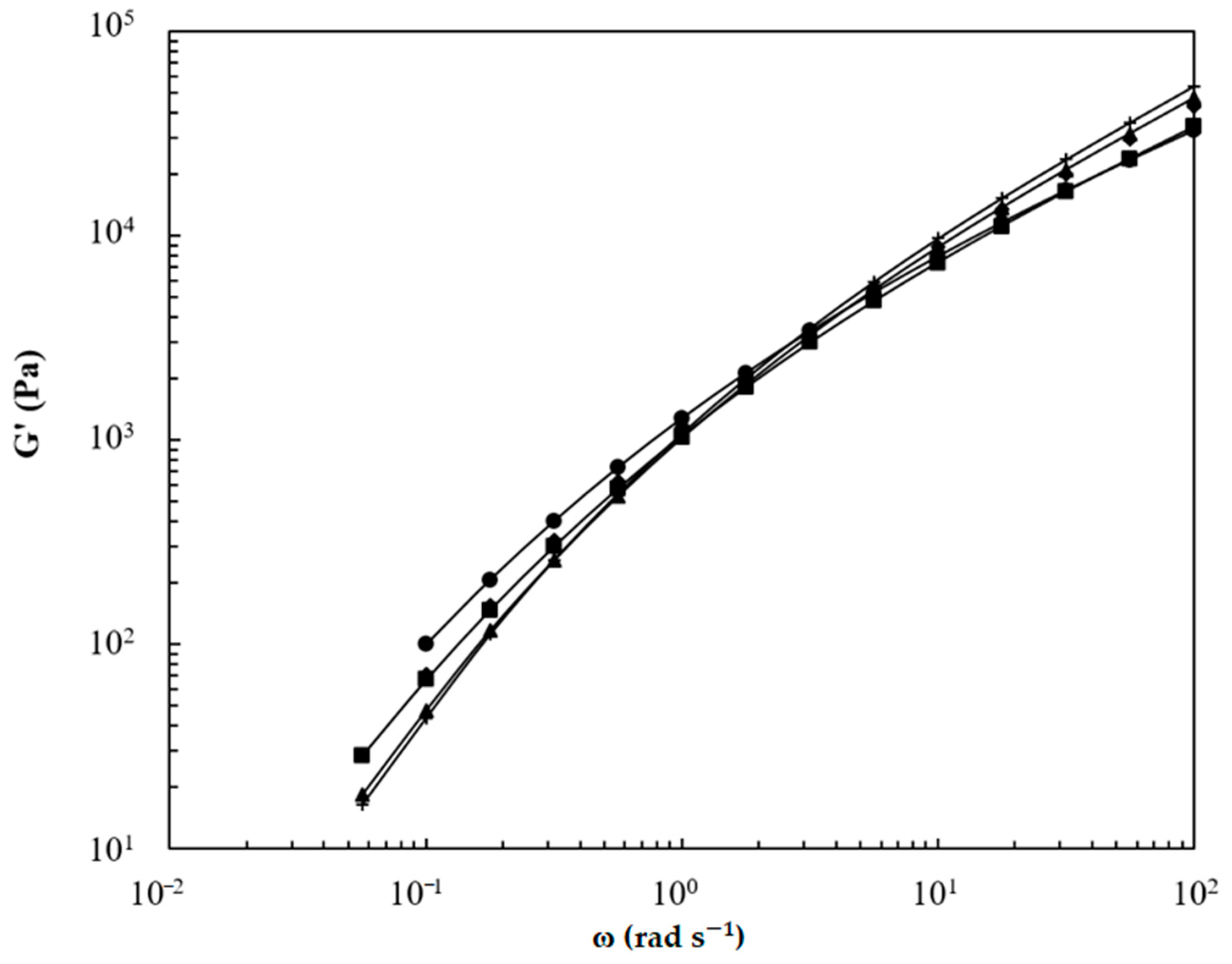
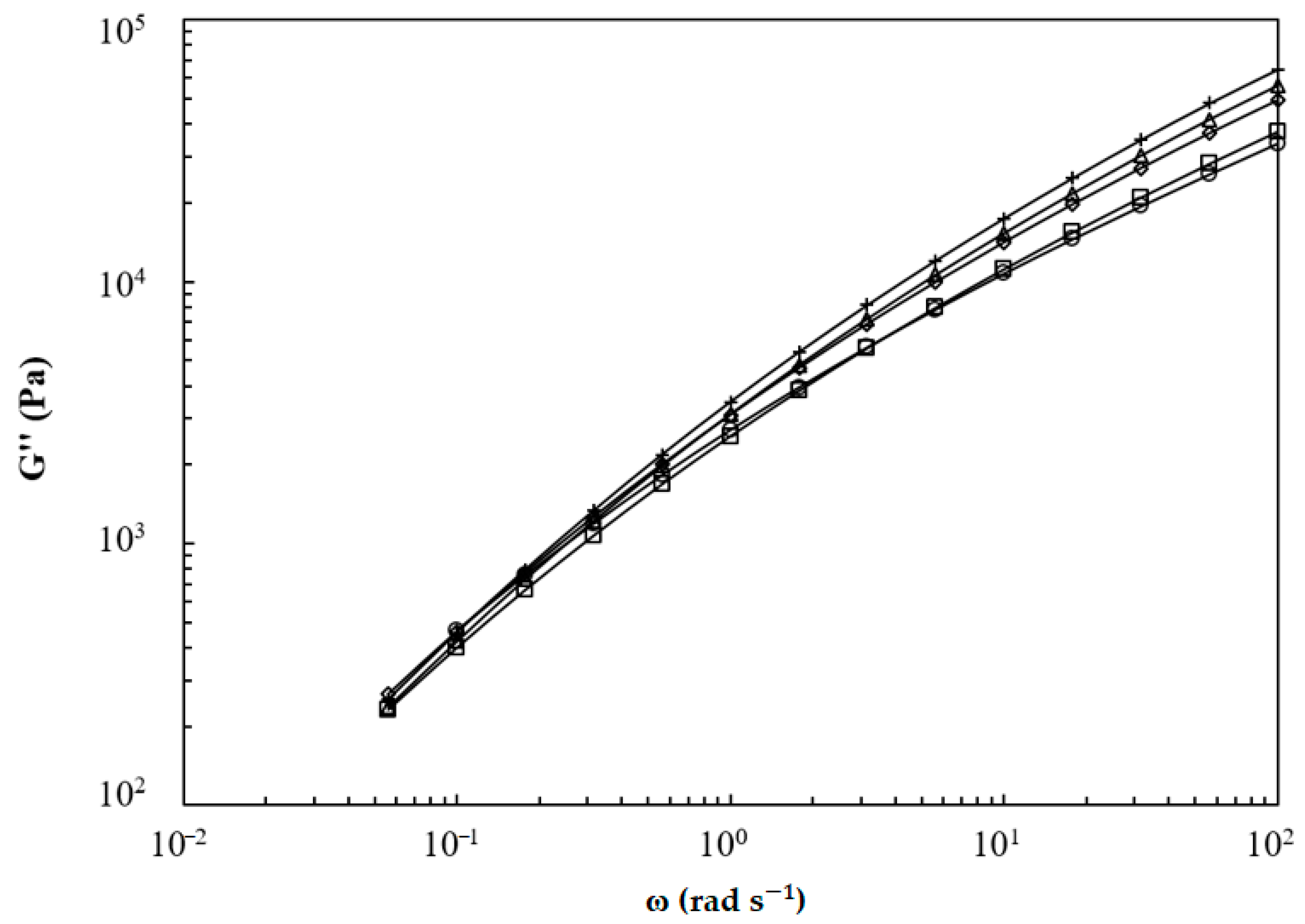
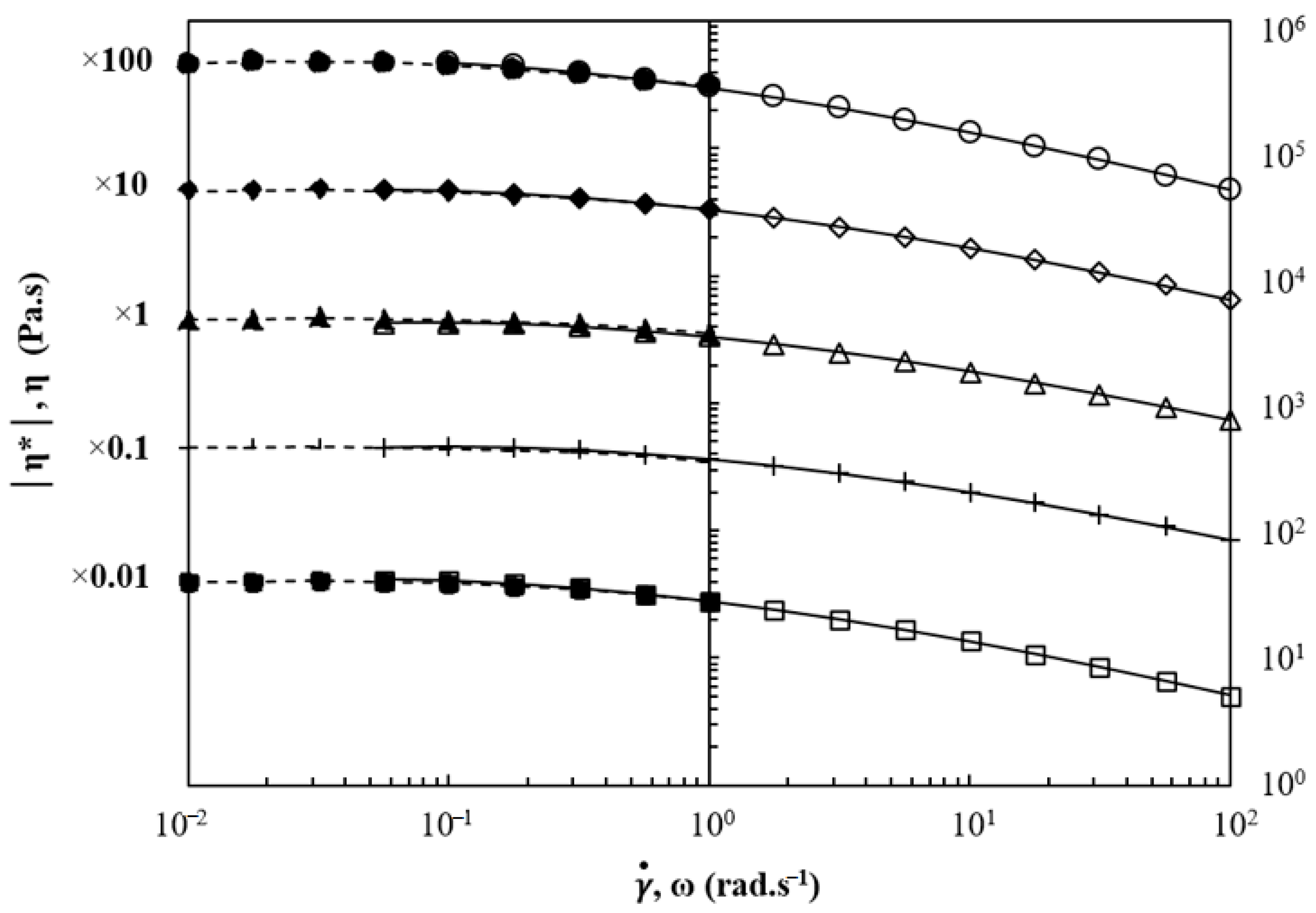
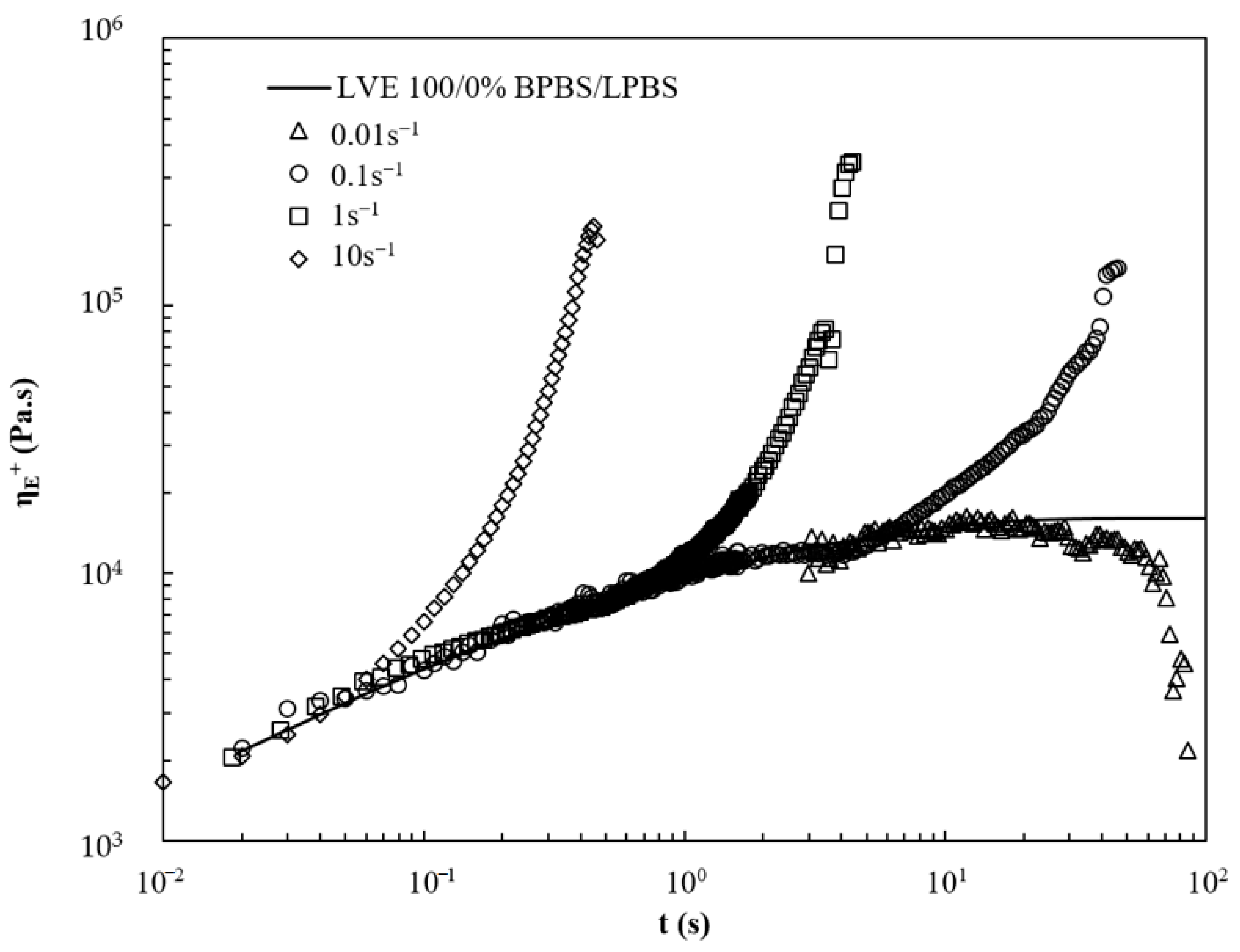
3.1. Analysis of the Macromolecular Architecture of Linear and Branched PBS
The values of weight- and number-average molecular weights (
Mw and
Mn respectively), polydispersity index (PI as the
Mw/
Mn ratio), intrinsic viscosity [
η] and Mark Houwink Sakurada (MHS) parameters related to each polymer are listed in
Table 2. The mean value of the radius of gyration (
rg) was directly calculated by the software from the measured value of the hydrodynamic radius
rh thanks to the Fox–Flory relationship [
25].
