Polydioxanone-Based Membranes for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Materials
2.3. Characterization
2.4. Biological Evaluation In Vitro
2.4.1. ASC Isolation and Expansion
2.4.2. Cell-Integration Capacity
2.4.3. Cell Migration
2.4.4. Cell Viability
2.4.5. Fluorescence Staining
2.4.6. The hASC Differentiation on Membranes
2.5. Biological Evaluation In Vivo
2.5.1. Sample Preparation for Systemic Toxicity Assays
2.5.2. Systemic Toxicity Assay
2.5.3. Subchronic Systemic-Toxicity Assay
2.5.4. Clinical Evaluation
2.5.5. Histological Evaluation
2.5.6. Blood Evaluation
2.6. Statistical Analysis
3. Results
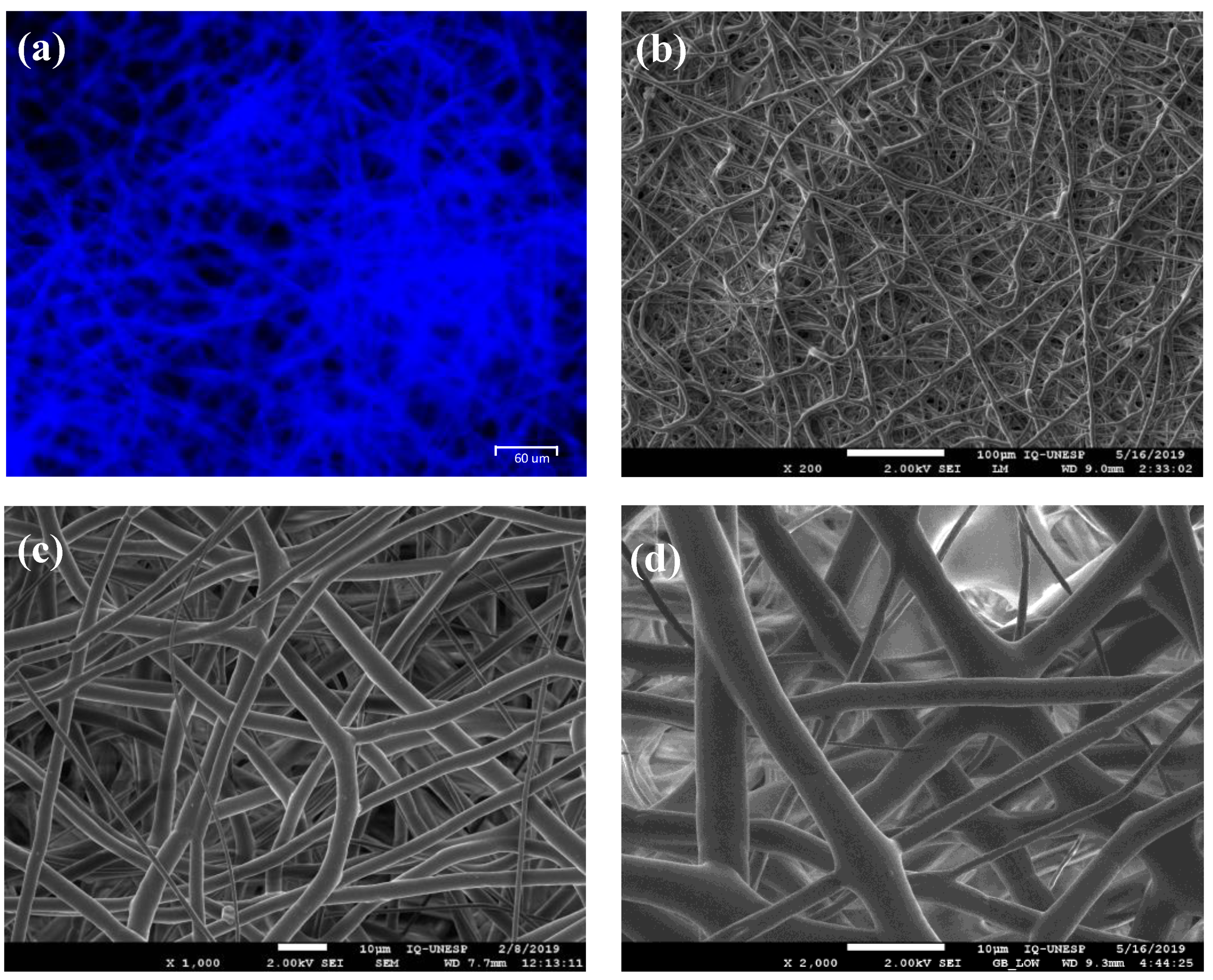
3.1. Membrane Characterization
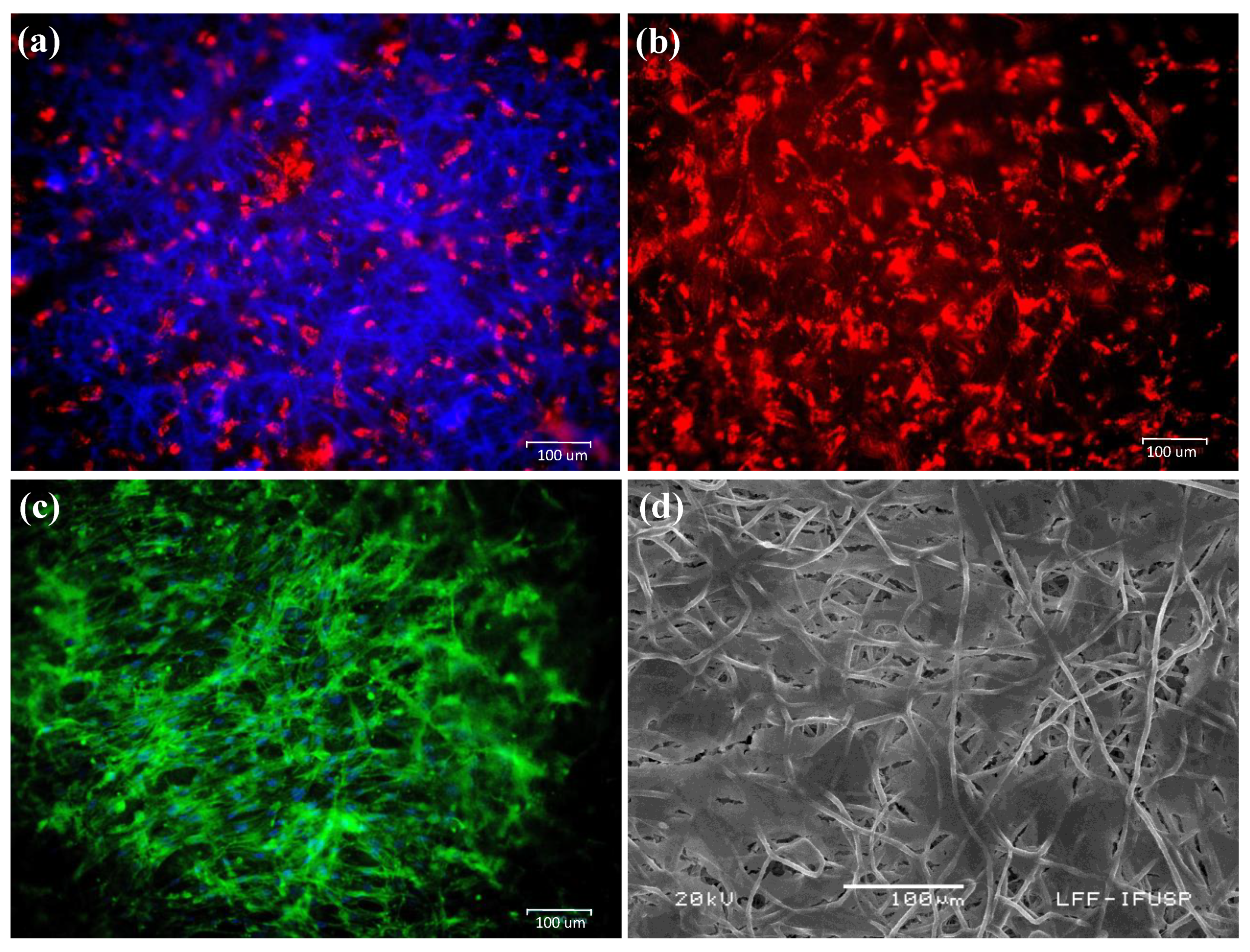
3.2. Integration and Migratory Capacity of hASCs on PDO-Based Membranes
3.3. Osteogenic Differentiation on PDO Membranes
3.4. Toxicity and Clinical Evaluation
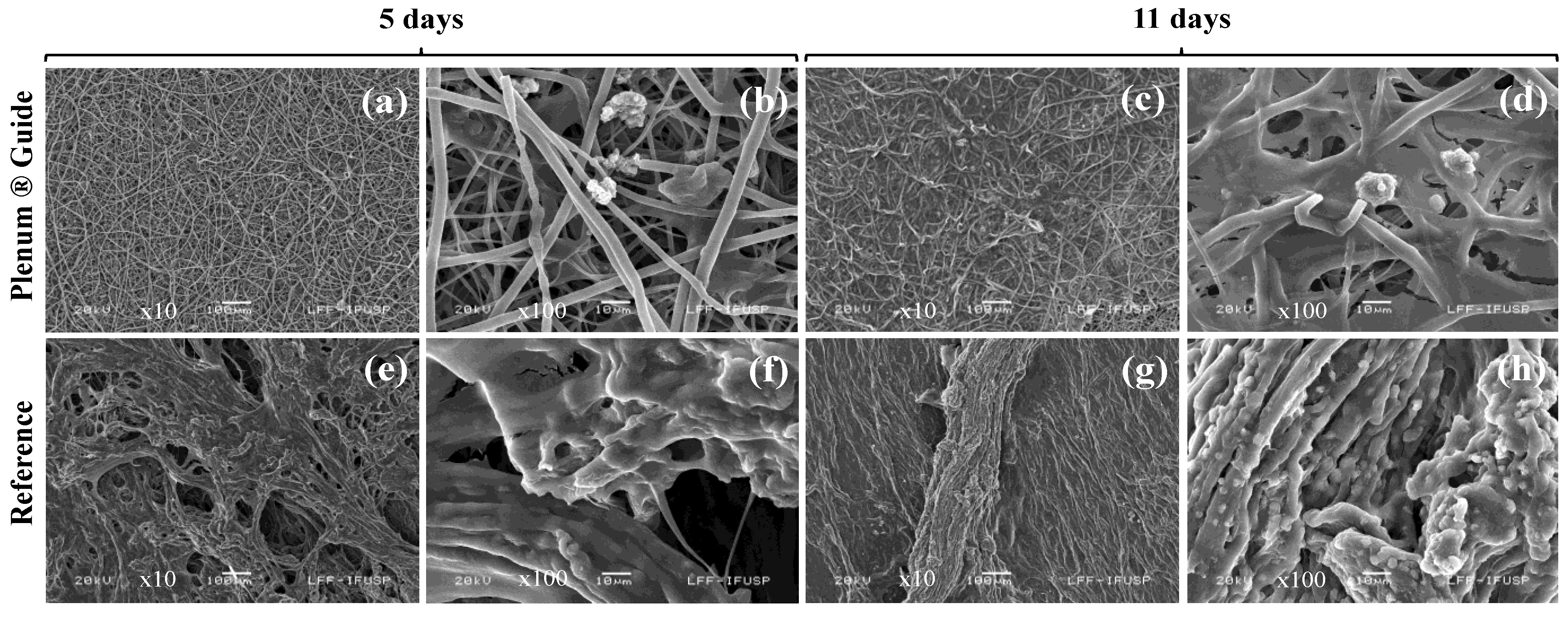
3.5. Histological and Blood Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Retzepi, M.; Donos, N. Guided bone regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.C.L.; Aquilina, T.; Klein, A.; Hakimi, O.; Alexis-Mouthuy, P.; Carr, A.J.; Snelling, S.J.B. A comparative evaluation of the effect of polymer chemistry and fiber orientation on mesenchymal stem cell differentiation. J. Biomed. Mater. Res. Part A 2016, 104, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, X.; Valenzuela, R.G.; Currier, B.L.; Yaszemski, M.J. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin. Orthop. Relat. Res. 2001, S251–S270. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Jing, X.; Guo, W.; Hao, C.; Zhang, Y.; Gao, S.; Chen, M.; Zhang, Z.; Zhang, X.; et al. Bone marrow and adipose tissue-derived mesenchymal stem cells: Characterization, differentiation, and applications in cartilage tissue engineering. Crit. Rev. Euk. Gene Exp. 2018, 28, 285–310. [Google Scholar] [CrossRef]
- Freed, L.E.; Grande, D.A.; Lingbin, Z.; Emmanual, J.; Marquis, J.C.; Langer, R. Joint resurfacing using allograft chondrocytes and synthetic biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1994, 28, 891–899. [Google Scholar] [CrossRef]
- Lee, C.H.; Single, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Lucke, S.; Hoene, A.; Walschus, U.; Kob, A.; Pissarek, J.W.; Schlosser, M. Acute and chronic local inflammatory reaction after implantation of different extracellular porcine dermis collagen matrices in rats. Biomed Res. Int. 2015, 2015, 938059. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.; van Donkelaar, C.C.; Ito, K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012, 347, 613–627. [Google Scholar] [CrossRef]
- Boland, E.D.; Coleman, B.D.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef]
- Goonoo, N.; Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef]
- Song, S.J.; Shin, Y.C.; Kim, S.E.; Kwon, I.K.; Lee, J.H.; Hyon, S.H.; Han, D.W.; Kim, B. Aligned laminin core-polydioxanone/collagen shell fiber matrices effective for neuritogenesis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boenisch, M.; Tamás, H.; Nolst Trenité, G.J. Influence of polydioxanone foil on growing septal cartilage after surgery in an animal model: New aspects of cartilage healing and regeneration (preliminary results). Arch. Facial Plast. Surg. 2003, 5, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Madurantakam, P.A.; McCool, J.M.; Sell, S.A.; Yang, H.; Moon, P.C.; Bowlin, G.L. Mineralization potential of electrospun PDO-hydroxyapatite-fibrinogen blended scaffolds. Int. J. Biomater. 2012, 2012, 159484. [Google Scholar] [CrossRef]
- Kim, T.H.; Oh, S.H.; Chun, S.Y.; Lee, J.H. Bone morphogenetic proteins-immobilized polydioxanone porous particles as an artificial bone graft. J. Biomed. Mater. Res. Part A 2014, 102, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1990, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R.; Pittenger, M.F.; et al. Multilineage potential of adult human mesenchymal stem cells. Science 2016, 284, 143–147. [Google Scholar] [CrossRef]
- Levi, B.; Longaker, M.T. Concise review: Adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 2011, 29, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Travničková, M.; Bačáková, L. Application of adult mesenchymal stem cells in bone and vascular tissue engineering. Physiol. Res. 2018, 67, 831–850. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 2009, 15, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Leslie, P.; Zhang, Y.; Dong, J. Stem cells in a three-dimensional scaffold environment. Springerplus 2014, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Pontailler, M.; Illangakoon, E.; Williams, G.R.; Marijon, C.; Bellamy, V.; Balvay, D.; Autret, G.; Vanneaux, V.; Larghero, J.; Planat-Benard, V.; et al. Polymer-based reconstruction of the inferior vena cava in rat: Stem cells or RGD peptide? Tissue Eng. Part A 2015, 21, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Rodriguez, I.A.; Wesner, D.; Schönherr, H.; Bowlin, G.L.; Jhurry, D. Poly(ester-ether)s: II. Properties of electrospun nanofibres from polydioxanone and poly(methyl dioxanone) blends and human fibroblast cellular proliferation. Biomater. Sci. 2014, 2, 339–351. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-18, Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D570-98, Standard Test Method for Water Absorption of Plastics; ASTM International: West Conshohocken, PA, USA, 2018.
