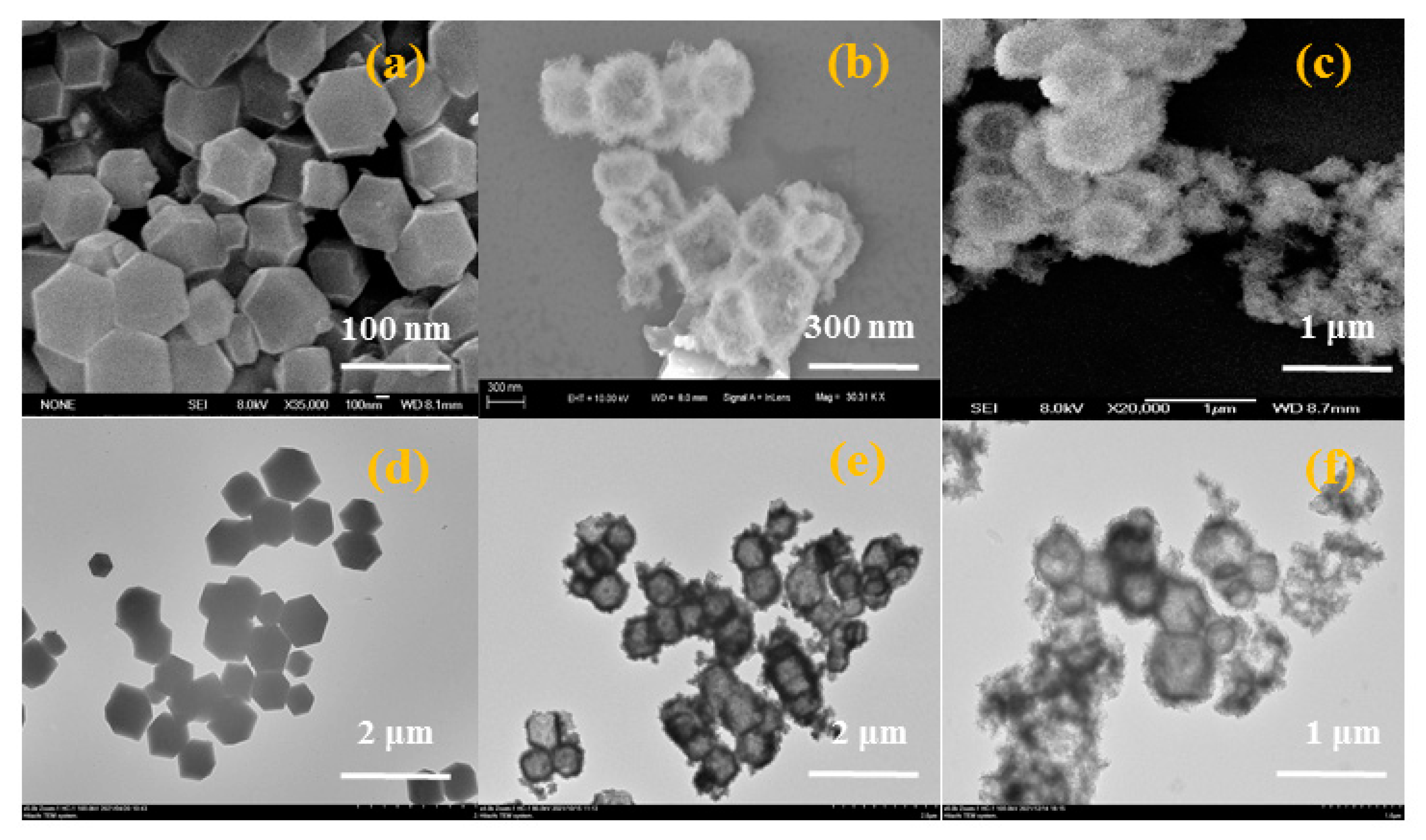
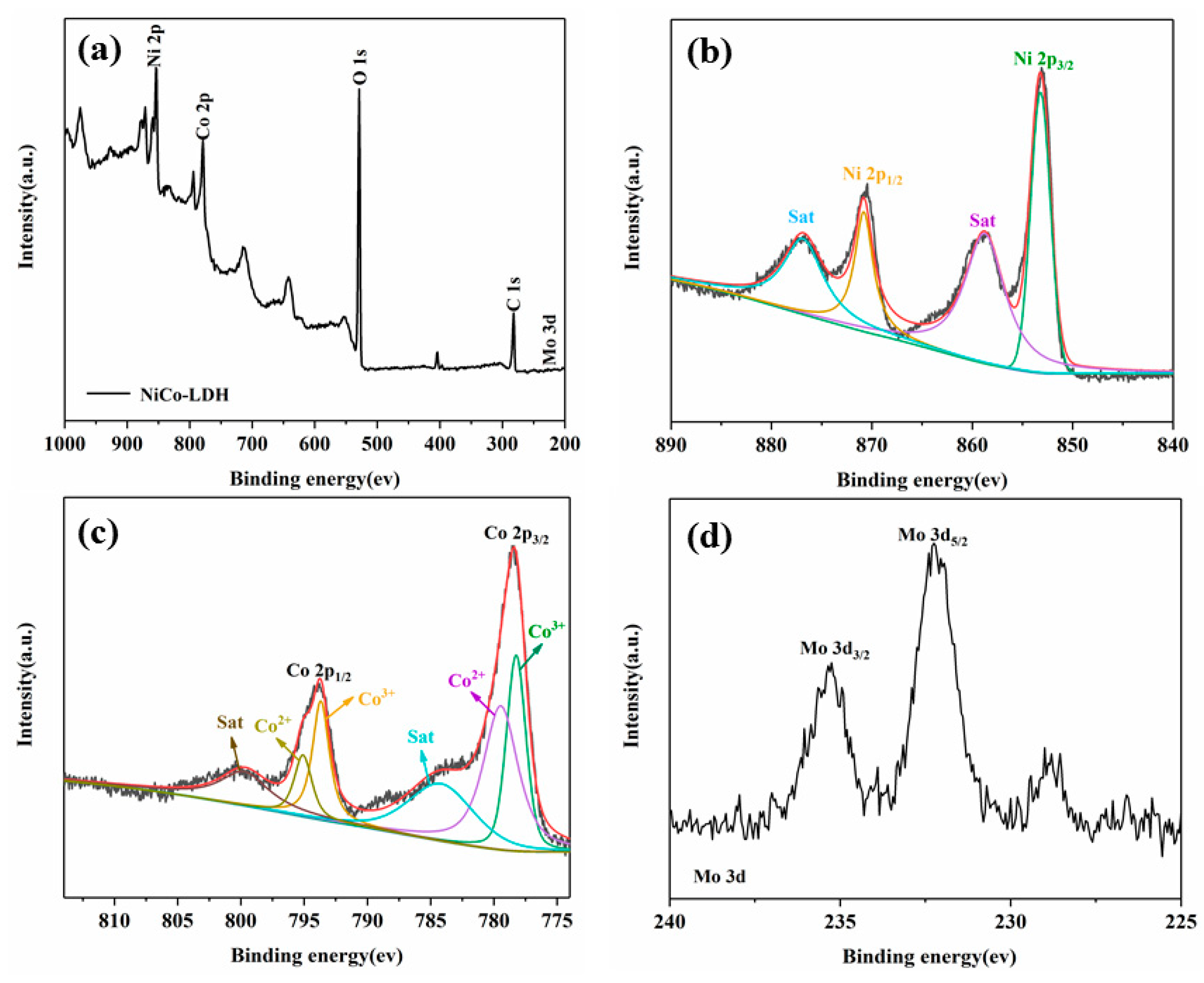
3.1. Characterization of Hollow NiCo-LDH and Its hybrid material
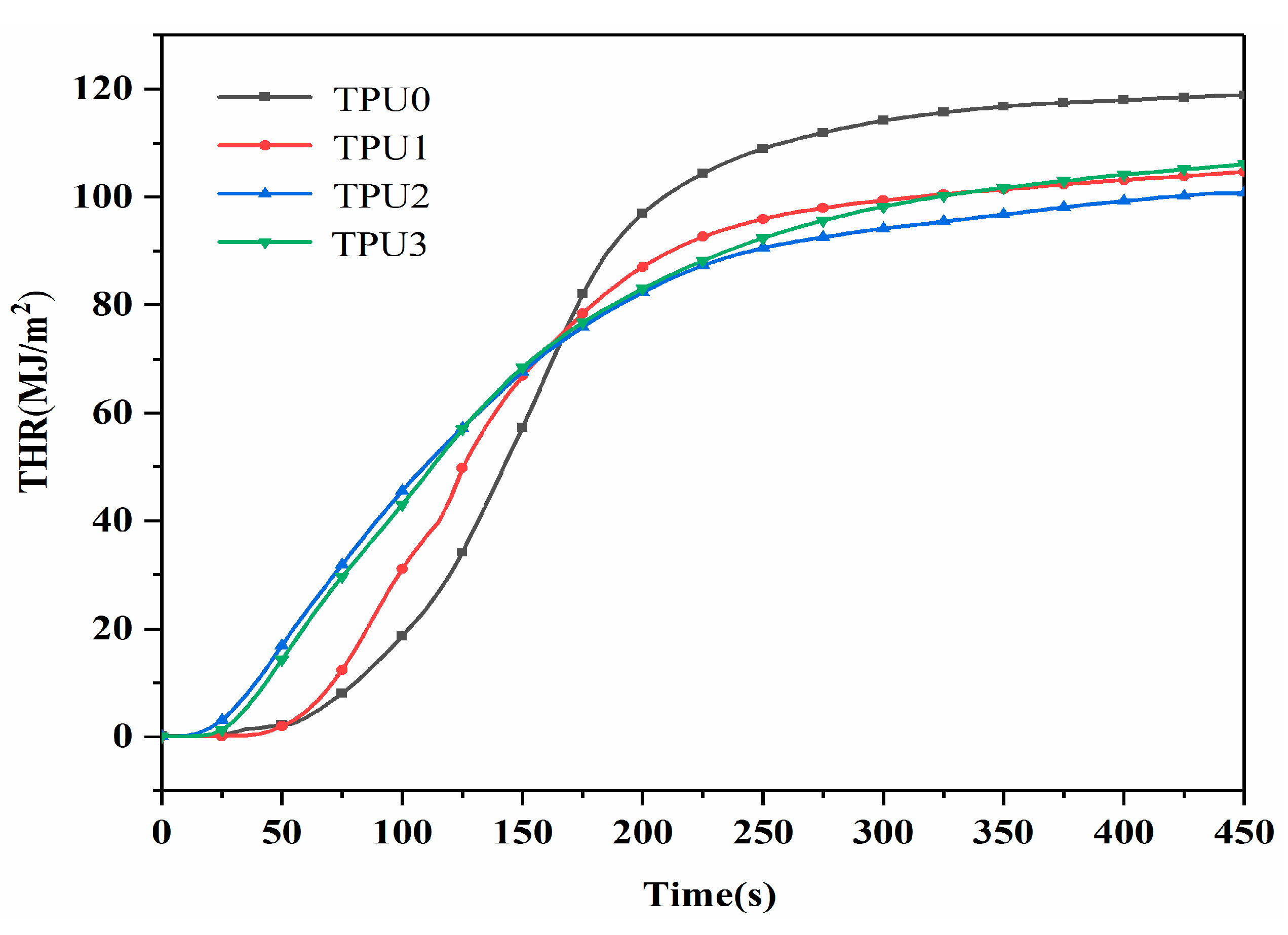
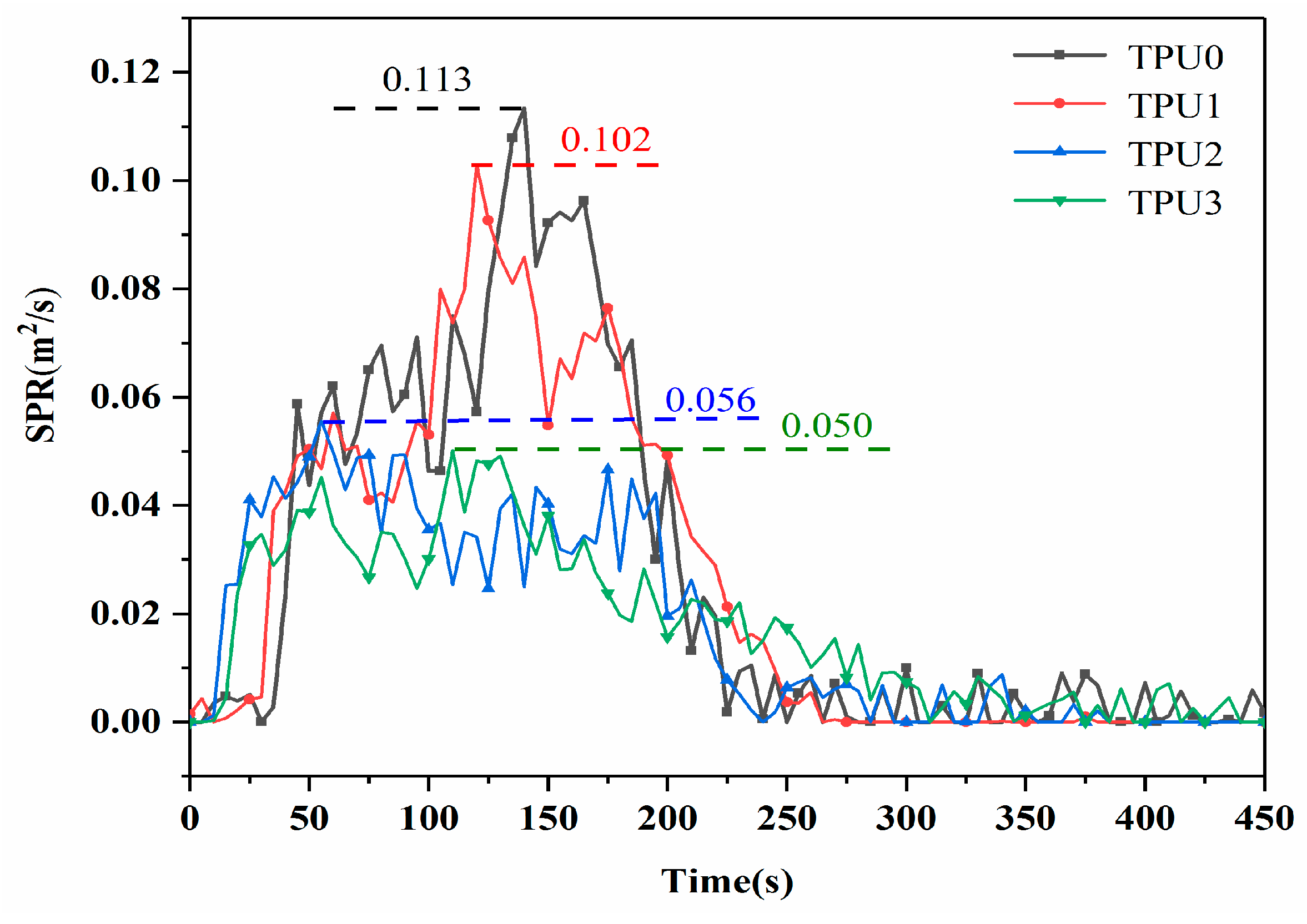
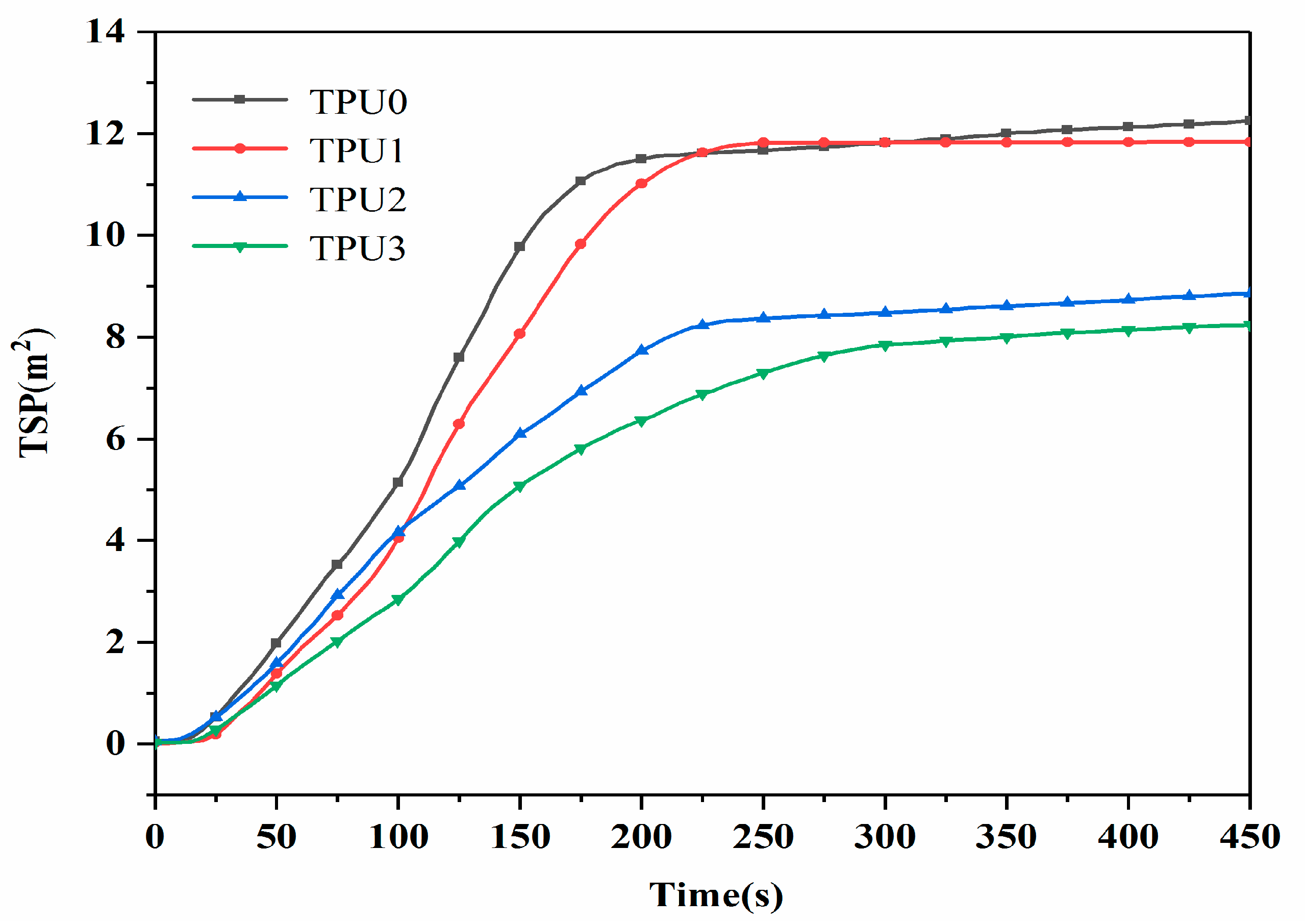
3.2. CCT Analysis
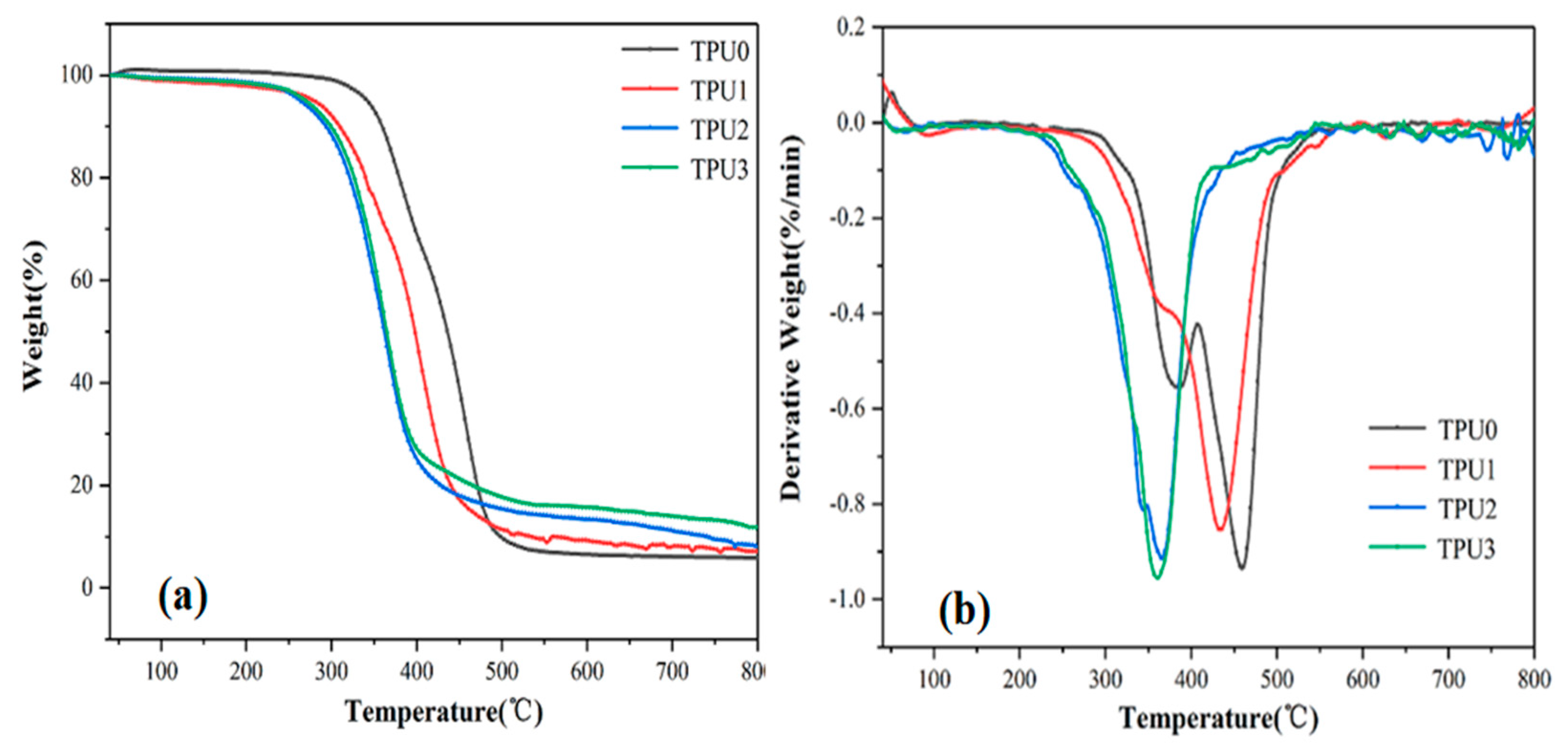
3.3. Thermal Stability Analysis
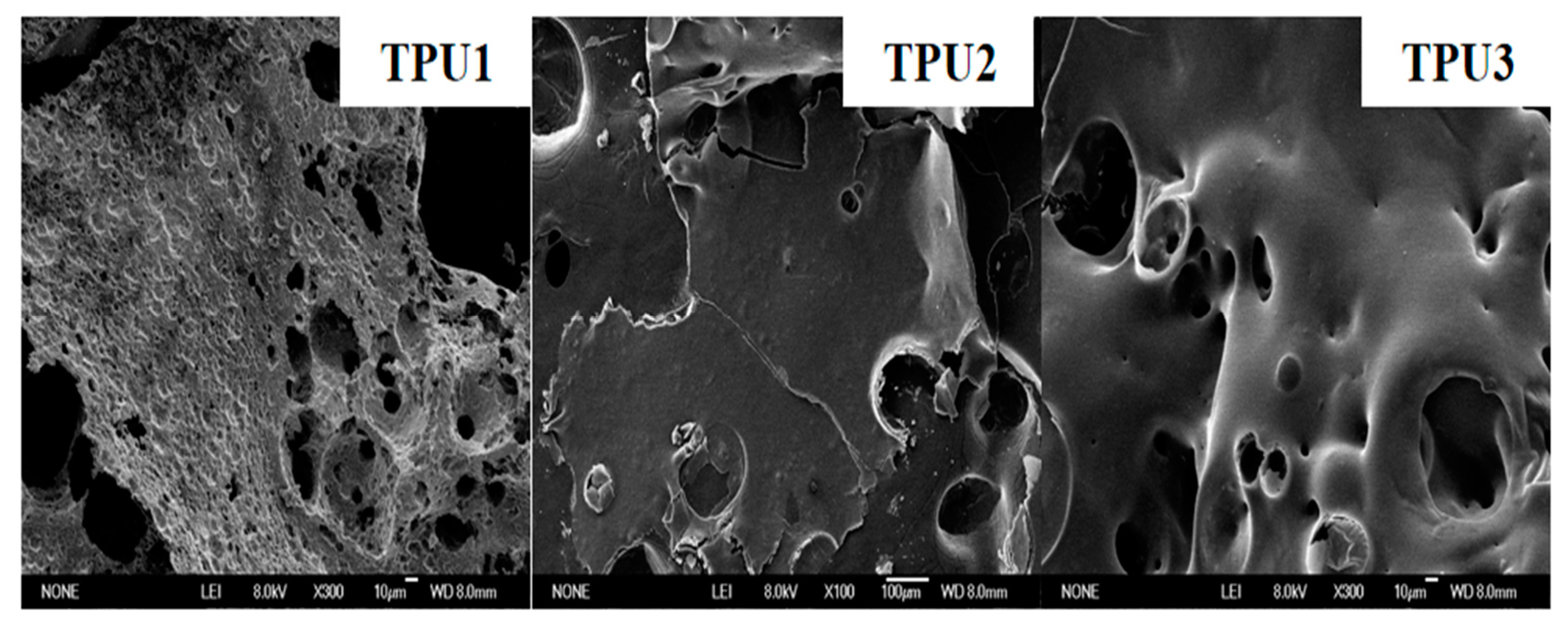
3.4. Char Residue Analysis
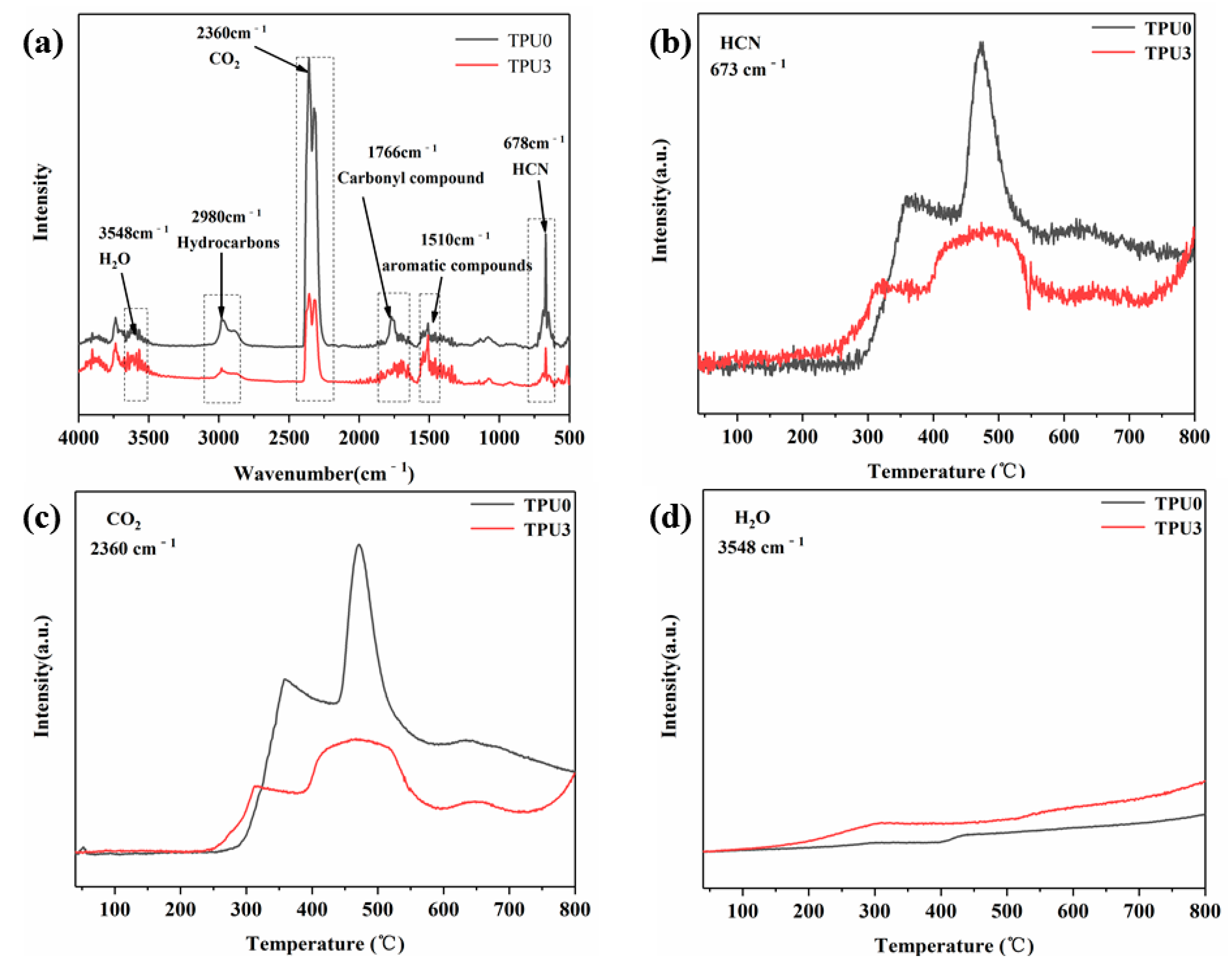
3.5. Thermal Decomposition Products Analysis
3.6. Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.; Jang, J.U.; Yin, W.B.; Lee, B.; Lee, K.J. Synthesis of highly functionalized thermoplastic polyurethanes and their potential applications. Polymer 2017, 116, 287–294. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, L.; Chen, X.; Guo, J.; Yang, F.; Wang, J.; Zheng, Y.; Hu, Y. Hypophosphite/graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef] [Green Version]
- Spontak, R.J.; Patel, N.P. Thermoplastic elastomers: Fundamentals and applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]
- Ghariniyat, P.; Leung, S.N. Development of thermally conductive thermoplastic polyurethane composite foams via CO2 foaming-assisted filler networking. Compos. Part B Eng. 2018, 143, 9–18. [Google Scholar] [CrossRef]
- Chen, H.; Deng, C.; Zhao, Z.Y.; Wan, L.; Yang, A.H.; Wang, Y.Z. Novel piperazine-containing oligomer as flame retardant and crystallization induction additive for thermoplastics polyurethane. Chem. Eng. J. 2020, 400, 125941. [Google Scholar] [CrossRef]