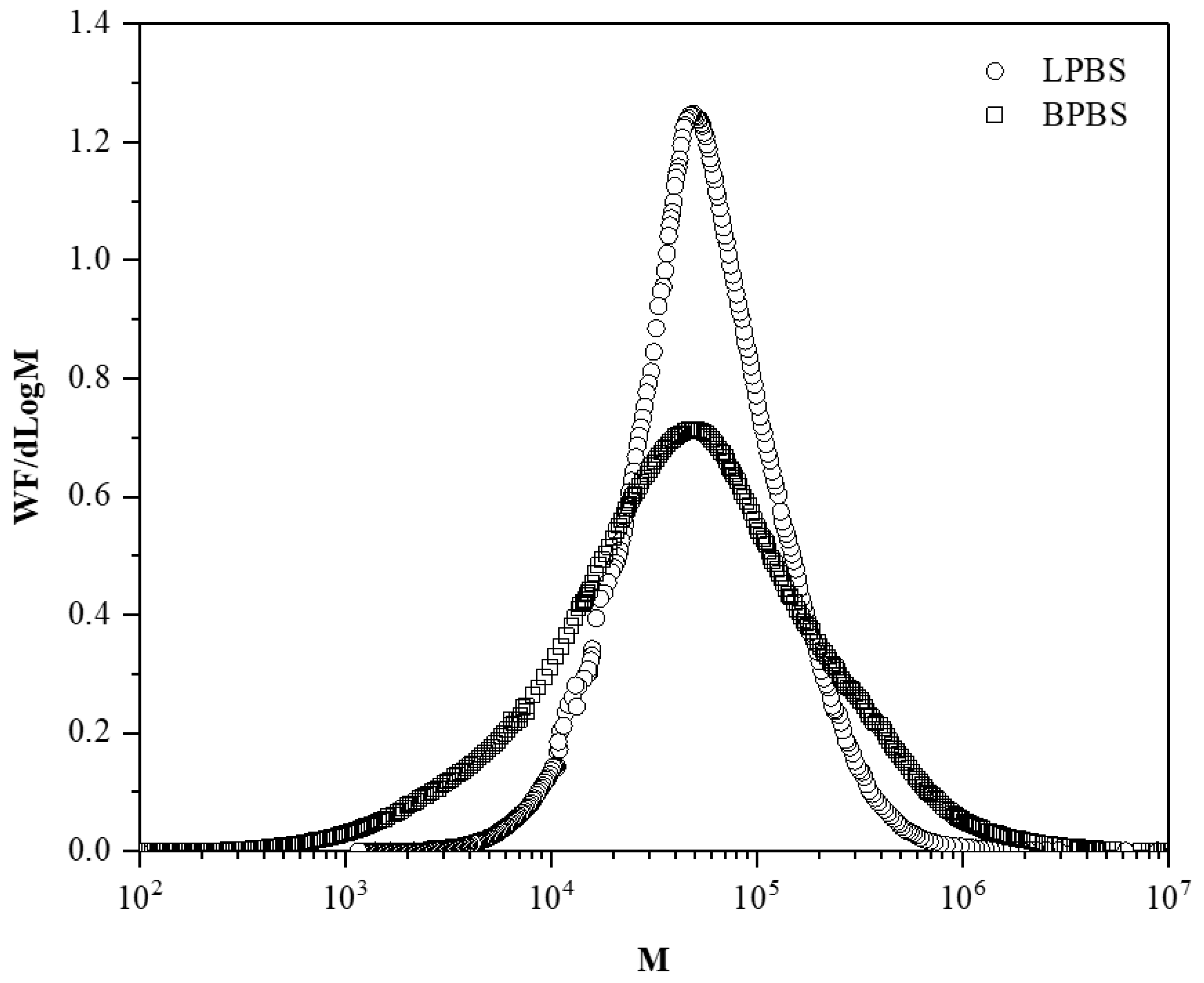
Molecular weight distributions of LPBS and BPBS samples are illustrated in
Figure 1 and show the relatively narrow distribution of the linear PBS (LPBS) compared to the broader distribution of the branched PBS (BPBS) that is in agreement with the polydispersity values. The high polydispersity index and broad molecular weight distribution of BPBS is a first evidence of the presence of LCB in this PBS grade [
26]. Both samples exhibit a log-Gaussian molar mass monomodal distribution. The intrinsic viscosities of LPBS and BPBS, denoted [
ηLPBS] and [
ηBPBS], respectively, were directly measured from the viscosity detector for each eluted fraction of polymer. In a semi-logarithmic graph, MHS parameters were determined from the slope and intercept of the intrinsic viscosity as a function of molecular weight:
where
K and
a are dependent on the considered polymer–solvent system and on the measurement temperature.
The exponent
a gives information on the conformation of the polymer chains in the solvent. A value between 0 and 0.5 is generally obtained for branched chains, and a value comprised between 0.5 and 0.8 for is obtained for flexible chains [
25]. The MHS parameters of PBS were reported only once by Garin et al. on an in-house synthetized linear PBS characterized by SEC coupled with multi-angle laser light scattering (SEC-MALLS). They found an average value of
a = 0.71 (+/−0.1) and
K = 39.94 × 10
−5 (+/−6.31 × 10
−5) dL·g
−1 [
27], which are in total agreement with our values for LPBS. However, to the best of our knowledge, no study has previously reported the MHS parameters for long-chain branched PBS. For BPBS, we found a much higher value of
K = 325 × 10
−5 dL·g
−1 and a lower value of
a = 0.528, indicating a lower flexibility of polymer chains related to branching. In their study on the role of the architecture on the conformation in dilute solution of in-house synthetized polystyrenes (PS), Kharchenko et al. proposed a deep characterization of linear, star, and hyperbranched PS. For the same branching density, they found that a star PS exhibited a lower value (
a = 0.68) and even much lower value for hyperbranched PS (
a = 0.39) compared to the linear counterpar of equivalent molecular weight.
