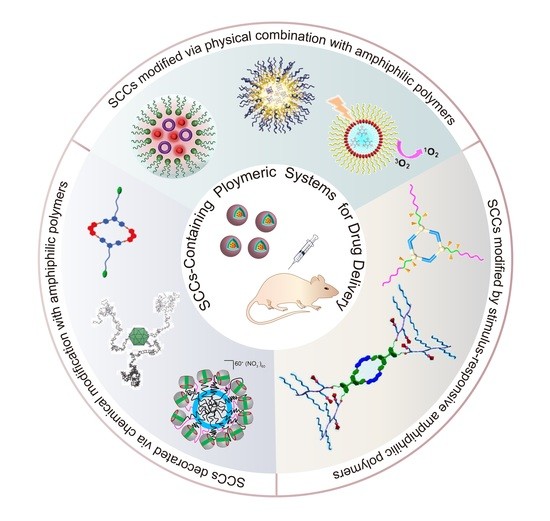
Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery
Abstract
1. Introduction
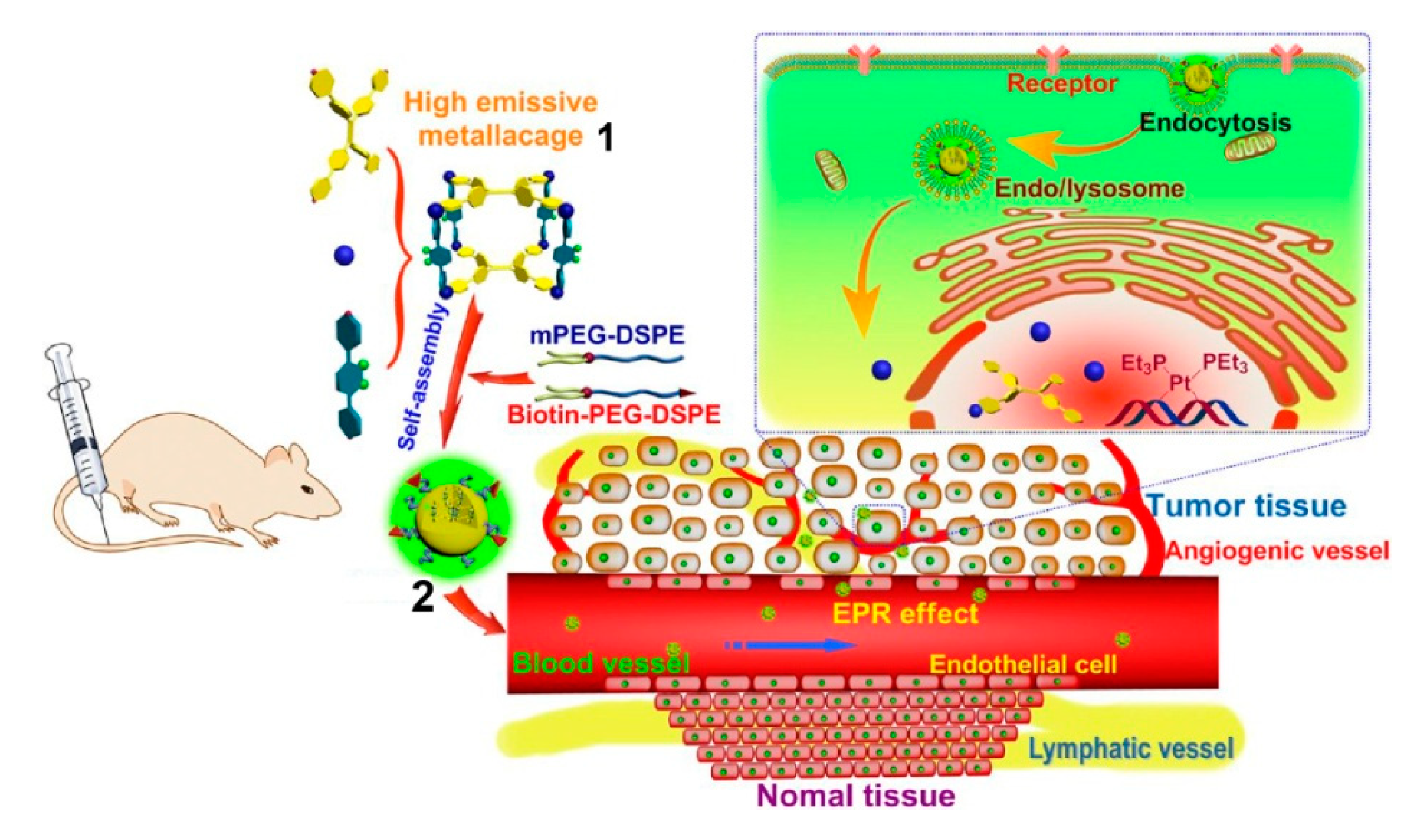
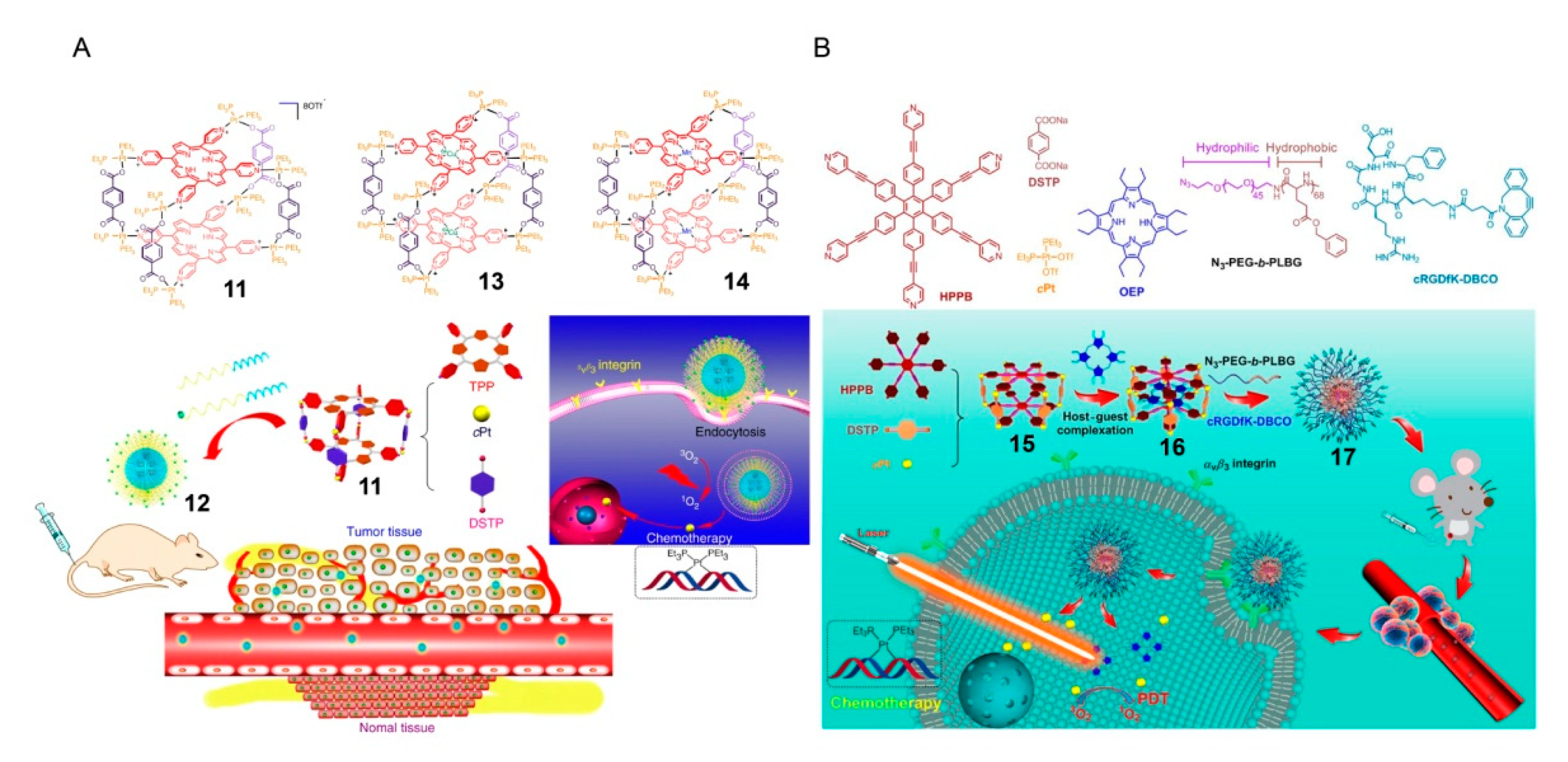
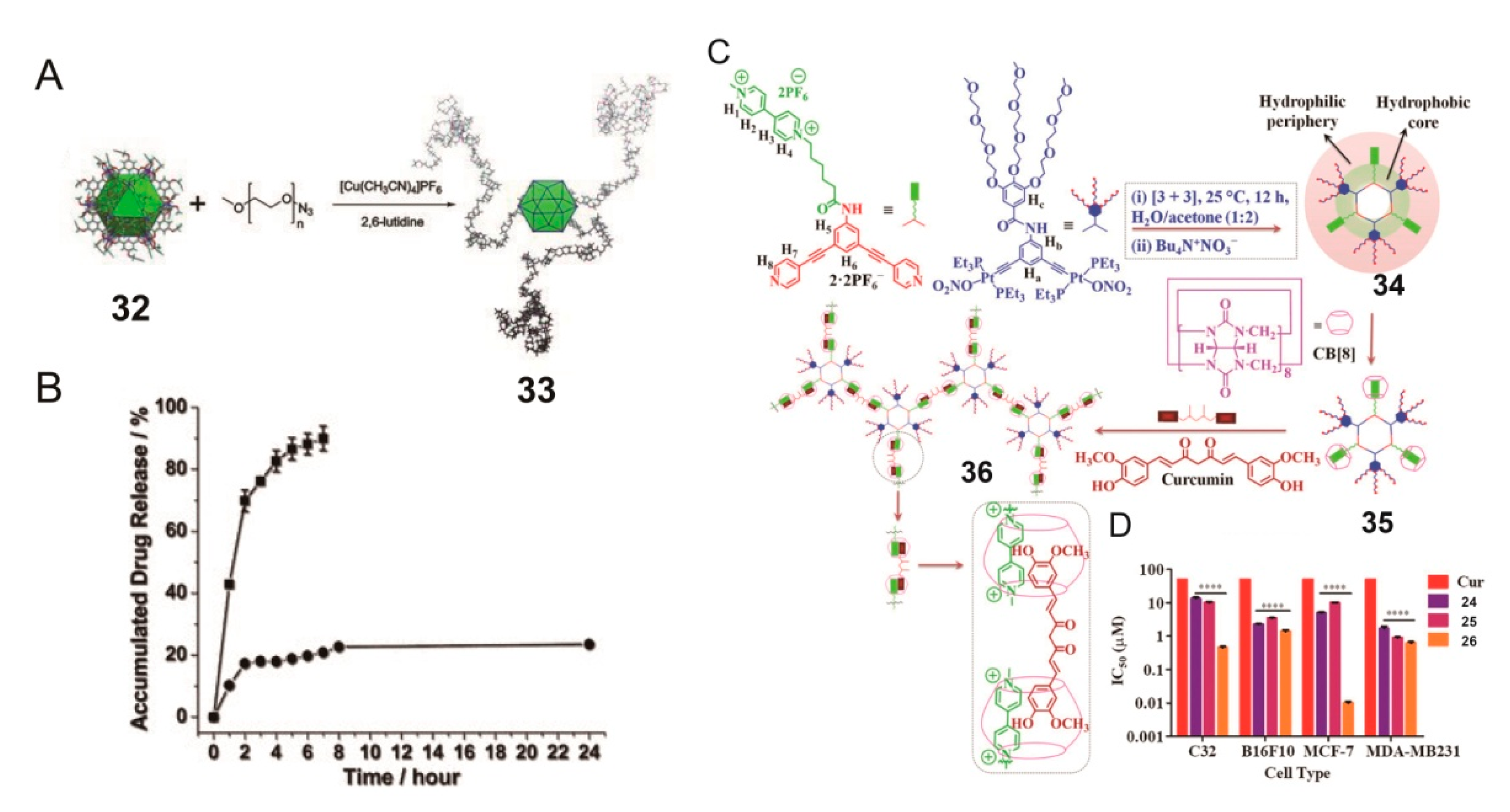
2. SCCs Modified via Physical Combination with Amphiphilic Polymers
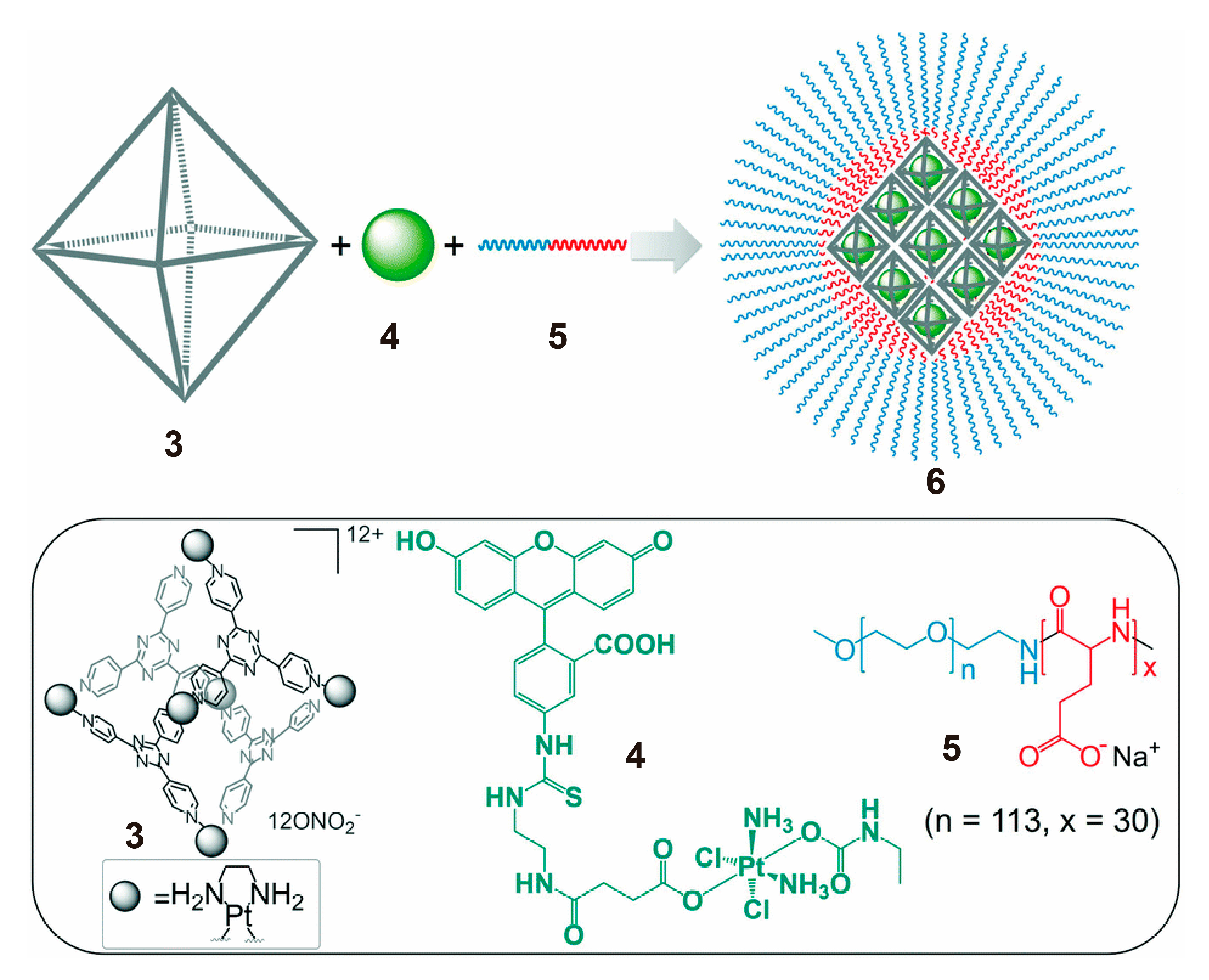
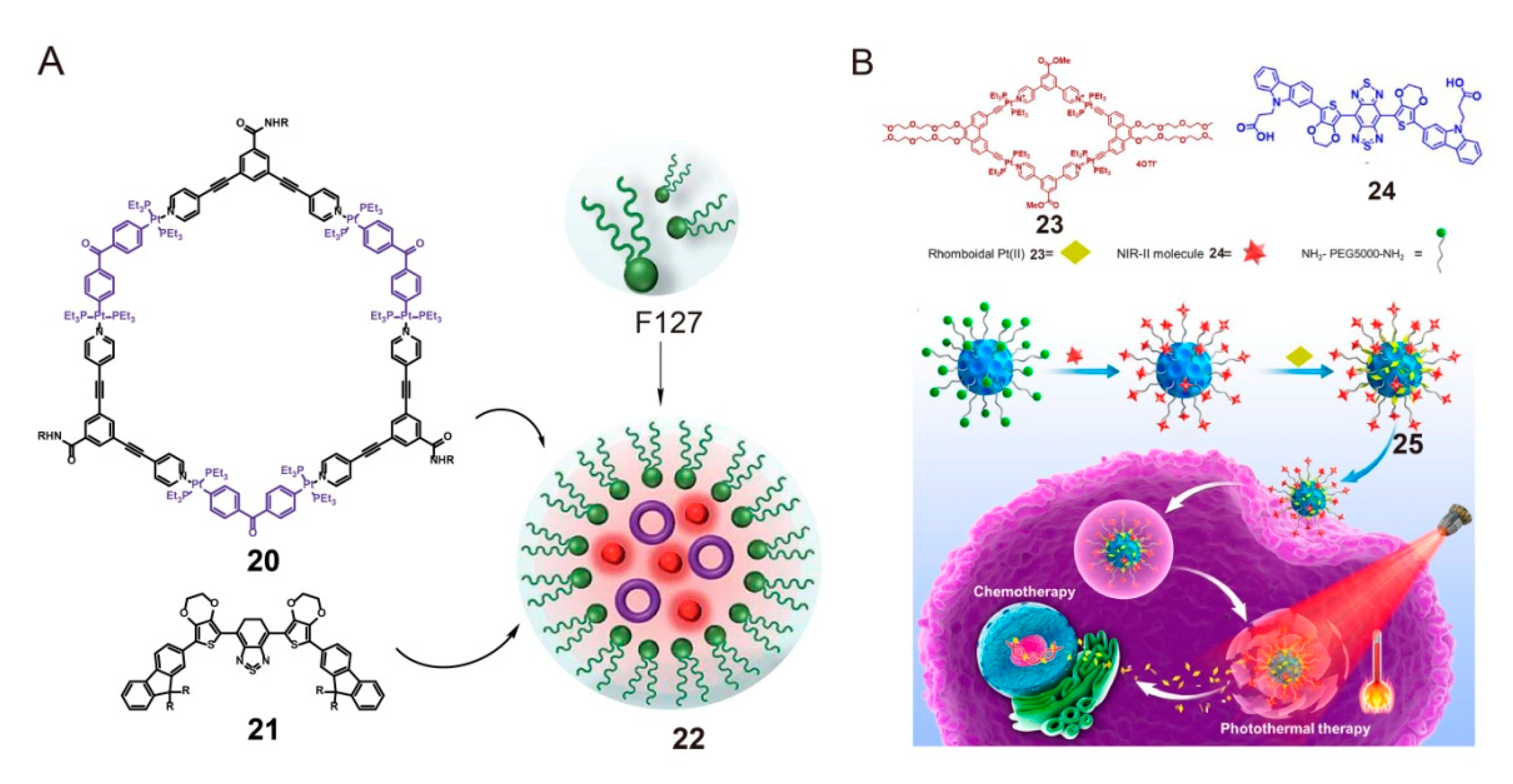
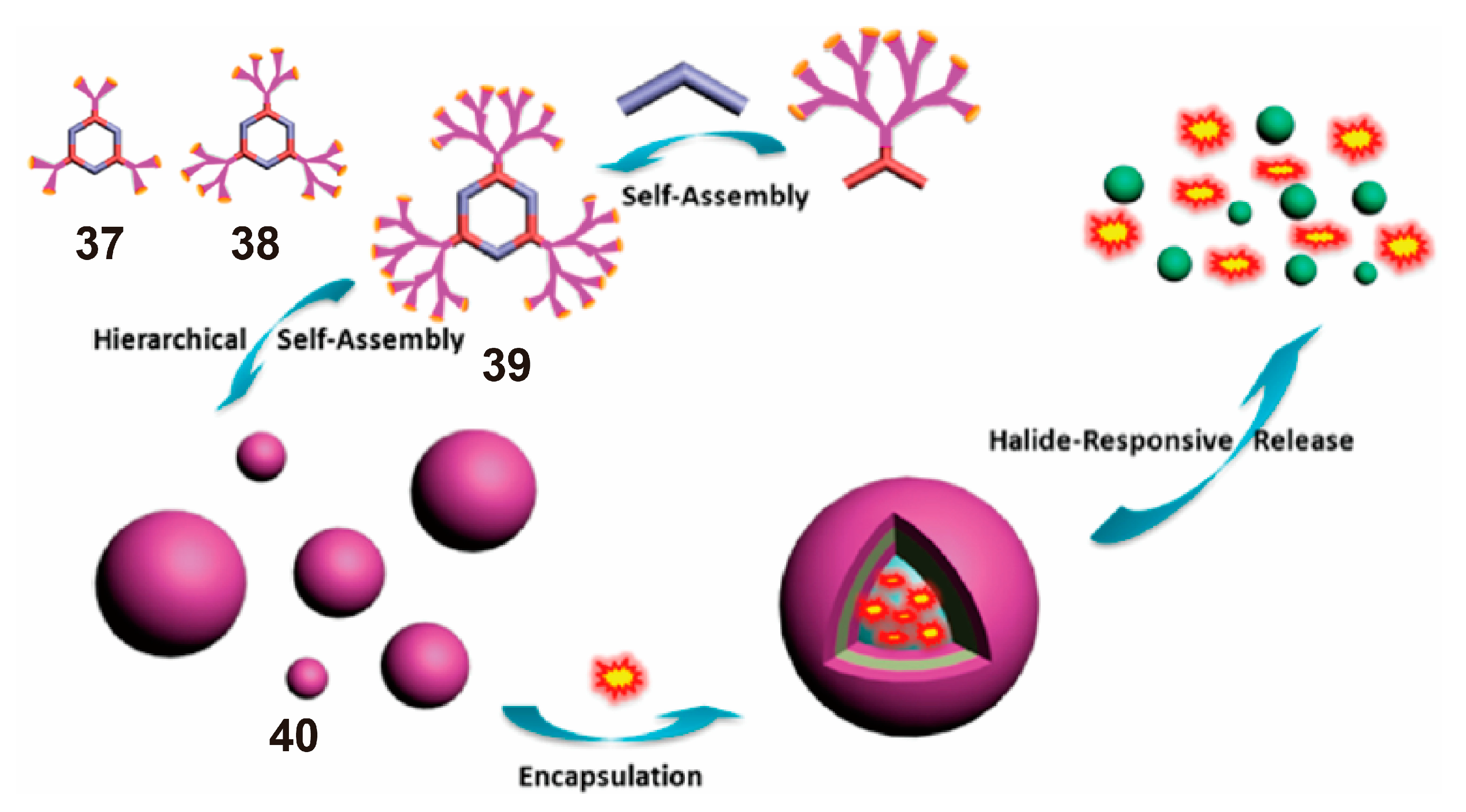
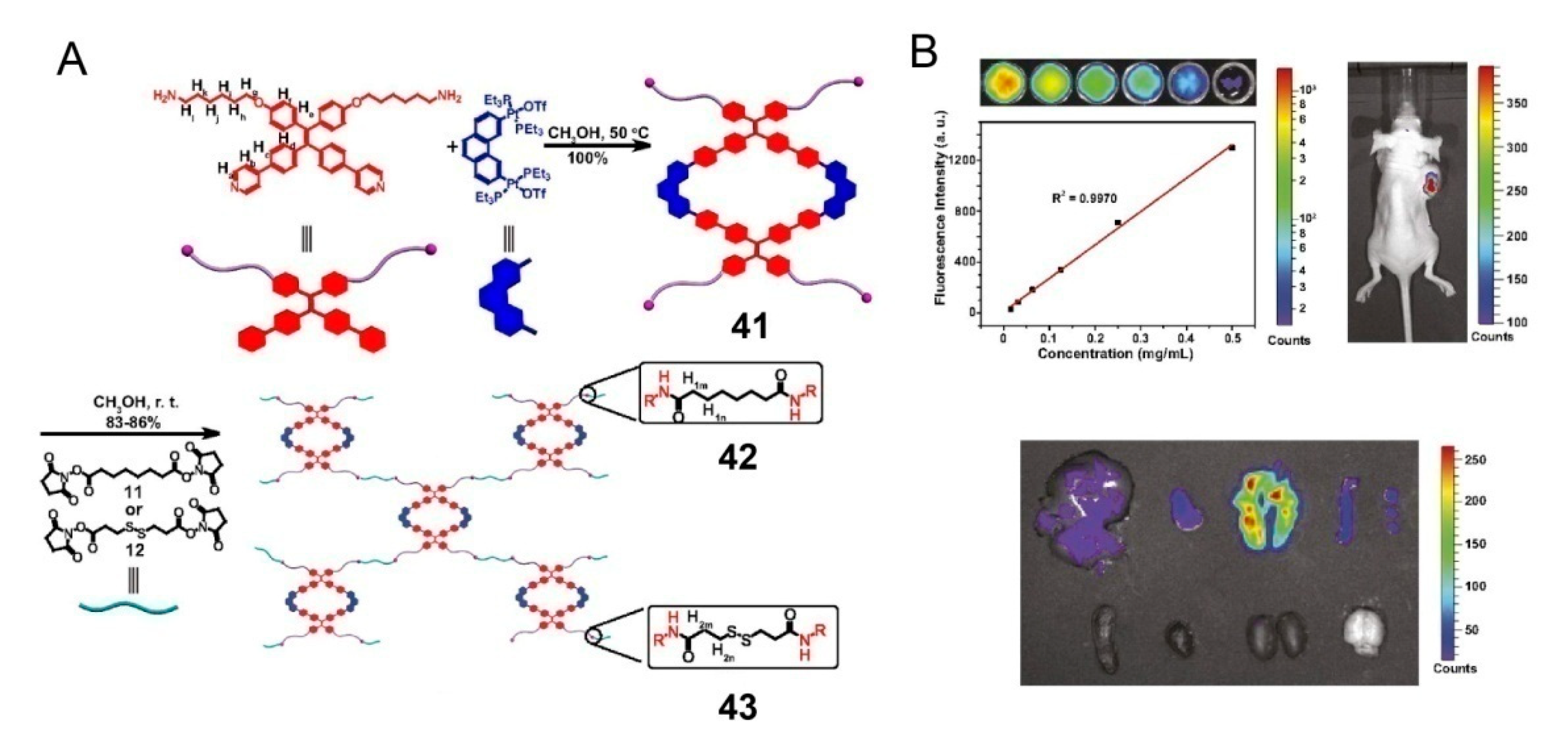
3. SCCs Decorated via Chemical Modification with Amphiphilic Polymers
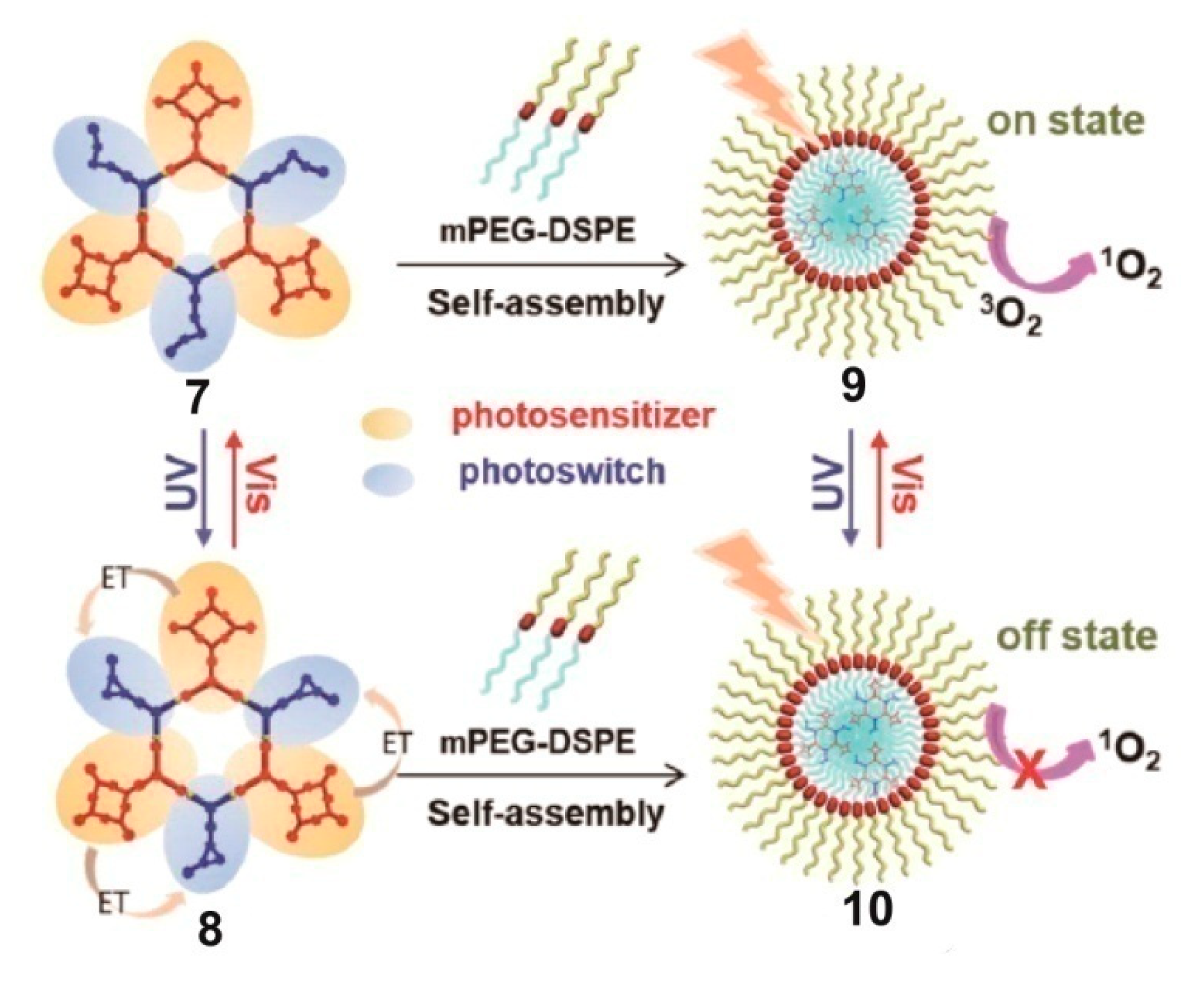
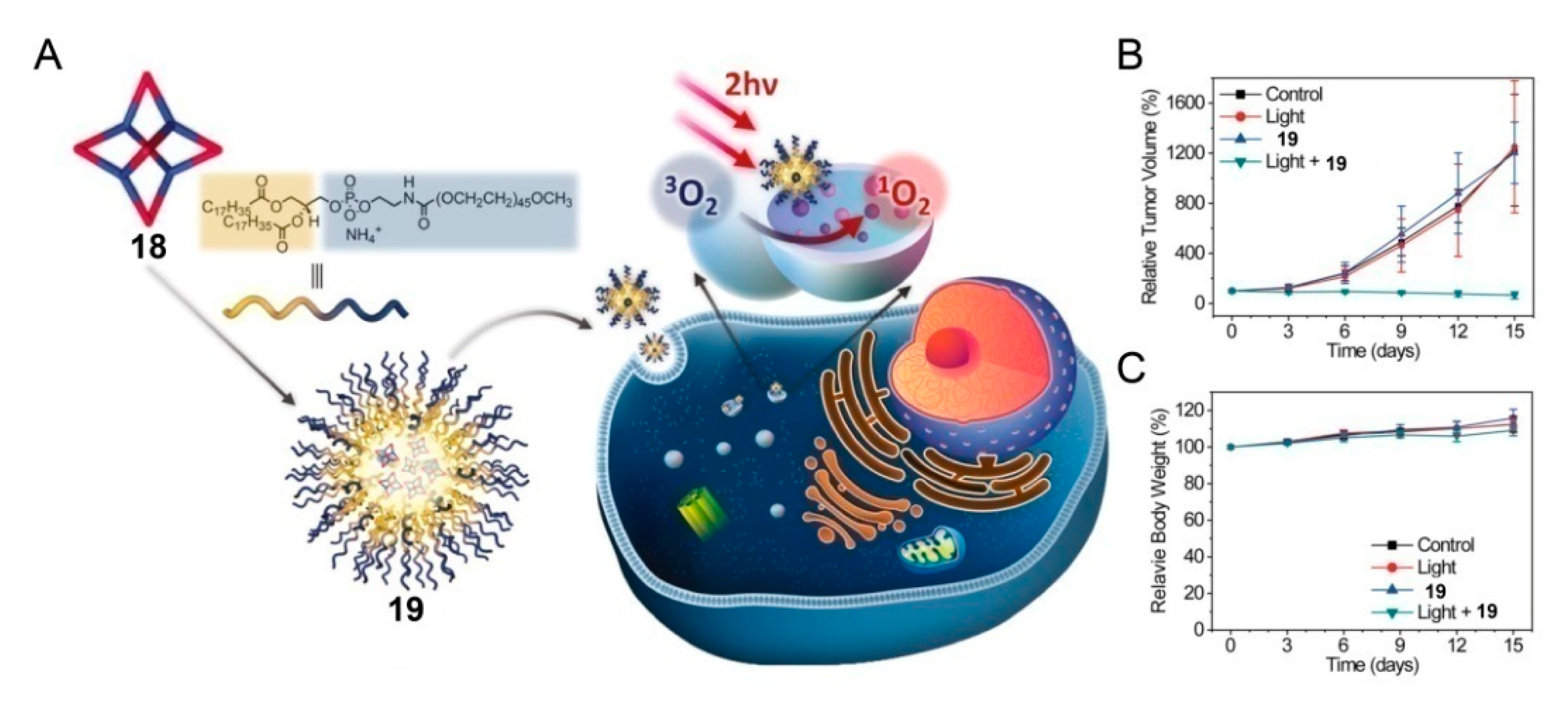
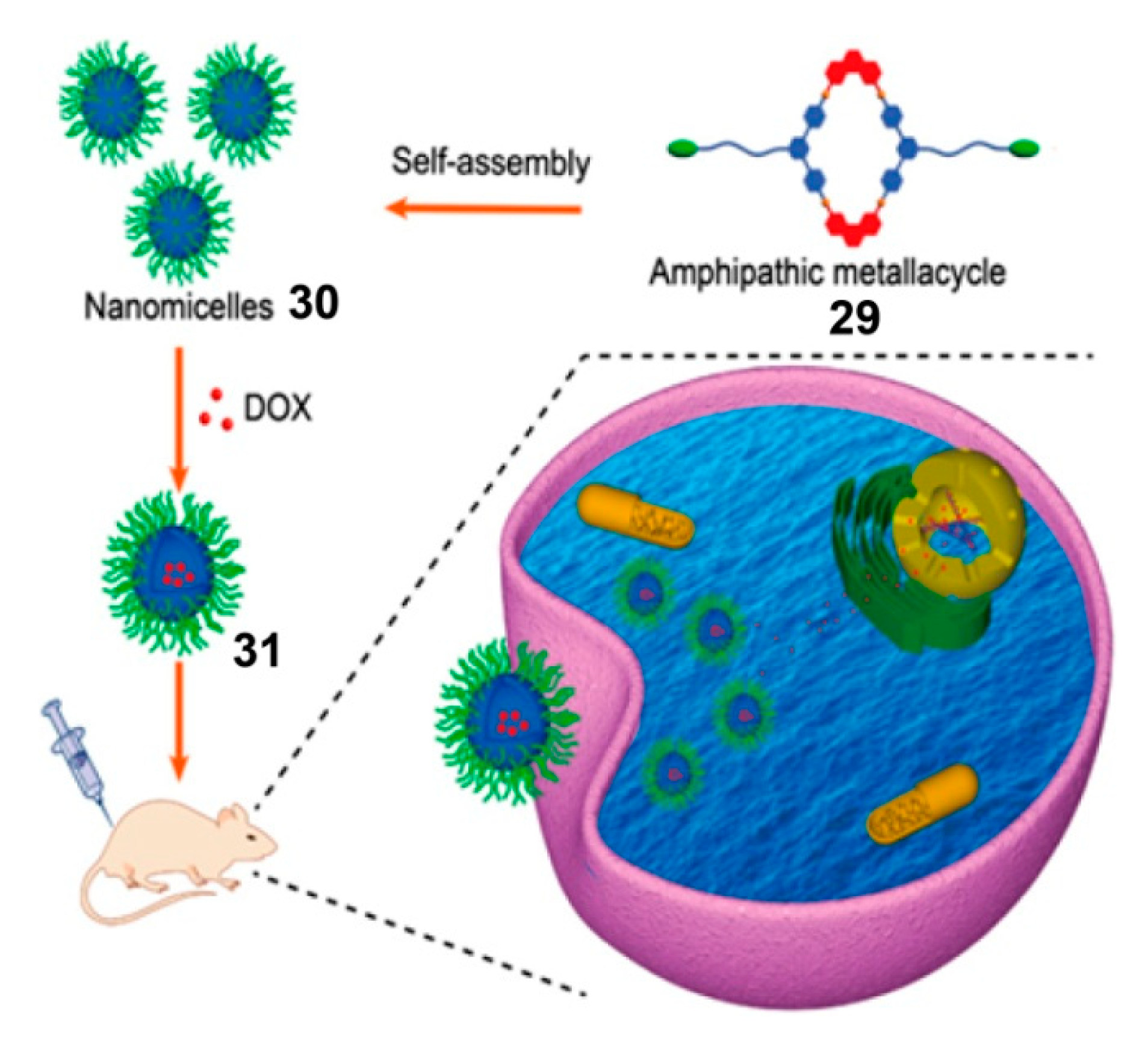
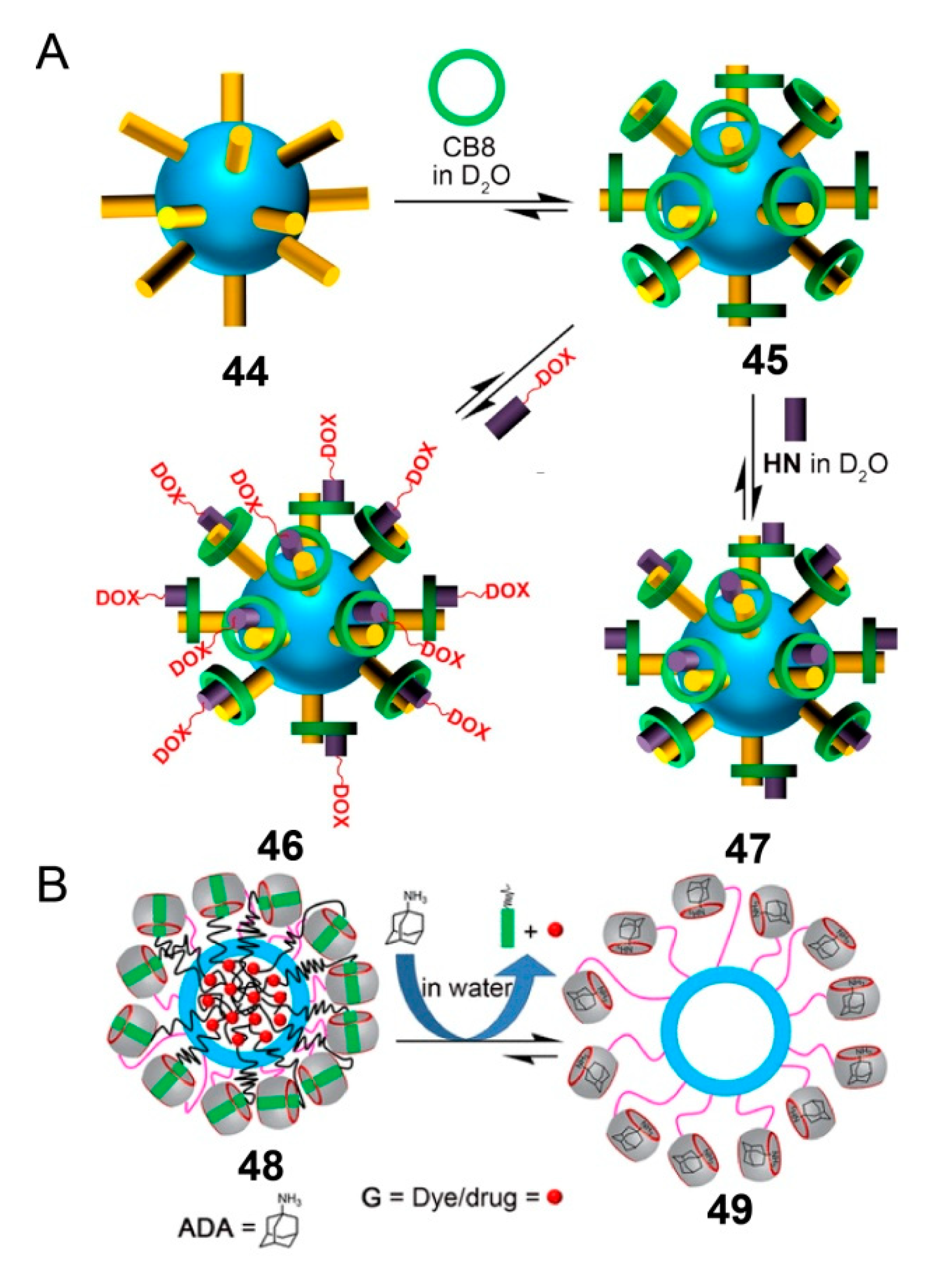
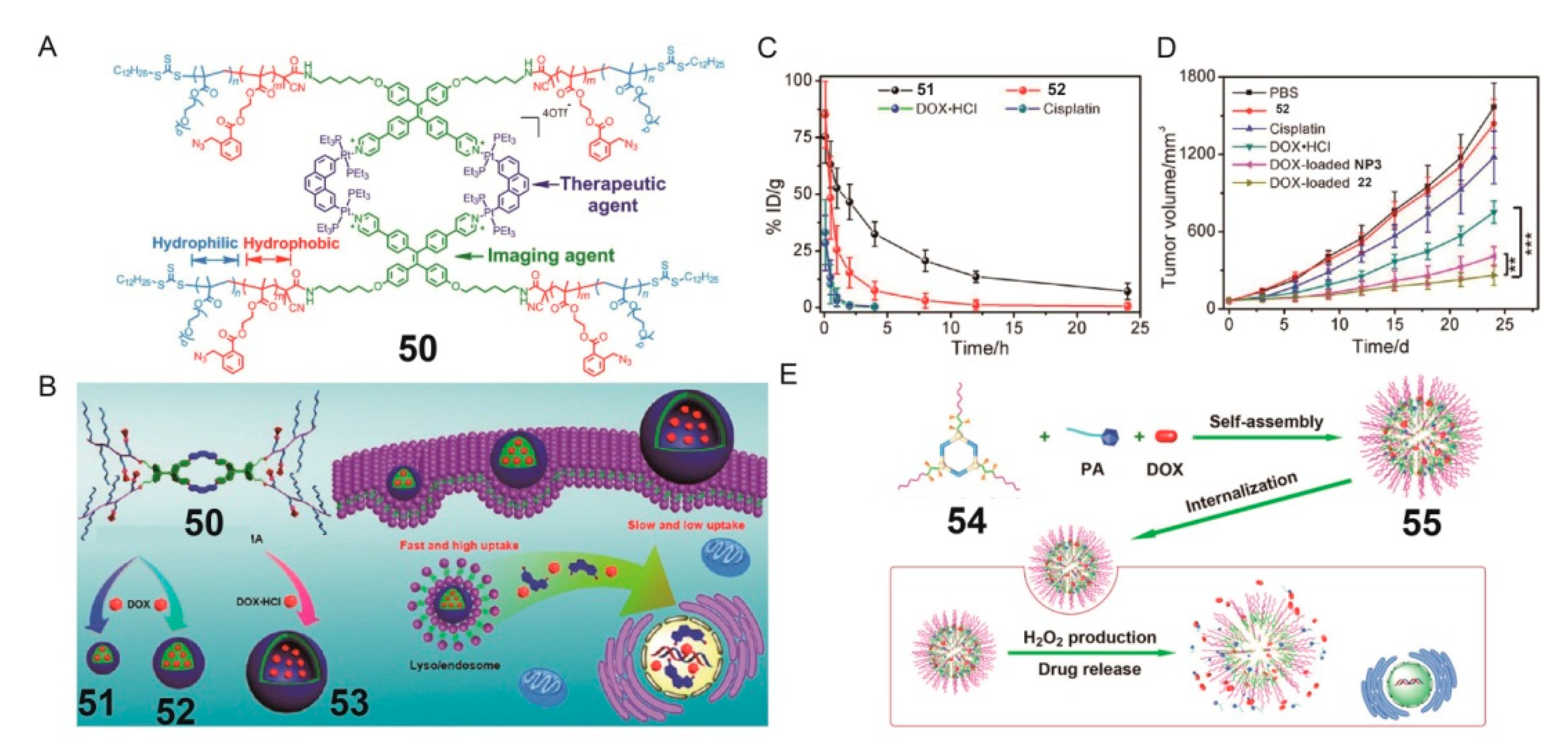
4. SCCs Modified by Stimulus-Responsive Amphiphilic Polymers
5. Perspectives and Challenges
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.-M.; Zong, Y.-N.; Cao, S.-M.; Xu, R.-H. Current Cancer Situation in China: Good or Bad News from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise Nanomedicine for Intelligent Therapy of Cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/Biocompatible Polymer-Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Accounts Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef]
- Pottanam Chali, S.; Ravoo, B.J. Polymer Nanocontainers for Intracellular Delivery. Angew. Chem. Int. Ed. 2020, 59, 2962–2972. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired Polymer Vesicles and Membranes for Biological and Medical Applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef]
- Szafraniec-Szczęsny, J.; Janik-Hazuka, M.; Odrobińska, J.; Zapotoczny, S. Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers. Polymers 2020, 12, 1999. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on Architectural Principles for Three-Dimensional Metallosupramolecular Construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.R.; Stang, P.J. High-Symmetry Coordination Cages via Self-Assembly. Accounts Chem. Res. 2002, 35, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Judge, N.; Wang, L.; Ho, Y.Y.L.; Wang, Y. Molecular Engineering of Metal-Organic Cycles/Cages for Drug Delivery. Macromol. Res. 2018, 26, 1074–1084. [Google Scholar] [CrossRef]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.; Stang, P.J. Soft Materials with Diverse Suprastructures via the Self-Assembly of Metal–Organic Complexes. Accounts Chem. Res. 2019, 52, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, T.; Fan, Y.; Yuan, X.; Qiu, H.; Yin, S. Recent Developments in the Construction of Metallacycle/Metallacage-Cored Supramolecular Polymers via Hierarchical Self-Assembly. Chem. Commun. 