- Wang, D.; Song, L.; Zhou, K.; Yu, X.; Hu, Y.; Wang, J. Anomalous nano-barrier effects of ultrathin molybdenum disulfide nanosheets for improving the flame retardance of polymer nanocomposites. J. Mater. Chem. A 2015, 3, 14307–14317. [Google Scholar] [CrossRef]
- Elbasuney, S. Surface engineering of layered double hydroxide (LDH) nanoparticles for polymer flame retardancy. Powder Technol. 2015, 277, 63–73. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Lomeda, J.R.; Morgan, A.B.; Tour, J.M. Graphite oxide flame-retardant polymer nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 2256–2261. [Google Scholar] [CrossRef]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhang, B.; Wang, X.; Wang, G. The flame retardancy and smoke suppression effect of a hybrid containing dihydrogen phosphate anion modified reduced graphene oxide/layered double hydroxide on epoxy resin. RSC Adv. 2017, 7, 19662–19673. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tao, Y.; Zhou, K.; Shi, Y.; Feng, X.; Jie, G.; Richard, K.K.; Hu, Y. The influence of typical layered inorganic compounds on the improved thermal stability and fire resistance properties of polystyrene nanocomposites. Polym. Compos. 2017, 38, E320–E330. [Google Scholar] [CrossRef]
- Thapa, S.; Meng, L.; Hettiarachchi, E.; Bader, Y.K.; Dickie, D.A.; Rubasinghege, G.; Ivanov, S.A.; Vreeland, E.C.; Qin, Y. Charge-Separated and Lewis Paired Metal-Organic Framework for Anion Exchange and CO2 Chemical Fixation. Chem. A Eur. J. 2020, 26, 13788–13791. [Google Scholar] [CrossRef]