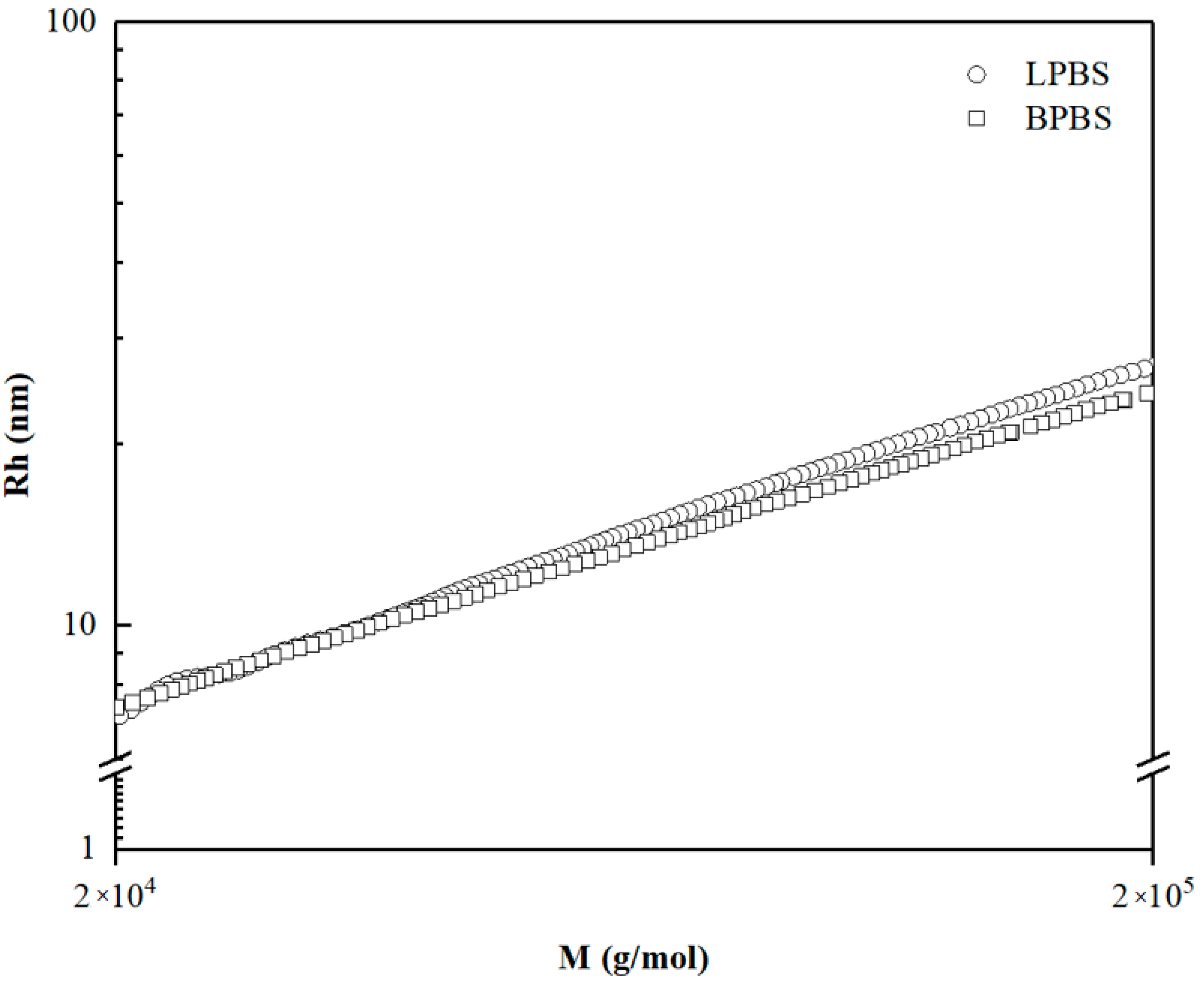
Figure 2 shows the hydrodynamic radius of LPBS and BPBS as a function of molecular weight
M. At low molecular weight, BPBS and LPBS exhibit the same value of hydrodynamic radius, meaning that the macromolecules below 40,000 g/mol are mostly linear. Above 40,000 g/mol, the radius of BPBS fall below that of LPBS, as expected by the lower volume occupied by the coil of a branched chain which is more compact than a linear one of the same molar mass [
4]. Thus, the continuous decrease of the radius depicts the increase of LCB as the molecular weight increases.