2019, 55, 8036–8059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Li, H.; Fan, Y.; He, T.; Qiu, H.; Yin, S. Metallacycle/Metallacage-Cored Fluorescent Supramolecular Assemblies with Aggregation-Induced Emission Properties. Adv. Opt. Mater. 2020, 8, 1902190. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional Molecular Flasks: New Properties and Reactions within Discrete, Self-Assembled Hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-Assembled Metal–Organic Polyhedra: An Overview of Various Applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Zhang, J.; Ni, R.; Xu, L.; He, T.; Lin, X.; Li, X.; Qiu, H.; Yin, S.; et al. Self-Healing Heterometallic Supramolecular Polymers Constructed by Hierarchical Assembly of Triply Orthogonal Interactions with Tunable Photophysical Properties. J. Am. Chem. Soc. 2019, 141, 17909–17917. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, M.; Tang, D.; Yan, X.; Zhang, Z.; Zhou, Z.; Song, B.; Wang, H.; Li, X.; Yin, S.; et al. Fluorescent Metallacage-Core Supramolecular Polymer Gel Formed by Orthogonal Metal Coordination and Host–Guest Interactions. J. Am. Chem. Soc. 2018, 140, 7674–7680. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, X.; Zhou, Z.; He, T.; Zhang, J.; Qiu, H.; Saha, M.L.; Yin, S.; Stang, P.J. Metallacycle-Cored Supramolecular Polymers: Fluorescence Tuning by Variation of Substituents. J. Am. Chem. Soc. 2018, 140, 16920–16924. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal–Organic Molecular Cages: Applications of Biochemical Implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef]
- Li, Z.; Yan, X.; Huang, F.; Sepehrpour, H.; Stang, P.J. Near-Infrared Emissive Discrete Platinum(II) Metallacycles: Synthesis and Application in Ammonia Detection. Org. Lett. 2017, 19, 5728–5731. [Google Scholar] [CrossRef]
- Xu, W.-Q.; Fan, Y.-Z.; Wang, H.-P.; Teng, J.; Li, Y.-H.; Chen, C.-X.; Fenske, D.; Jiang, J.-J.; Su, C.-Y. Investigation of Binding Behavior between Drug Molecule 5-Fluoracil and M4L4-Type Tetrahedral Cages: Selectivity, Capture, and Release. Chem. Eur. J. 2017, 23, 3542–3547. [Google Scholar] [CrossRef]
