Incorporation of Elastin to Improve Polycaprolactone-Based Scaffolds for Skeletal Muscle via Electrospinning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospining Process
2.3. Characterization of Nanofibrous Scaffolds
2.3.1. Morphological Evaluation
2.3.2. Physicochemical Evaluation
2.3.3. Mechanical Evaluation
2.3.4. Biological Evaluation
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results & Discussion
3.1. Morphological Evaluation of Nanofibrous Scaffolds
3.2. Physicochemical Evaluation of Nanofibrous Scaffolds
3.3. Mechanical Characterization of Nanofibrous Scaffolds
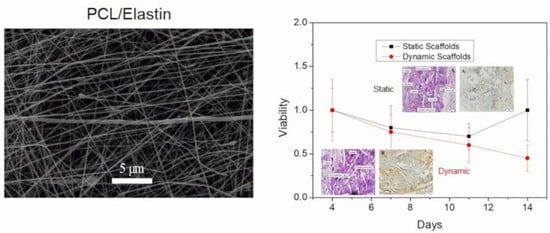
3.4. Biological Evaluation of Nanofibrous Scaffolds
3.4.1. “In Vitro” Evaluation
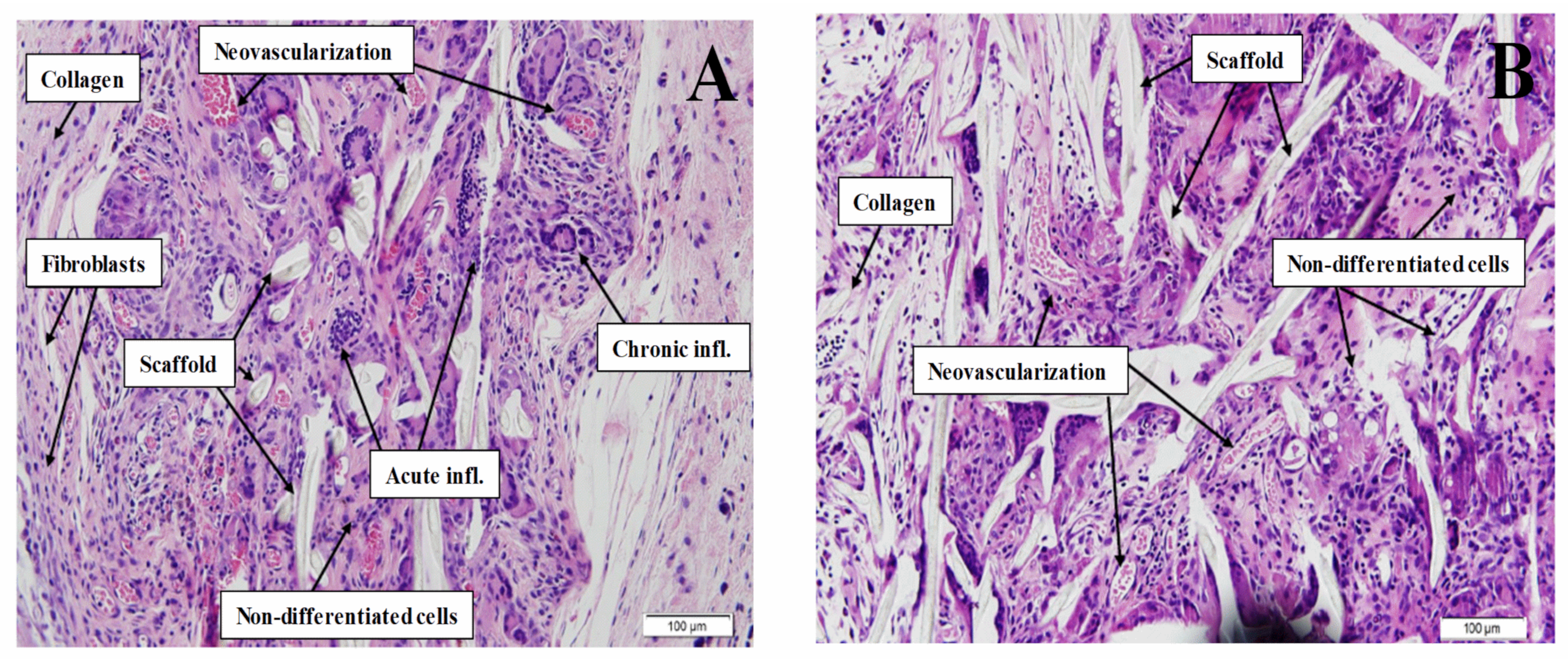
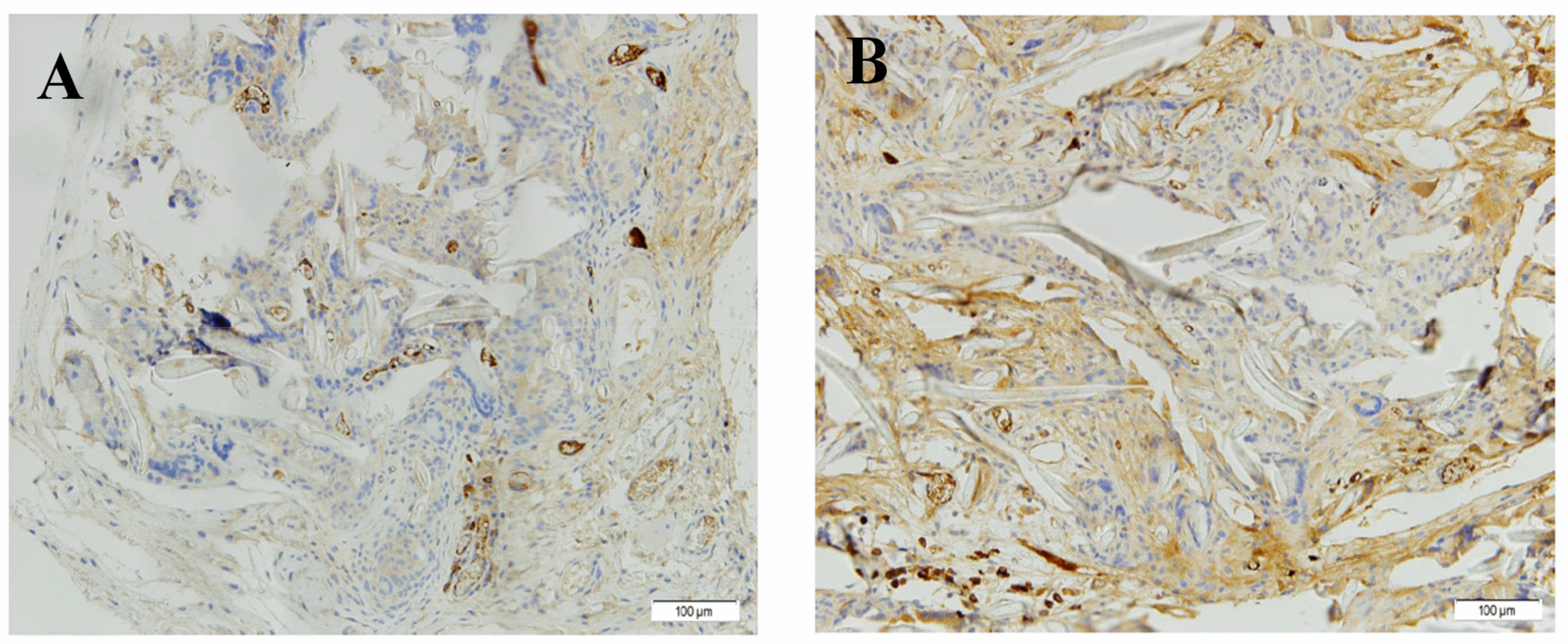
3.4.2. “In Vivo” Evaluation
H&E Staining
Immunohistochemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.P. Allowable limits for toxic leachables: Practical use of ISO 10993-17 standard. The recommendations offered in this chapter should not be construed as guidance from the US Food and Drug Administration (FDA). The mention of commercial products, their sour. In Biocompatibility and Performance of Medical Devices; Biomatech/Elsevier: Sawston, UK, 2012; pp. 95–119. [Google Scholar]
- Lalan, S.; Pomerantseva, I.; Vacanti, J.P. Tissue Engineering and Its Potential Impact on Surgery. World J. Surg. 2001, 25, 1458–1466. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Xing, J.; Lu, Y.; Cui, Y.; Zhu, X.; Luo, F.; Xie, Z.; Wu, X.; Deng, M.; Xu, J.; Hou, T. A Standardized and Quality-Controllable Protocol of Constructing Individual Tissue-Engineered Grafts Applicable to Treating Large Bone Defects. Tissue Eng. Part C Methods 2019, 25, 137–147. [Google Scholar] [CrossRef]
- Paliwal, P.; Pishesha, N.; Wijaya, D.; Conboy, I.M. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging 2012, 4, 553–566. [Google Scholar] [CrossRef]