- Meng, L.; Yu, B.; Qin, Y. Templated interfacial synthesis of metal-organic framework (MOF) nano-and micro-structures with precisely controlled shapes and sizes. Commun. Chem. 2021, 4, 82. [Google Scholar] [CrossRef]
- Xu, H.; Shan, C.; Wu, X.; Sun, M.; Huang, B.; Tang, Y.; Yan, C.H. Fabrication of layered double hydroxide microcapsules mediated by cerium doping in metal-organic frameworks for boosting water splitting. Energy Environ. Sci. 2020, 13, 2949–2956. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Qin, Z.; Sun, H.; Jiao, X.; Chen, D. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale 2013, 5, 11770–11775. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Wang, D.Y. Hierarchical layered double hydroxide nanosheets/phosphorus-containing organosilane functionalized hollow glass microsphere towards high performance epoxy composite: Enhanced interfacial adhesion and bottom-up charring behavior. Polymer 2020, 210, 123018. [Google Scholar] [CrossRef]
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Eksik, O.; Gao, J.; Shojaee, S.A.; Thomas, A.; Chow, P.; Bartolucci, S.F.; Lucca, D.A.; Koratkar, N. Epoxy nanocomposites with two-dimensional transition metal dichalcogenide additives. ACS Nano 2014, 8, 5282–5289. [Google Scholar] [CrossRef]