- Kaiser, F.; Schmidt, A.; Heydenreuter, W.; Altmann, P.J.; Casini, A.; Sieber, S.A.; Kühn, F.E. Self-Assembled Palladium and Platinum Coordination Cages: Photophysical Studies and Anticancer Activity. Eur. J. Inorg. Chem. 2016, 2016, 5189–5196. [Google Scholar] [CrossRef]
- Preston, D.; Lewis, J.E.M.; Crowley, J.D. Multicavity [PdnL4]2n+ Cages with Controlled Segregated Binding of Different Guests. J. Am. Chem. Soc. 2017, 139, 2379–2386. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Lippard, S.J. Encapsulation of Pt(IV) Prodrugs within a Pt(II) Cage for Drug Delivery. Chem. Sci. 2015, 6, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Jeong, Y.J.; Jo, J.H.; Woo, S.; Kim, D.H.; Kim, H.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Anticancer Activity and Autophagy Involvement of Self-Assembled Arene–Ruthenium Metallacycles. Organometallics 2015, 34, 4507–4514. [Google Scholar] [CrossRef]
- Mishra, A.; Jeong, Y.J.; Jo, J.-H.; Kang, S.C.; Lah, M.S.; Chi, K.-W. Anticancer Potency Studies of Coordination Driven Self-Assembled Arene–Ru-Based Metalla-Bowls. ChemBioChem 2014, 15, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The Development of Anticancer Ruthenium(II) Complexes: From Single Molecule Compounds to Nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Lv, F.; Yu, B.; Shen, Y.; Cong, H. Recent Advances in Ruthenium and Platinum Based Supramolecular Coordination Complexes for Antitumor Therapy. Colloids Surf. B 2019, 182, 110373. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Jana, A.; Bhowmick, S.; Kumar, S.; Singh, K.; Garg, P.; Das, N. Self-Assembly of Pt(II) Based Nanoscalar Ionic Hexagons and Their Anticancer Potencies. Inorg. Chim. Acta 2019, 484, 19–26. [Google Scholar] [CrossRef]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-Responsive Metal–Ligand Assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Yan, X.; Zhou, Z.; Saha, M.L.; Wang, Y.-C.; Stang, P.J. Fluorescent Metallacycle-Cored Polymers via Covalent Linkage and Their Use as Contrast Agents for Cell Imaging. Proc. Natl. Acad. Sci. USA 2016, 113, 11100–11105. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, M.; Saha, M.L.; Mao, Z.; Chen, J.; Yao, Y.; Zhou, Z.; Liu, Y.; Gao, C.; Huang, F.; et al. Antitumor Activity of a Unique Polymer That Incorporates a Fluorescent Self-Assembled Metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yu, G.; Crawley, M.R.; Fulong, C.R.P.; Friedman, A.E.; Sengupta, S.; Sun, J.; Li, Q.; Huang, F.; et al. Highly Emissive Self-Assembled BODIPY-Platinum Supramolecular Triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, P.-P.; Xu, Y.-L.; Ding, F.; Yang, W.-C.; Sun, Y.; Li, X.-P.; Yin, G.-Q.; Xu, L.; Yang, G.-F. Photoacoustic Imaging-Guided Chemo-Photothermal Combinational Therapy Based on Emissive Pt(II) Metallacycle-Loaded Biomimic Melanin Dots. Sci. China Chem. 2020. [Google Scholar] [CrossRef]
- Zhu, H.; Shangguan, L.; Shi, B.; Yu, G.; Huang, F. Recent Progress in Macrocyclic Amphiphiles and Macrocyclic Host-Based Supra-Amphiphiles. Mater. Chem. Front. 2018, 2, 2152–2174. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking Cancer Nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; McNeill, S.M.; Lewis, J.E.M.; Giles, G.I.; Crowley, J.D. Enhanced Kinetic Stability of [Pd2L4]4+ Cages through Ligand Substitution. Dalton Trans. 2016, 45, 8050–8060. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent Progress in Biomedical Applications of Pluronic (PF127): Pharmaceutical Perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent Advances in Polymeric Micelles for Anti-Cancer Drug Delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-Based Nanovehicles: Recent Advances in Anticancer Therapeutic Applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Yu, G.; Cook, T.R.; Li, Y.; Yan, X.; Wu, D.; Shao, L.; Shen, J.; Tang, G.; Huang, F.; Chen, X.; et al. Tetraphenylethene-Based Highly Emissive Metallacage as a Component of Theranostic Supramolecular Nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 13720–13725. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, H.; Bowers, D.J.; Gao, M.; Stilgenbauer, M.; Nielsen, F.; Shelley, J.T.; Zheng, Y.-R. Nanoparticles of Metal–Organic Cages Designed to Encapsulate Platinum-Based Anticancer Agents. Dalton Trans. 2018, 47, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Master, A.; Livingston, M.; Sen Gupta, A. Photodynamic Nanomedicine in the Treatment of Solid Tumors: Perspectives and Challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Q.; He, S.; Xiong, H.; Zhang, Q.; Xu, W.; Ricotta, V.; Bai, L.; Zhang, Q.; Yu, Z.; et al. Recent Advances in Delivery of Photosensitive Metal-Based Drugs. Coord. Chem. Rev. 2019, 387, 154–179. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.-y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A Smart and Versatile Theranostic Nanomedicine Platform Based on Nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome Nanovesicles Generated by Porphyrin Bilayers for Use as Multimodal Biophotonic Contrast Agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Denoyelle-Di-Muro, E.; Mbakidi, J.-P.; Leroy-Lhez, S.; Sol, V.; Therrien, B. Delivery of Porphin to Cancer Cells by Organometallic Rh(III) and Ir(III) Metalla-Cages. J. Organomet. Chem. 2015, 787, 44–50. [Google Scholar] [CrossRef]
- Schmitt, F.; Freudenreich, J.; Barry, N.P.E.; Juillerat-Jeanneret, L.; Süss-Fink, G.; Therrien, B. Organometallic Cages as Vehicles for Intracellular Release of Photosensitizers. J. Am. Chem. Soc. 2012, 134, 754–757. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Z.; Yang, H.; Shan, C.; Yu, H.; Wojtas, L.; Zhang, M.; Mao, Z.; Wang, M.; Stang, P.J. Self-Assembly of Porphyrin-Containing Metalla-Assemblies and Cancer Photodynamic Therapy. Inorg. Chem. 2020, 59, 7380–7388. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.-J.; Dong, F.; Jiang, S.-T.; Yin, G.-Q.; Li, X.; Tian, Y.; Yang, H.-B. Light-Controlled Generation of Singlet Oxygen within a Discrete Dual-Stage Metallacycle for Cancer Therapy. J. Am. Chem. Soc. 2019, 141, 8943–8950. [Google Scholar] [CrossRef]
- Yu, G.; Yu, S.; Saha, M.L.; Zhou, J.; Cook, T.R.; Yung, B.C.; Chen, J.; Mao, Z.; Zhang, F.; Zhou, Z.; et al. A Discrete Organoplatinum(II) Metallacage as a Multimodality Theranostic Platform for Cancer Photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhu, B.; Shao, L.; Zhou, J.; Saha, M.L.; Shi, B.; Zhang, Z.; Hong, T.; Li, S.; Chen, X.; et al. Host−Guest Complexation-Mediated Codelivery of Anticancer Drug and Photosensitizer for Cancer Photochemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 6618–6623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Zheng, Y.; Tan, C.-P.; Sun, J.-H.; Zhang, W.; Ji, L.-N.; Mao, Z.-W. Graphene Oxide Decorated with Ru(II)–Polyethylene Glycol Complex for Lysosome-Targeted Imaging and Photodynamic/Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 6761–6771. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, B.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly Charged Ruthenium(II) Polypyridyl Complexes as Lysosome-Localized Photosensitizers for Two-Photon Photodynamic Therapy. Angew. Chem. Int. Ed. 2015, 54, 14049–14052. [Google Scholar]
