Characterization of Zinc Oxide-Urea Formaldehyde Nano Resin and Its Impact on the Physical Performance of Medium-Density Fiberboard
Abstract
1. Introduction
2. Materials and Methods
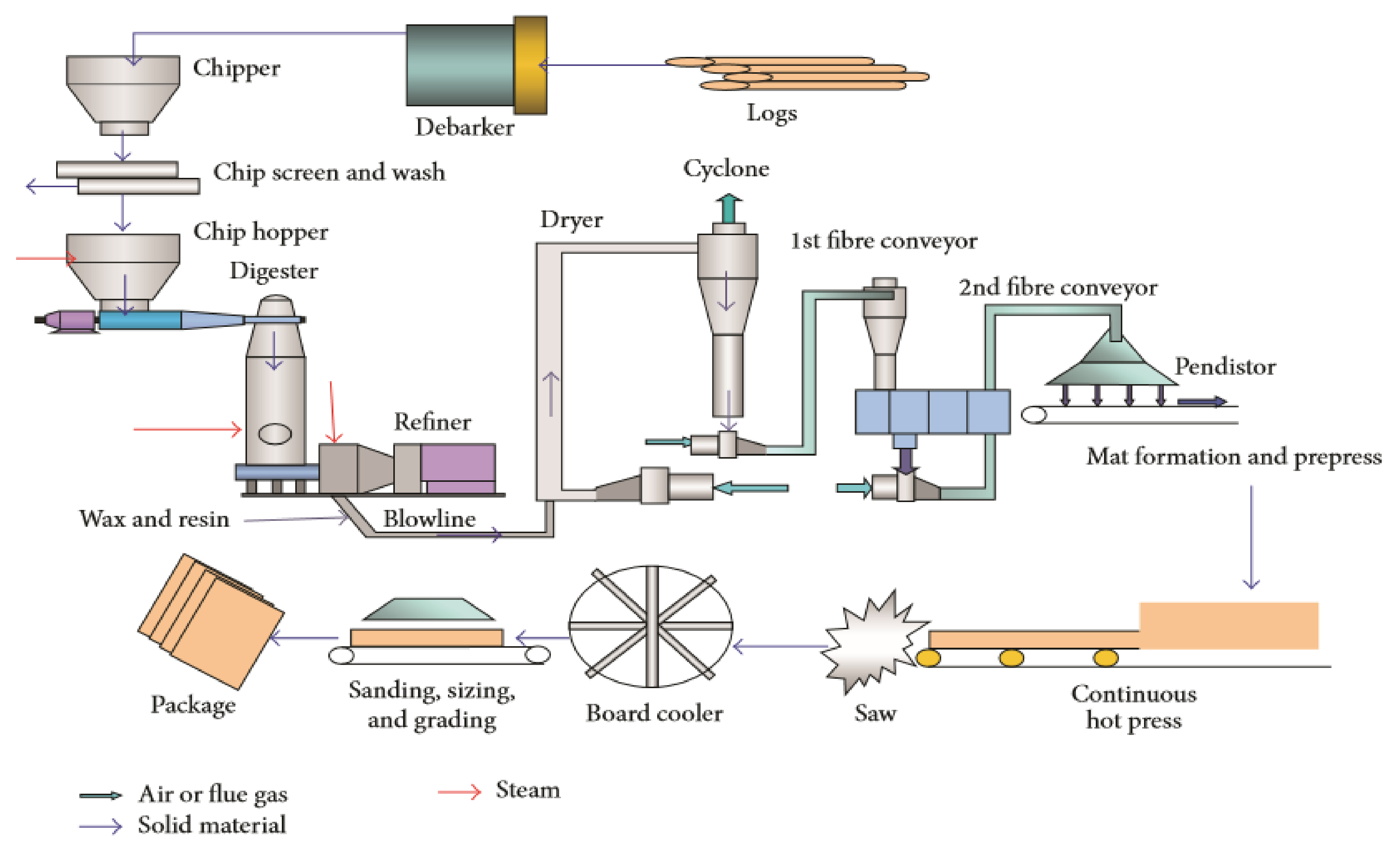
2.1. Materials
2.2. Urea Formaldehyde and Zinc Oxide (UF-ZnO) Nanofiller Preparation
2.3. Design of Nanocomposite Containing (UF-ZnO) Nanofiller
3. Characterization
4. Results and Discussion
4.1. Scanning Electron Microscopy (SEM) of ZnO Nanoparticles
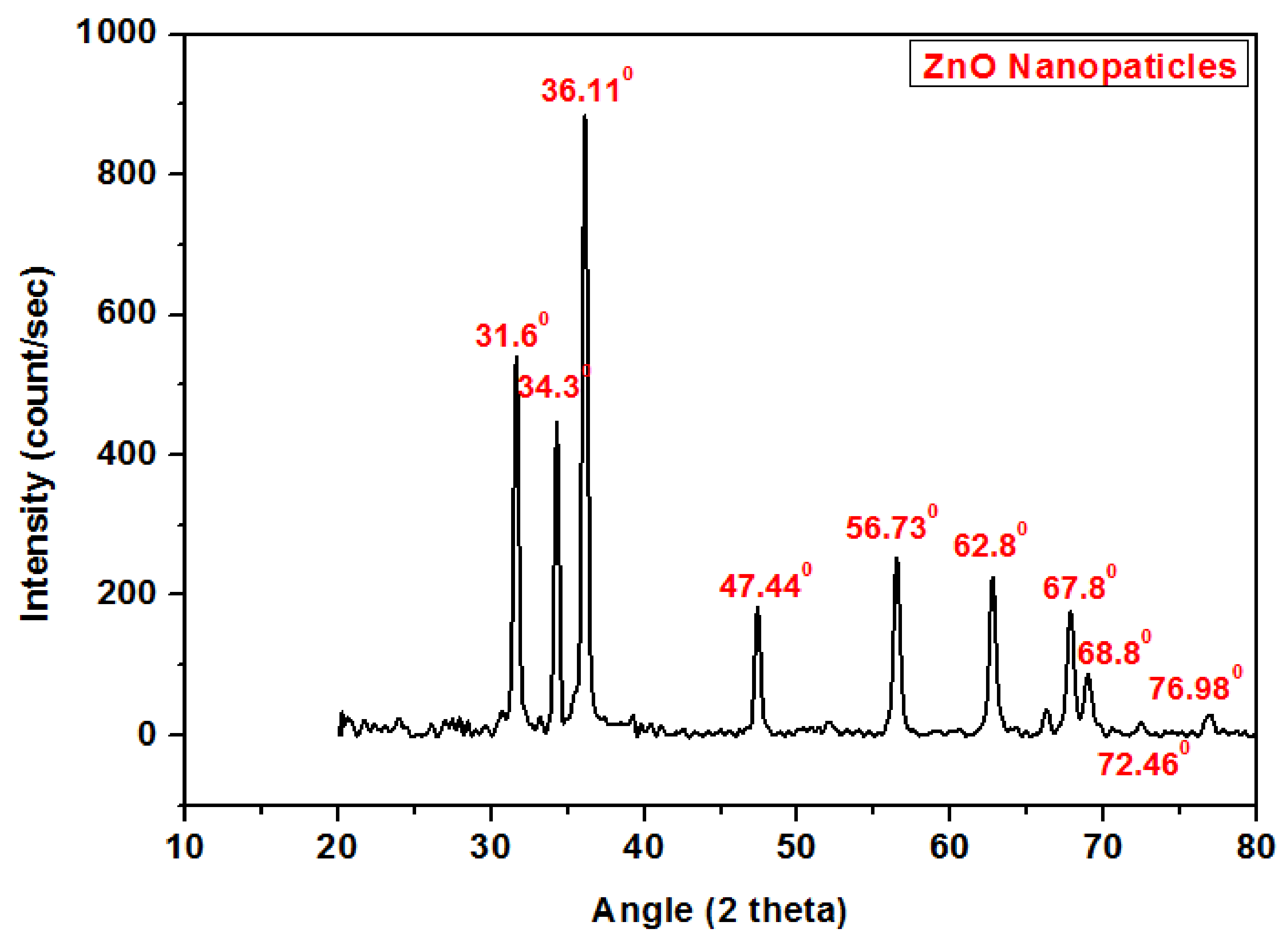
4.2. X-ray Diffraction Analysis of ZnO Nanoaprticles
4.3. Scanning Electron Microscopy of Cured UF-ZnO Nanofiller
4.4. X-ray Diffraction Analysis of UF-ZnO Nanofiller
4.5. Fourier-Transform Infrared (FT-IR) Spectroscopy of UF and UF-ZnO Nanofillers
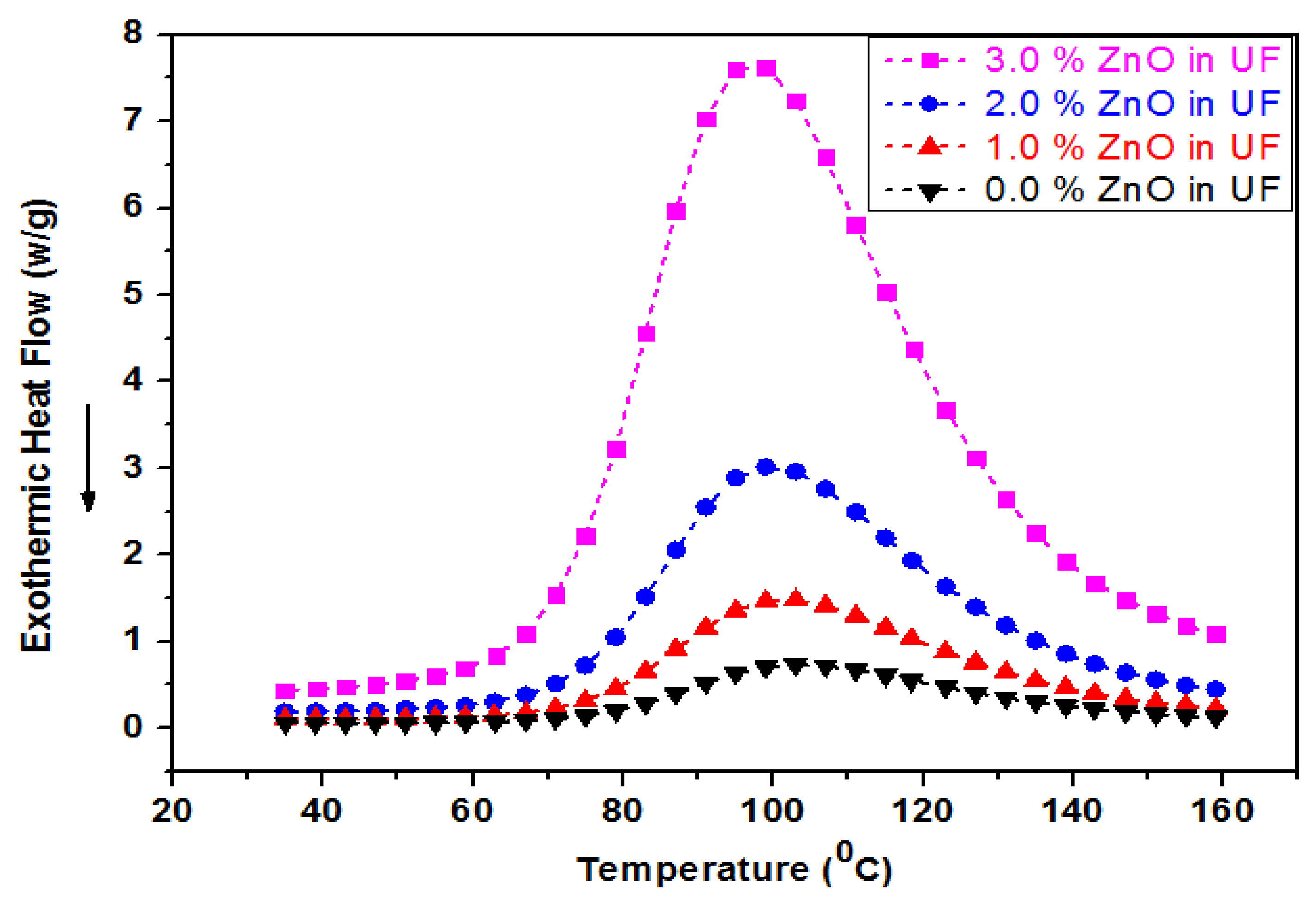
4.6. Differential Scan Calorimetry (DSC) of UF-ZnO Nanofillers
4.7. Thermogravmetric Analysis (TGA)Analysis of of UF-ZnO Nanofillers
4.8. Analysis of Variance (ANOVA) of Nano-MDF for Physical Properties
4.9. Final Average Physical Properties of UF-ZnO-Based MDF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Taghiyari, H.R.; Hosseini, G.; Tarmian, A.; Papadopoulos, A.N. Fluid Flow in Nano Silver-Impregnated Heat-Treated Beech Wood in Different Mediums. Appl. Sci. 2020, 10, 1919. [Google Scholar] [CrossRef]