- Koning, M.; Harmsen, M.C.; van Luyn, M.J.A.; Werker, P.M.N. Current opportunities and challenges in skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2009, 3, 407–415. [Google Scholar] [CrossRef]
- Liao, H.; Zhou, G.-Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng. Part B. Rev. 2009, 15, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, G.; To, F.; Butler, J.R.; Claude, A.; McLaughlin, R.M.; Williams, L.N.; de Jongh Curry, A.L.; Liao, J. Myocardial scaffold-based cardiac tissue engineering: Application of coordinated mechanical and electrical stimulations. Langmuir 2013, 29, 11109–11117. [Google Scholar] [CrossRef]
- Arrigoni, C.; Petta, D.; Bersini, S.; Mironov, V.; Candrian, C.; Moretti, M. Engineering complex muscle-tissue interfaces through microfabrication. Biofabrication 2019, 11, 032004. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Johnson, J.; Ghosh, A.; Lannutti, J. Microstructure-property relationships in a tissue-engineering scaffold. J. Appl. Polym. Sci. 2007, 104, 2919–2927. [Google Scholar] [CrossRef]
- Minuth, W.W.; Schumacher, K.; Strehl, R.; Kloth, S. Physiological and cell biological aspects of perfusion culture technique employed to generate differentiated tissues for long term biomaterial testing and tissue engineering. J. Biomater. Sci. Polym. Ed. 2000, 11, 495–522. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Ghezzi, C.E.; McNamara, S.L.; Black, L.D., III; Kaplan, D.L. Clinical Applications of Naturally Derived Biopolymer-Based Scaffolds for Regenerative Medicine. Ann. Biomed. Eng. 2015, 43, 657–680. [Google Scholar] [CrossRef]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwander, P.; Cossu, G.; Mantero, S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Brown, T.D.; Upton, Z.; Hutmacher, D.W.; Dalton, P.D.; Dargaville, T.R. Dermal fibroblast infiltration of poly(ε-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication 2013, 5, 025001. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Nano/microscale topographically designed alginate/PCL scaffolds for inducing myoblast alignment and myogenic differentiation. Carbohydr. Polym. 2019, 223, 115041. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ɛ-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef]
- Aguirre-Chagala, Y.E.; Altuzar, V.; León-Sarabia, E.; Tinoco-Magaña, J.C.; Yañez-Limón, J.M.; Mendoza-Barrera, C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater. Sci. Eng. C 2017, 76, 897–907. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar] [CrossRef]
- Swindle-Reilly, K.E.; Paranjape, C.S.; Miller, C.A. Electrospun poly(caprolactone)-elastin scaffolds for peripheral nerve regeneration. Prog. Biomater. 2014, 3, 20. [Google Scholar] [CrossRef]
- Knightly, J.J.; Agostino, D.; Cliffton, E.E. The effect of fibrinolysin and heparin on the formation of peritoneal adhesions. Surgery 1962, 52, 250–258. [Google Scholar] [CrossRef]
- Kohn, D.F.; Martin, T.E.; Foley, P.L.; Morris, T.H.; Swindle, M.M.; Vogler, G.A.; Wixson, S.K. Guidelines for the assessment and management of pain in rodents and rabbits. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 97–108. [Google Scholar]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Composite Mats of Poly [(D,L-lactide)-co-glycolide] and Collagen with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Macromol. Mater. Eng. 2009, 294, 611–619. [Google Scholar] [CrossRef]
- Ng, R.; Zhang, X.; Liu, N.; Yang, S.-T. Modifications of nonwoven polyethylene terephthalate fibrous matrices via NaOH hydrolysis: Effects on pore size, fiber diameter, cell seeding and proliferation. Process Biochem. 2009, 44, 992–998. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Guo, B.; Ma, P.X. Nanofiber Yarn/Hydrogel Core–Shell Scaffolds Mimicking Native Skeletal Muscle Tissue for Guiding 3D Myoblast Alignment, Elongation, and Differentiation. ACS Nano 2015, 9, 9167–9179. [Google Scholar] [CrossRef]