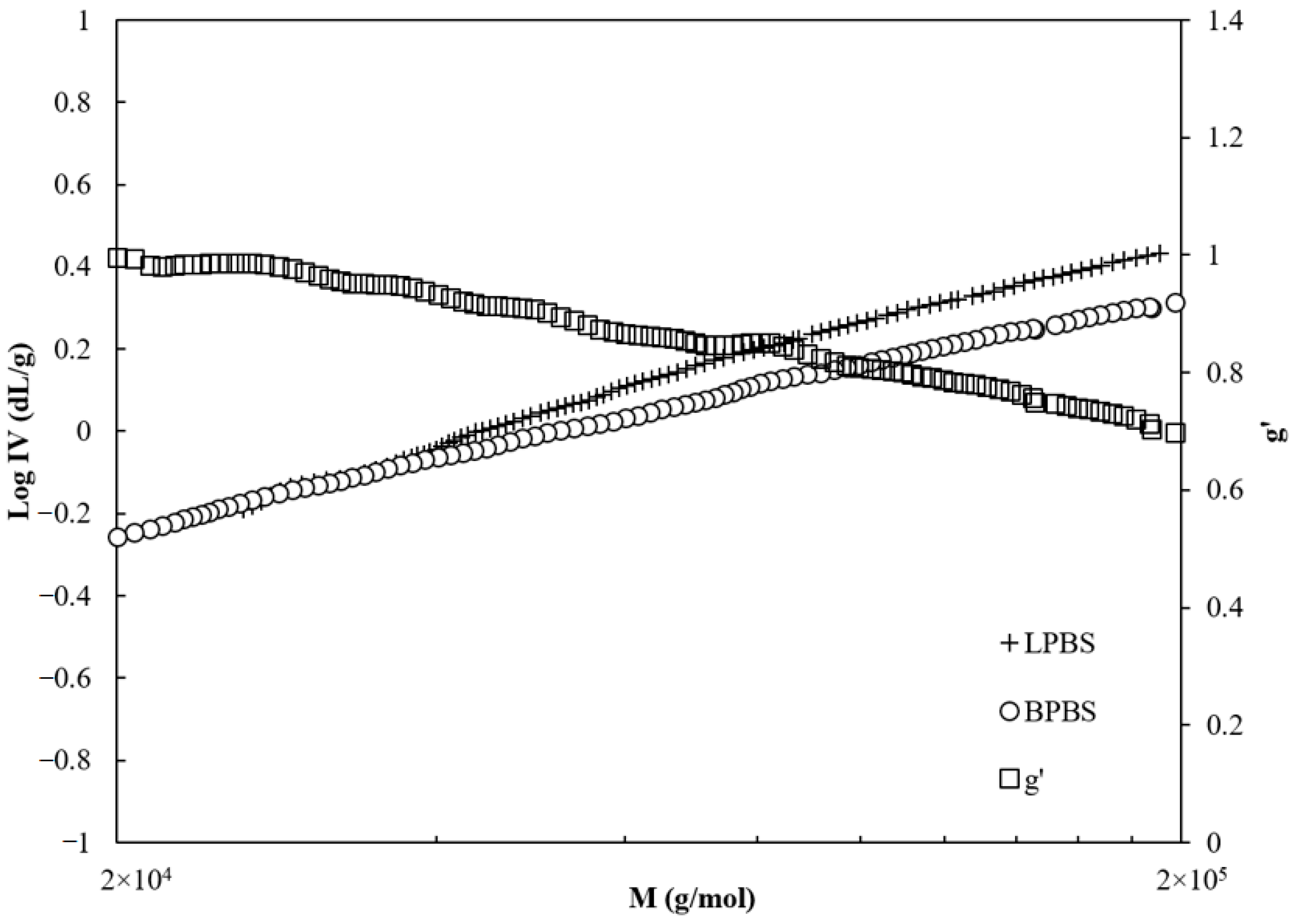
Consequently, to the reduction of the hydrodynamic radius in this range of molecular weight (i.e., >40,000 g/mol), the intrinsic viscosity of BPBS is lower than that of LPBS, as shown in
Figure 3. At the same time, the viscosity branching index (
) (given by Equation (2)) decreases almost monotonically from 0.9 (
would be equal to 1 for linear chains) to 0.65 on the range of molecular weights accordingly with the BPBS decreasing viscosity.
where [
η] is the intrinsic viscosity and the subscripts
b and
l correspond to the branched and linear counterpart, respectively, which are considered at the same molecular weight.
The decrease of viscosity branching index indicates that the chains are mostly linear below 40,000 g/mol and that higher molecular weight molecules have a larger branching degree.
Assuming that the BPBS is randomly branched, which is the case for most branched polyesters in nature [
28], a Zimm–Stockmayer model for randomly distributed branch points per molecule with a random distribution of branch length (polydispersed) tri-functional polymers [
29] (Equation (3)) was used to determine the branching characteristics of the polymer.
The former relationship links the branching index (
) to the weight-average number of branch points per molecule (
Bw) and the LCB frequency (λ) (average number of branch points per 1000 monomers) given by:
where
M0 is the molecular weight of a monomer and
Mn is the number-average molecular weight. For PBS,
M0 = 172 g/mol.