- Lizier, N.F.; Kerkis, A.; Gomes, C.M.; Hebling, J.; Oliveira, C.F.; Caplan, A.I.; Kerkis, I. Scaling-Up of Dental Pulp Stem Cells Isolated from Multiple Niches. PLoS ONE 2012, 7, e39885. [Google Scholar]
- Debnath, T.; Chelluri, L.K. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol. Transfus. Cell Ther. 2018, 41, 7–16. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Kaplan, D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef]
- ISO 10993-11: Biological Evaluation of Medical Devices. Part 11: Tests for Systemic Toxicity; The International Organization for Standardization: Genrva, Switzerland, 2017.
- ISO 10993-6: Biological Evaluation of Medical Devices. Part 6: Tests for Local Effects after Implantation; The International Organization for Standardization: Genrva, Switzerland, 2010.
- Sabino, M.A.; González, S.; Márquez, L.; Feijoo, J.L. Study of the hydrolytic degradation of polydioxanone PPDX. Polym. Degrad. Stab. 2000, 69, 209–216. [Google Scholar] [CrossRef]
- Liu, X.; Feng, S.; Wang, X.; Qi, J.; Lei, D.; Li, Y.; Bai, W. Tuning the mechanical properties and degradation properties of polydioxanone isothermal annealing. Turk. J. Chem. 2020, 44, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.; Hürzeler, M.B.; Schliephake, H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int. J. Oral Maxillofac. Implants 1996, 11, 667–678. [Google Scholar] [PubMed]
- Suckow, M.A.; Stevens, K.A.; Wilson, R.P. (Eds.) The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; American College of Laboratory Animal Medicine, Elsevier: London, UK, 2012; ISBN 978-0-12-380920-9. [Google Scholar]
- Fatkhudinov, T.; Tsedik, L.; Arutyunyan, I.; Lokhonina, A.; Makarov, A.; Korshunov, A.; Elchaninov, A.; Kananykhina, E.; Vasyukova, O.; Usman, N.; et al. Evaluation of resorbable polydioxanone and polyglycolic acid meshes in a rat model of ventral hernia repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 652–663. [Google Scholar] [CrossRef]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. Ist. Super Sanità 2015, 51, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Coïc, M.; Placet, V.; Jacquet, E.; Meyer, C. Propriétés mécaniques des membranes de collagne. Rev. Stomatol. Chir. Maxillofac. 2010, 111, 286–290. [Google Scholar] [CrossRef]
- Charulatha, V.; Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003, 24, 759–767. [Google Scholar] [CrossRef]
- Becker, S.T.; Terheyden, H.; Fabel, M.; Kandzia, C.; Möller, B.; Wiltfang, J. Comparison of collagen membranes and polydioxanone for reconstruction of the orbital floor after fractures. J. Craniofac. Surg. 2010, 21, 1066–1068. [Google Scholar] [CrossRef]
- Rimmer, J.; Ferguson, L.M.; Saleh, H.A. Versatile applications of the polydioxanone plate in rhinoplasty and septal surgery. Arch. Facial Plast. Surg. 2012, 14, 323–330. [Google Scholar] [CrossRef] [PubMed]
- San-Marina, S.; Sharma, A.; Voss, S.G.; Janus, J.R.; Hamilton, G.S. Assessment of scaffolding properties for chondrogenic differentiation of adipose-derived mesenchymal stem cells in nasal reconstruction. JAMA Facial Plast. Surg. 2017, 19, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Dörfer, C.E.; Kim, T.S.; Steinbrenner, H.; Holle, R.; Eickholz, P. Regenerative periodontal surgery in interproximal intrabony defects with biodegradable barriers. J. Clin. Periodontol. 2000, 27, 162–168. [Google Scholar] [CrossRef]