- Gul, W.; Akbar, S.R.; Khan, A.; Ahmed, S. Investigation of the surface morphology and structural characterization of MDF & HDF. In Proceedings of the 5th International Conference on Advances in Mechanical Engineering, Istanbul, Turkey, 17–19 December 2019. [Google Scholar]
- Gul, W.; Khan, A.; Shakoor, A. Impact of hot pressing temperature on medium density fiberboard (MDF) performance. Adv. Mater. Sci. Eng. 2017, 2017, 4056360. [Google Scholar] [CrossRef]
- Szwajka, K.; Zielińska-Szwajka, J.; Trzepiecinski, T. Experimental Study on Drilling MDF with Tools Coated with TiAlN and ZrN. Materials 2019, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, Y.; Jing, W.; Woźniak, M.; Damaševičius, R.; Scherer, R.; Wei, W. Quality Control of the Continuous Hot Pressing Process of Medium Density Fiberboard Using Fuzzy Failure Mode and Effects Analysis. Appl. Sci. 2020, 10, 4627. [Google Scholar] [CrossRef]
- Zhang, J.; Ying, J.; Cheng, F.; Liu, H.; Luo, B.; Li, L. Investigating the Sanding Process of Medium-Density Fiberboard and Korean Pine for Material Removal and Surface Creation. Coatings 2018, 8, 416. [Google Scholar] [CrossRef]