- Whited, B.M.; Rylander, M.N. The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 2014, 111, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Kai, D.; Ramakrishna, S. Biocompatibility evaluation of protein-incorporated electrospun polyurethane-based scaffolds with smooth muscle cells for vascular tissue engineering. J. Mater. Sci. 2013, 48, 5113–5124. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ɛ-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef]
- Debelle, L.; Alix, A.J.P.; Wei, S.M.; Jacob, M.-P.; Huvenne, J.-P.; Berjot, M.; Legrand, P. The secondary structure and architecture of human elastin. Eur. J. Biochem. 1998, 258, 533–539. [Google Scholar] [CrossRef]
- Hendrikson, W.J.; Rouwkema, J.; Van Blitterswijk, C.A.; Moroni, L. Influence of PCL molecular weight on mesenchymal stromal cell differentiation. RSC Adv. 2015, 5, 54510–54516. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Mathia, T.G. Anisotropic Wetting of Hydrophobic and Hydrophilic Surfaces–Modelling by Lattice Boltzmann Method. Procedia Eng. 2014, 79, 45–48. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surfaces B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, S.M.; Kang, M.; Shin, H.S.; Youk, J.H. Preparation of hydrophilic PCL nanofiber scaffolds via electrospinning of PCL/PVP-b-PCL block copolymers for enhanced cell biocompatibility. Polymer 2015, 69, 95–102. [Google Scholar] [CrossRef]
- Lee, J.-J.; Yu, H.-S.; Hong, S.-J.; Jeong, I.; Jang, J.-H.; Kim, H.-W. Nanofibrous membrane of collagen–polycaprolactone for cell growth and tissue regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 1927–1935. [Google Scholar] [CrossRef]
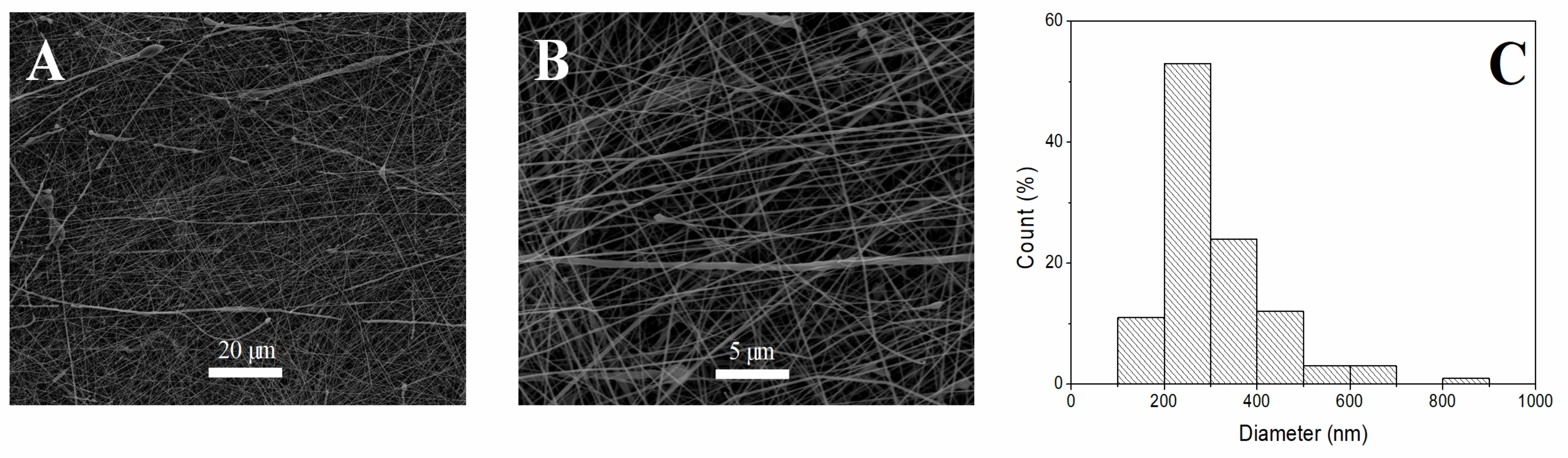


| System | Fiber Diameter (nm) | Alignment (%) | Protein Content (%) | Contact Angle (°) | Young’s Modulus (MPa) | Strain at Break (%) | Maximum Stress (MPa) |