In the present study, only the viscosity branching index
was determined. The viscosity branching index and the branching index
are related through
ε known as the shielding factor (sometimes called the drainage factor) equal to 3/2 in the non-draining case. This factor is known to be dependent on the branching structure and has shown to be equal to 0.5 for star polymers while
ε is closer to 1.5 for comb-shaped structures [
30]:
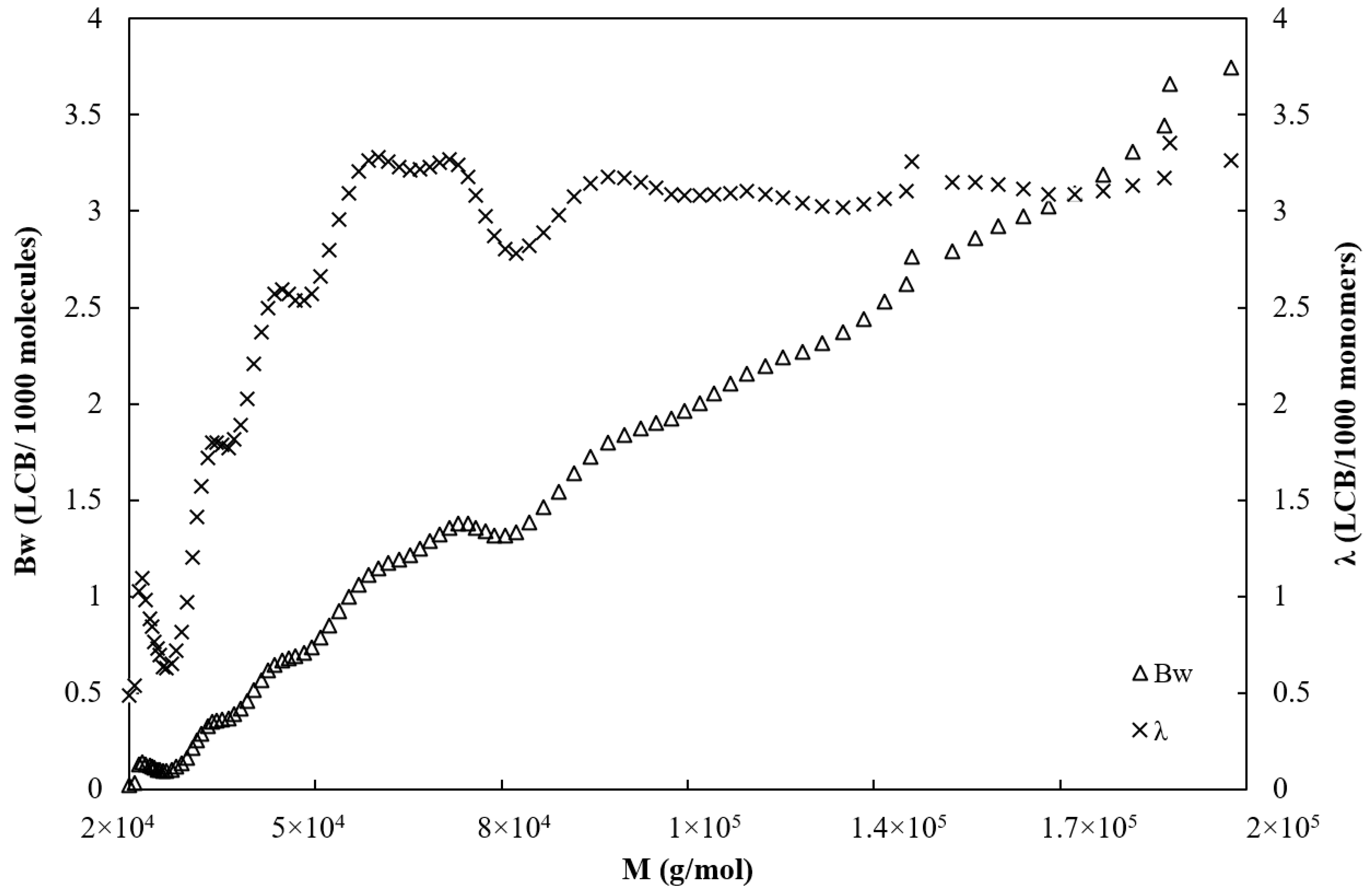
Figure 4 shows the average number of branch points per molecule
Bw and the LCB frequency λ plotted versus the molecular weight. The number of branches is ranged from 0 and 0.5 between 20,000 and 40,000 g/mol and starts to grow sharply above 40,000 g/mol from 0.5 to 3.7 at 200,000 g/mol.
Meanwhile, the branching frequency increases sharply from 0.5 LCB per 1000 units to approximately 3 LCB per 1000 units in the range of molecular weight comprised between 20,000 and 60,000 g/mol and then remains constant at 3 LCB/1000 units on the range of 60,000 and 200,000 g/mol.
Values of the average viscosity branching index, average number of LCB per molecule, and LCB frequency calculated by OmniSEC software are given in
Table 3.
Only very scarce studies were carried out on the characterization of long-chain branched polybutylene succinate. Although Wang et al. [
31] studied the synthesis, characterization, and properties of long-chain branched PBS, they did not evaluate the branching characteristics and only determined the conventional molecular features (
Mw,
Mn, PI) by the use of conventional calibration (leading to possible misinterpretation of
Mw). Kim et al. [
32] used SEC-MALLS to determine the molecular characteristics of in-house synthetized branched PBS in chloroform. Unfortunately, they did not report any branching characteristics nor MHS coefficients. Two decades ago, Yoshikawa et al. performed SEC coupled with multi-angle laser light scattering (MALLS) detector on the same polymer grades used in this study [
24]. The authors did not report the distribution of the number of LCB per molecule as a function of molecular weight and did not report the branching frequency.