- Daniloska, V.; Keddie, J.L.; Asua, J.M.; Tomovska, R. MoS2 nanoplatelet fillers for enhancement of the properties of waterborne pressure-sensitive adhesives. ACS Appl. Mater. Interfaces 2014, 6, 22640–22648. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Wang, D.; Fu, S. Simultaneous exfoliation and functionalization of MoS2 nanosheets by molecular-designed poly (ionic liquid): Integrated interfacial crosslinking effect for mechanical and flame retardance enhancement of polyacrylonitrile composite fiber. Compos. Commun. 2021, 27, 100902. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Rui, X.; Liu, H.; Yadian, B.; Zhou, K.; Wei, J.; Yan, Q.; Feng, X.; Long, Y.; et al. Zeolitic Imidazolate framework 67-derived high symmetric porous Co3O4 hollow dodecahedra with highly enhanced lithium storage capability. Small 2014, 10, 1932–1938. [Google Scholar] [CrossRef]
- Wan, C.; Ding, S.; Zhang, C.; Tan, X.; Zou, W.; Liu, X.; Yang, X. Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Sep. Purif. Technol. 2017, 180, 1–12. [Google Scholar] [CrossRef]
- Sanikop, R.; Budumuru, A.K.; Gautam, S.; Chae, K.H.; Sudakar, C. Robust ferromagnetism in Li-intercalated and deintercalated MoS2 nanosheets: Implications for 2D spintronics. ACS Appl. Nano Mater. 2020, 3, 11825–11837. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Rui, X.; Zhang, W.; Yan, Q.; Chen, P.; Hou, F.; Huang, W.; Dong, X. MOF-directed templating synthesis of a porous multicomponent dodecahedron with hollow interiors for enhanced lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 8483–8488. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, S.; Hu, Y.; Kundu, C.K.; Gui, Z.; Hu, W. Construction of bimetallic ZIF-derived Co-Ni LDHs on the surfaces of GO or CNTs with a recyclable method: Toward reduced toxicity of gaseous thermal decomposition products of unsaturated polyester resin. ACS Appl. Mater. Interfaces 2018, 10, 18359–18371. [Google Scholar] [CrossRef]