- Liu, J.; Chen, Y.; Li, G.; Zhang, P.; Jin, C.; Zeng, L.; Ji, L.; Chao, H. Ruthenium(II) Polypyridyl Complexes as Mitochondria-Targeted Two-Photon Photodynamic Anticancer Agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [PubMed]
- Zhou, Z.; Liu, J.; Huang, J.; Rees, T.W.; Wang, Y.; Wang, H.; Li, X.; Chao, H.; Stang, P.J. A Self-Assembled Ru–Pt Metallacage as a Lysosome-Targeting Photosensitizer for 2-Photon Photodynamic Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 20296–20302. [Google Scholar] [PubMed]
- Ding, F.; Zhan, Y.; Lu, X.; Sun, Y. Recent Advances in Near-Infrared II Fluorophores for Multifunctional Biomedical Imaging. Chem. Sci. 2018, 9, 4370–4380. [Google Scholar]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 1–22. [Google Scholar]
- Miao, Q.; Pu, K. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second Near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. 2018, 30, 1801778. [Google Scholar]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.-X.; Zhang, F. NIR-II Bioluminescence for In Vivo High Contrast Imaging and In Situ ATP-Mediated Metastases Tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar]
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. NIR-II Nanoprobes In-Vivo Assembly to Improve Image-Guided Surgery for Metastatic Ovarian Cancer. Nat. Commun. 2018, 9, 2898. [Google Scholar]
- Sun, Y.; Ding, F.; Zhou, Z.; Li, C.; Pu, M.; Xu, Y.; Zhan, Y.; Lu, X.; Li, H.; Yang, G.; et al. Rhomboidal Pt(II) Metallacycle-Based NIR-II Theranostic Nanoprobe for Tumor Diagnosis and Image-Guided Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 1968–1973. [Google Scholar]
- Ding, F.; Chen, Z.; Kim, W.Y.; Sharma, A.; Li, C.; Ouyang, Q.; Zhu, H.; Yang, G.; Sun, Y.; Kim, J.S. A Nano-Cocktail of an NIR-II Emissive Fluorophore and Organoplatinum(II) Metallacycle for Efficient Cancer Imaging and Therapy. Chem. Sci. 2019, 10, 7023–7028. [Google Scholar]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Yang, X.; Li, X.; Lv, S.; Zhang, H.; Sun, J.; Li, L.; Wang, L.; Qu, B.; Peng, X.; et al. Photoacoustic-Imaging-Guided Therapy of Functionalized Melanin Nanoparticles: Combination of Photothermal Ablation and Gene Therapy against Laryngeal Squamous Cell Carcinoma. Nanoscale 2019, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fan, Q.; Yang, M.; Cheng, K.; Lu, X.; Zhang, L.; Huang, W.; Cheng, Z. Engineering Melanin Nanoparticles as an Efficient Drug-Delivery System for Imaging-Guided Chemotherapy. Adv. Mater. 