- Pretzl, B.; Kim, T.S.; Steinbrenner, H.; Dörfer, C.; Himmer, K.; Eickholz, P. Guided tissue regeneration with bioabsorbable barriers III 10-year results in infrabony defects. J. Clin. Periodontol. 2009, 36, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Burggasser, G.; Gauss, N.; Ewers, R. Orbital floor reconstruction with an alloplastic resorbable polydioxanone sheet. Int. J. Oral Maxillofac. Surg. 2002, 31, 367–373. [Google Scholar] [CrossRef]
- Kontio, R.; Suuronen, R.; Salonen, O.; Paukku, P.; Konttinen, Y.T.; Lindqvist, C. Effectiveness of operative treatment of internal orbital wall fracture with polydioxanone implant. Int. J. Oral Maxillofac. Surg. 2001, 30, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Merten, H.A.; Luhr, H.G. Resorbable synthetics (PDS foils) for bridging extensive orbital wall defects in an animal experiment comparison. Fortschr Kiefer Gesichtschir 1994, 39, 186–190. [Google Scholar]
- Kontio, R.; Ruuttila, P.; Lindroos, L.; Suuronen, R.; Salo, A.; Lindqvist, C.; Virtanen, I.; Konttinen, Y.T. Biodegradable polydioxanone and poly (L/D) lactide implants: An experimental study on peri-implant tissue response. Int. J. Oral Maxillofac. Surg. 2005, 34, 766–776. [Google Scholar] [CrossRef]
- Schmitt, S.C.; Wiedmann-Al-Ahmad, M.; Kuschnierz, J.; Al-Ahmad, A.; Huebner, U.; Schmelzeisen, R.; Gutwald, R. Comparative in vitro study of the proliferation and growth of ovine osteoblast-like cells on various alloplastic biomaterials manufactured for augmentation and reconstruction of tissue or bone defects. J. Mater. Sci. Mater. Med. 2008, 19, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, F.; Behrens, E.; Kern, M.; Gassling, V.; Wiltfang, J. Mechanical properties of collagen membranes: Are they sufficient for orbital floor reconstructions? J. Cranio Maxillofac. Surg. 2015, 43, 260–263. [Google Scholar] [CrossRef]
- Birkenfeld, F.; Flörke, C.; Behrens, E.; Rohnen, M.; Kern, M.; Gassling, V.; Wiltfang, J. Mechanical properties of collagen membranes modified with pores—are they still sufficient for orbital floor reconstruction? Br. J. Oral Maxillofac. Surg. 2015, 53, 957–962. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Bhowmick, S.; Scharnweber, D.; Koul, V. Co-cultivation of keratinocyte-human mesenchymal stem cell (hMSC) on sericin loaded electrospun nanofibrous composite scaffold (cationic gelatin/hyaluronan/chondroitin sulfate) stimulates epithelial differentiation in hMSCs: In vitro study. Biomaterials 2016, 88, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kendal, A.; Snelling, S.; Dakin, S.; Stace, E.; Mouthuy, P.A.; Carr, A. Resorbable electrospun polydioxanone fibres modify the behaviour of cells from both healthy and diseased human tendons. Eur. Cells Mater. 2017, 33, 169–182. [Google Scholar] [CrossRef]
- Fleischer, S.; Miller, J.; Hurowitz, H.; Shapira, A.; Dvir, T. Effect of fiber diameter on the assembly of functional 3D cardiac patches. Nanotechnology 2015, 26, 291002. [Google Scholar] [CrossRef]
- Schorn, L.; Handschel, J.; Lommen, J.; Von Beck, F.P.; Depprich, R.; Kübler, N.; Holtmann, H. Evaluation of biocompatibility of different membrane surfaces using unrestricted somatic stem cells. In Vivo 2019, 33, 1447–1454. [Google Scholar] [CrossRef]
- Açil, Y.; Zhang, X.; Nitsche, T.; Möller, B.; Gassling, V.; Wiltfang, J.; Gierloff, M. Effects of different scaffolds on rat adipose tissue derived stroma cells. J. Cranio Maxillofac. Surg. 2014, 42, 825–834. [Google Scholar] [CrossRef]