- Kim, T. Production Planning to Reduce Production Cost and Formaldehyde Emission in Furniture Production Process Using Medium-Density Fiberboard. Processes 2019, 7, 529. [Google Scholar] [CrossRef]
- Hagel, S.; Saake, B. Fractionation of Waste MDF by Steam Refining. Molecules 2020, 25, 2165. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Wan, S.; Wu, Z.; Wang, Z. Functional surface coating on cellulosic flexible substrates with improved water-resistant and antimicrobial properties by use of ZnO nanoparticles. J. Nanomater. 2017, 2017, 9689035. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Rangavar, H.; Bibalan, O.F. Effect of nano-silver on reduction of hot-pressing time and improvement in physical and mechanical properties of particleboard. BioResources 2011, 6, 4067–4075. [Google Scholar]
- Xian, D.; Semple, K.E.; Haghdan, S.; Smith, G.D. Properties and wood bonding capacity of nanoclay-modified urea and melamine formaldehyde resins. Wood Fiber Sci. 2013, 45, 383–395. [Google Scholar]
- Taghiyari, H.R.; Ghorbanali, M.; Tahir, P.M. Effects of the improvement in thermal conductivity coefficient by nano-wollastonite on physical and mechanical properties in medium-density fiberboard (MDF). BioResources 2014, 9, 4138–4149. [Google Scholar] [CrossRef][Green Version]