- Pan, Y.T.; Wan, J.; Zhao, X.; Li, C.; Wang, D.Y. Interfacial growth of MOF-derived layered double hydroxide nanosheets on graphene slab towards fabrication of multifunctional epoxy nanocomposites. Chem. Eng. J. 2017, 330, 1222–1231. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, F.; Shang, J.; Iqbal, M.; Han, A.; Sun, X.; Xu, H.; Liu, J. Ternary NiCoFe-layered double hydroxide hollow polyhedrons as highly efficient electrocatalysts for oxygen evolution reaction. J. Energy Chem. 2020, 43, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, X.; He, H.; Chen, W. Fabrication of hollow LDH nanocages using ZIF-67 template as superb adsorbent for anionic organic pollutant. J. Porous Mater. 2021, 28, 471–480. [Google Scholar] [CrossRef]
- Ren, H.; Qing, K.; Chen, Y.; Lin, Y.; Duan, X. Smoke suppressant in flame retarded thermoplastic polyurethane composites: Synergistic effect and mechanism study. Nano Res. 2021, 14, 3926–3934. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Mathys, Z.; Gibson, A.G. Heat release of polymer composites in fire. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1040–1054. [Google Scholar] [CrossRef]
- Wen, X.; Gong, J.; Yu, H.; Liu, Z.; Wan, D.; Liu, J.; Jiang, Z.; Tang, T. Catalyzing carbonization of poly (L-lactide) by nanosized carbon black combined with Ni2O3 for improving flame retardancy. J. Mater. Chem. 2012, 22, 19974–19980. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Song, L.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Aluminum hypophosphite in combination with expandable graphite as a novel flame retardant system for rigid polyurethane foams. Polym. Adv. Technol. 2015, 25, 1034–1043. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wang, D.; Wilkie, C.A. Flame-retarded polystyrene: Investigating chemical interactions between ammonium polyphosphate and MgAl layered double hydroxide. Polym. Degrad. Stab. 2008, 93, 1656–1663. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Su, W.; Li, L.; Fu, H.; Li, J.; Zhang, Y. Synthesis of 3D Hollow Layered Double Hydroxide-Molybdenum Disulfide Hybrid Materials and Their Application in Flame Retardant Thermoplastic Polyurethane. Polymers 2022, 14, 1506. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, G.; Yuan, H.; Yu, W.; Jiang, S. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly (methyl methacrylate). J. Hazard. Mater. 2012, 209, 34–39. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Zhang, J. Thermal and combustion behavior of ethylene-vinyl acetate/aluminum trihydroxide/Fe-montmorillonite composites. Polym. Eng. Sci. 2012, 52, 414–419. [Google Scholar] [CrossRef]