- Trojahn Kølle, S.F.; Oliveri, R.S.; Glovinski, P.V.; Elberg, J.J.; Fischer-Nielsen, A.; Drzewiecki, K.T. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J. Plast. Surg. Hand Surg. 2012, 46, 59–68. [Google Scholar] [CrossRef] [PubMed]


| Score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Bone neoformation/response | Osteoblast rhyme | 0 | Rare, 1–5 | 5–10 | Infiltrate | Accumulated |
| Osteoclasts | 0 | Rare, 1–5 | 5–10 | Infiltrate | Accumulated | |
| Immature bone | 0 | Minimum | Middle | Moderate | Severe | |
| Non-mineralized osteoid matrix | 0 | Minimum | Middle | Moderate | Severe | |
| Necrosis | 0 | Minimum | Middle | Moderate | Severe | |
| Tissue response | Neovascularization | 0 | Minimal capillary proliferation, focal 1–3 shoots | Groups of 4–7 capillaries with fibroblastic support structures | Broad bands of capillaries with support structures | Extensive range of capillaries with fibroblastic support structures |
| Fibrosis | 0 | Narrow range | Moderate | Thick band | Extensive | |
| Fatty Infiltrate | 0 | Minimum amount of fat associated with fibrosis | Several layers of fat and accumulation of fibrous tissue | Elongated and wide fat cells on the implant site | Extensive fat that completely involves the implant | |
| Tm (°C) | ∆Hm (J/g) | %Xc | ||
|---|---|---|---|---|
| Before sterilization | Mean | 102.36 | −74.62 | 56.39 |
| SD | 0.43 | 4.19 | 0.70 | |
| After sterilization | Mean | 107.35 | −79.62 | 52.86 |
| SD | 0.48 | 0.99 | 2.97 |
| Membrane | Migration Rate |
|---|---|
| PDO | +++ |
| Collagen | ++ |
| Bone Neoformation/Response | Treated Group | Control Group |
|---|---|---|
| Osteoblast rhyme (sum of the group) | 15 | 13 |
| Osteoclasts (sum of the group) | 1 | 0 |
| Immature bone (sum of the group) | 18 | 17 |
| Non-mineralized osteoid matrix (sum of the group) | 13 | 12 |
| Necrosis (sum of the group) | 0 | 0 |
| Subtotal (2×) | 94 | 84 |
| Neovascularization (sum of the group) | 2 | 1 |
| Fibrosis (sum of the group) | 1 | 0 |
| Fatty infiltrate (sum of the group) | 2 | 1 |
| Subtotal | 5 | 2 |
| Group total | 99 | 86 |
| Group mean | 9.9 | 8.6 |
| Mean index 1 | 1.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saska, S.; Pilatti, L.; Silva, E.S.d.S.; Nagasawa, M.A.; Câmara, D.; Lizier, N.; Finger, E.; Dyszkiewicz Konwińska, M.; Kempisty, B.; Tunchel, S.; et al. Polydioxanone-Based Membranes for Bone Regeneration. Polymers 2021, 13, 1685. https://doi.org/10.3390/polym13111685
Saska S, Pilatti L, Silva ESdS, Nagasawa MA, Câmara D, Lizier N, Finger E, Dyszkiewicz Konwińska M, Kempisty B, Tunchel S, et al. Polydioxanone-Based Membranes for Bone Regeneration. Polymers. 2021; 13(11):1685. https://doi.org/10.3390/polym13111685
Chicago/Turabian StyleSaska, Sybele, Livia Pilatti, Edvaldo Santos de Sousa Silva, Magda Aline Nagasawa, Diana Câmara, Nelson Lizier, Eduardo Finger, Marta Dyszkiewicz Konwińska, Bartosz Kempisty, Samy Tunchel, and et al. 2021. "Polydioxanone-Based Membranes for Bone Regeneration" Polymers 13, no. 11: 1685. https://doi.org/10.3390/polym13111685
APA StyleSaska, S., Pilatti, L., Silva, E. S. d. S., Nagasawa, M. A., Câmara, D., Lizier, N., Finger, E., Dyszkiewicz Konwińska, M., Kempisty, B., Tunchel, S., Blay, A., & Shibli, J. A. (2021). Polydioxanone-Based Membranes for Bone Regeneration. Polymers, 13(11), 1685. https://doi.org/10.3390/polym13111685