2015, 27, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, F.; Chen, Z.; Zhang, R.; Li, C.; Xu, Y.; Zhang, Y.; Ni, R.; Li, X.; Yang, G.; et al. Melanin-Dot-Mediated Delivery of Metallacycle for NIR-II/Photoacoustic Dual-Modal Imaging-Guided Chemo-Photothermal Synergistic Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 16729–16735. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, R.; Shi, Y.; Cai, Y.; Chen, J.; Sun, S.; Zhang, W.; Tang, R. 2D Amphiphilic Organoplatinum(II) Metallacycles: Their Syntheses, Self-Assembly in Water and Potential Application in Photodynamic therapy. Chem. Commun. 2018, 54, 8068–8071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Yu, J.; Fan, Y.; He, T.; Lu, S.; Li, X.; Qiu, H.; Yin, S. Amphiphilic Rhomboidal Organoplatinum(II) Metallacycles with Encapsulated Doxorubicin for Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 8061–8068. [Google Scholar] [CrossRef]
- Zhao, D.; Tan, S.; Yuan, D.; Lu, W.; Rezenom, Y.H.; Jiang, H.; Wang, L.-Q.; Zhou, H.-C. Surface Functionalization of Porous Coordination Nanocages Via Click Chemistry and Their Application in Drug Delivery. Adv. Mater. 2011, 23, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The Aqueous Supramolecular Chemistry of Cucurbit[n]urils, Pillar[n]arenes and Deep-Cavity Cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.-J.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Accounts Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a New Function of Curcumin Which Enhances Its Anticancer Therapeutic Potency. Sci. Rep. 2016, 6, 30962. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Misra, S.K.; Saha, M.L.; Lahiri, N.; Louie, J.; Pan, D.; Stang, P.J. Orthogonal Self-Assembly of an Organoplatinum(II) Metallacycle and Cucurbit[8]uril That Delivers Curcumin to Cancer Cells. Proc. Natl. Acad. Sci. USA 2018, 115, 8087–8092. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications—Reflections on the Field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Metallocenyl Dendrimers and Their Applications in Molecular Electronics, Sensing, and Catalysis. Accounts Chem. Res. 2008, 41, 841–856. [Google Scholar] [CrossRef]