|---|---|---|---|---|---|---|---|
| PCL-Elastin | 269 ± 84 | 50 ± 7 | 2.5 ± 0.5 | 67.5 ± 3.2 | 120.0 ± 28.5 | 25.3 ± 4.2 | 18.1 ± 3.9 |
| PCL | 451 ± 62 | * | * | 102 ± 11 | 35 ± 2.4 | 55 ± 6.7 | 10.6 |
| Parameters | Acute Inflammation | Chronic Inflammation | Collagen Deposition | Fibroblast ACTIVITY | Neovascularization | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Static Scaffolds | Dynamic Scaffolds | Static Scaffolds | Dynamic Scaffolds | Static Scaffolds | Dynamic Scaffolds | Static Scaffolds | Dynamic Scaffolds | Static Scaffolds | Dynamic Scaffolds |
| Slight | 8 | 8 | 5 | 6 | 5 | 7 | 5 | 7 | 7 | 5 |
| Moderate | 1 | 2 | 4 | 3 | 4 | 1 | 5 | 1 | 3 | 3 |
| Severe | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 2 |
| TOTAL | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Puyana, V.; Villanueva, P.; Jiménez-Rosado, M.; de la Portilla, F.; Romero, A. Incorporation of Elastin to Improve Polycaprolactone-Based Scaffolds for Skeletal Muscle via Electrospinning. Polymers 2021, 13, 1501. https://doi.org/10.3390/polym13091501
Perez-Puyana V, Villanueva P, Jiménez-Rosado M, de la Portilla F, Romero A. Incorporation of Elastin to Improve Polycaprolactone-Based Scaffolds for Skeletal Muscle via Electrospinning. Polymers. 2021; 13(9):1501. https://doi.org/10.3390/polym13091501
Chicago/Turabian StylePerez-Puyana, Victor, Paula Villanueva, Mercedes Jiménez-Rosado, Fernando de la Portilla, and Alberto Romero. 2021. "Incorporation of Elastin to Improve Polycaprolactone-Based Scaffolds for Skeletal Muscle via Electrospinning" Polymers 13, no. 9: 1501. https://doi.org/10.3390/polym13091501
APA StylePerez-Puyana, V., Villanueva, P., Jiménez-Rosado, M., de la Portilla, F., & Romero, A. (2021). Incorporation of Elastin to Improve Polycaprolactone-Based Scaffolds for Skeletal Muscle via Electrospinning. Polymers, 13(9), 1501. https://doi.org/10.3390/polym13091501