- Tang, Q.; Song, Y.; He, J.; Yang, R. Synthesis and characterization of inherently flame-retardant and anti-dripping thermoplastic poly(imides-urethane)s. J. Appl. Polym. Sci. 2014, 131, 776–781. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Yu, B.; Meng, Y.; Cao, X.; Zhang, Q.; Wu, W.; Shi, D.; Jiang, T.; Li, R.K. Facile preparation of phosphorus containing hyperbranched polysiloxane grafted graphene oxide hybrid toward simultaneously enhanced flame retardancy and smoke suppression of thermoplastic polyurethane nanocomposites. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106614. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Gong, X.; Sun, Q.; Cao, X.; Su, Y.; Yu, B.; Li, R.K.; Vellaisamy, R.A. Surface decoration of halloysite nanotubes with POSS for fire-safe thermoplastic polyurethane nanocomposites. J. Mater. Sci. Technol. 2022, 101, 107–117. [Google Scholar] [CrossRef]
- Cao, X.; Chi, X.; Deng, X.; Liu, T.; Yu, B.; Wang, B.; Anthony, C.Y.; Wu, W.; Li, R.K. Synergistic effect of flame retardants and graphitic carbon nitride on flame retardancy of polylactide composites. Polym. Adv. Technol. 2020, 31, 1661–1670. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, R.; Qian, X. Self-assembly of exfoliated molybdenum disulfide (MoS2) nanosheets and layered double hydroxide (LDH): Towards reducing fire hazards of epoxy. J. Hazard. Mater. 2017, 338, 343–355. [Google Scholar] [CrossRef] [PubMed]


| Sample Code | TPU (wt%) | MoS2 (wt%) | NiCo-LDH (wt%) | NiCo-LDH/MoS2 (wt%) |
|---|---|---|---|---|
| TPU0 | 100 | 0 | 0 | 0 |
| TPU1 | 98 | 2 | 0 | 0 |
| TPU2 | 98 | 0 | 2 | 0 |
| TPU3 | 98 | 0 | 0 | 2 |
| Sample Code | PHRR kW/m2 | THR MJ/m2 | PSPR m2/s | TSP m2 |
|---|---|---|---|---|