- Chen, L.-J.; Zhao, G.-Z.; Jiang, B.; Sun, B.; Wang, M.; Xu, L.; He, J.; Abliz, Z.; Tan, H.; Li, X.; et al. Smart Stimuli-Responsive Spherical Nanostructures Constructed from Supramolecular Metallodendrimers via Hierarchical Self-Assembly. J. Am. Chem. Soc. 2014, 136, 5993–6001. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhang, X. Amphiphilic Building Blocks for Self-Assembly: From Amphiphiles to Supra-Amphiphiles. Accounts Chem. Res. 2012, 45, 608–618. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Symons, H.E.; Xu, J.-F.; Faul, C.F.J.; Zhang, X. Molecular Engineering of Polymeric Supra-Amphiphiles. Chem. Soc. Rev. 2019, 48, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Moncelet, D.; Briken, V.; Isaacs, L. Metal–Organic Polyhedron Capped with Cucurbit[8]uril Delivers Doxorubicin to Cancer Cells. J. Am. Chem. Soc. 2016, 138, 14488–14496. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Quigley, J.; Vinciguerra, B.; Briken, V.; Isaacs, L. Cucurbit[7]uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J. Am. Chem. Soc. 2017, 139, 9066–9074. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef]
- Zhang, Q.; Re Ko, N.; Kwon Oh, J. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Yang, J.; Shi, B.; Ye, B.; Wang, M.; Huang, F.; Stang, P.J. Polymeric Nanoparticles Integrated from Discrete Organoplatinum(II) Metallacycle by Stepwise Post-assembly Polymerization for Synergistic Cancer Therapy. Chem. Mater. 2020, 32, 4564–4573. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Li, Y.; Lin, X.; Qiu, H.; Yin, S. Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery. Polymers 2021, 13, 370. https://doi.org/10.3390/polym13030370
Chen F, Li Y, Lin X, Qiu H, Yin S. Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery. Polymers. 2021; 13(3):370. https://doi.org/10.3390/polym13030370
Chicago/Turabian StyleChen, Feng, Yang Li, Xiongjie Lin, Huayu Qiu, and Shouchun Yin. 2021. "Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery" Polymers 13, no. 3: 370. https://doi.org/10.3390/polym13030370
APA StyleChen, F., Li, Y., Lin, X., Qiu, H., & Yin, S. (2021). Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery. Polymers, 13(3), 370. https://doi.org/10.3390/polym13030370