They only give a mean value of the branching index as a function of the molecular weight calculated from pretty scattered data. They found an average number of branch points close to 2 LCB per molecule, which is consistent with our results and could confirm the shielding factor hypothesis we made. Finally, with a mean value of 2 LCB per molecule, Yoshikawa et al. suggested that the branching architecture could be an H-shaped molecule.
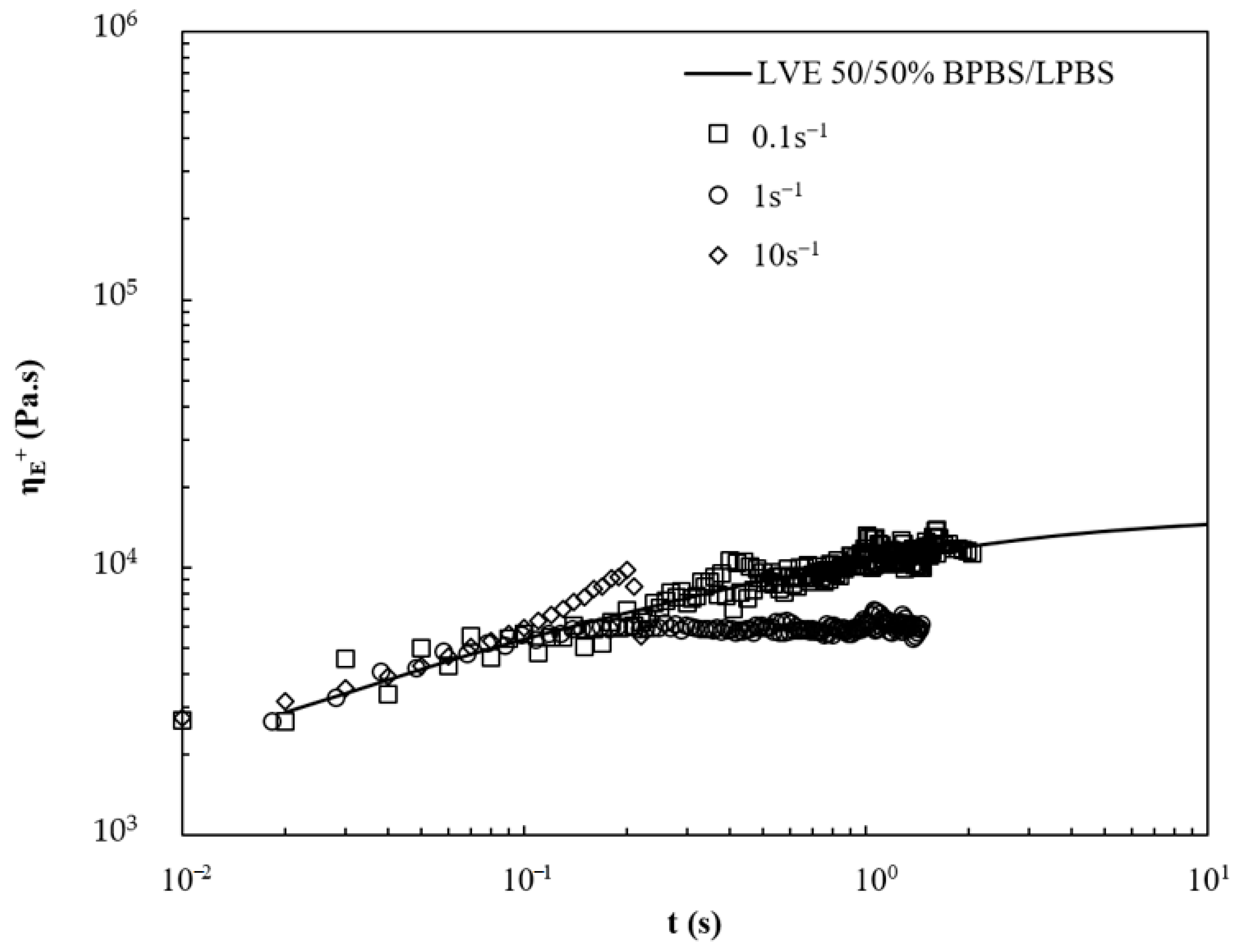
Focusing on the studies reporting the branching structure of polyolefins, the rheological behavior of the blends of linear and branched polymers depended heavily on the amount of LCB, on the molecular weight, on the type of branching (or topology), and on the relative composition of the mixture.
Stange et al. studied the impact of blending linear and long-chain branched PP on the shear and elongational flow behavior [
4]. They showed that increasing the amount of LCB in the LCB-PP/linear PP blend resulted in a more pronounced shear thinning behavior, which is beneficial for processing. This effect was attributed to both the higher polydispersity and the increasing amount of LCB with the addition of LCB-PP in the blend. The type of architecture has also been proven to greatly influence the rheological behavior. For instance, the Zero-Shear rate Viscosity (ZSV) of long-chain branched PE with a moderate degree of star-like molecular structure has been shown to be enhanced over that of its linear counterpart, whereas a highly tree-like long-chain branched one exhibits a lower ZSV than its linear homologue [
4].
Despite the difficulty of understanding the relationships between the molecular architecture and the resulting rheological properties, especially regarding the commercial nature and the impact of blending architectures, we show, in the following, a relationship with the rheological characterization of these architectures and blends.