| TPU0 | 1135 | 118.8 | 0.113 | 12.3 |
| TPU1 | 804 | 104.6 | 0.102 | 11.9 |
| TPU2 | 734 | 100.7 | 0.056 | 8.8 |
| TPU3 | 648 | 106.1 | 0.050 | 8.2 |
| Sample Code | T−5% (°C) | Tmax (°C) | Char Yield (%) |
|---|---|---|---|
| TPU0 | 333 | 454 | 5.85 |
| TPU1 | 317 | 432 | 7.93 |
| TPU2 | 264 | 366 | 8.02 |
| TPU3 | 270 | 360 | 11.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Su, W.; Li, L.; Zhao, R.; Fu, H.; Li, J.; Zhang, P.; Guo, Q.; Ma, J. Cooperative Effect of ZIF-67-Derived Hollow NiCo-LDH and MoS2 on Enhancing the Flame Retardancy of Thermoplastic Polyurethane. Polymers 2022, 14, 2204. https://doi.org/10.3390/polym14112204
Qian Y, Su W, Li L, Zhao R, Fu H, Li J, Zhang P, Guo Q, Ma J. Cooperative Effect of ZIF-67-Derived Hollow NiCo-LDH and MoS2 on Enhancing the Flame Retardancy of Thermoplastic Polyurethane. Polymers. 2022; 14(11):2204. https://doi.org/10.3390/polym14112204
Chicago/Turabian StyleQian, Yi, Wenyuan Su, Long Li, Rongmin Zhao, Haoyan Fu, Jiayin Li, Peidong Zhang, Qingjie Guo, and Jingjing Ma. 2022. "Cooperative Effect of ZIF-67-Derived Hollow NiCo-LDH and MoS2 on Enhancing the Flame Retardancy of Thermoplastic Polyurethane" Polymers 14, no. 11: 2204. https://doi.org/10.3390/polym14112204
APA StyleQian, Y., Su, W., Li, L., Zhao, R., Fu, H., Li, J., Zhang, P., Guo, Q., & Ma, J. (2022). Cooperative Effect of ZIF-67-Derived Hollow NiCo-LDH and MoS2 on Enhancing the Flame Retardancy of Thermoplastic Polyurethane. Polymers, 14(11), 2204. https://doi.org/10.3390/polym14112204