- Candan, Z.; Akbulut, T. Physical and mechanical properties of nano reinforced particleboard composites. Maderas. Cienc. Tecnol. 2015, 17, 319–334. [Google Scholar]
- Taghiyari, H.R.; Mohammad-Panah, B.; Morrell, J.J. Effects of wollastonite on the properties of medium-density fiberboard (MDF) made from wood fibers and camel-thorn. Maderas. Cienc. Tecnol. 2016, 18, 157–166. [Google Scholar] [CrossRef]
- Smita, N.; Lokesh, C. Effects of different nanoclay loadings on the physical and mechanical properties of Melia composite particle board. Bois. Forets Des. Trop. 2017, 334, 7–12. [Google Scholar]
- Chen, Y.; Cai, T.; Dang, B.; Wang, H.; Xiong, Y.; Yao, Q.; Jin, C. The properties of fiberboard based on nano lignocelluloses / CaCO 3/PMMA composite synthesized through mechano-chemical method. Sci. Rep. 2018, 8, 5121. [Google Scholar] [CrossRef]
- Alabduljabbar, H.; Alyousef, R.; Gul, W.; Shah, S.R.A.; Khan, A.; Khan, R.; Alaskar, A. Effect of Alumina Nano-Particles on Physical and Mechanical Properties of Medium Density Fiberboard. Materials 2020, 13, 4207. [Google Scholar] [CrossRef]
- BS EN 323. 1993 Wood-Based Panels-Determination of Density in Fiberboard; British Standards Institution: London, UK, 1993.
- EN 317. Particleboards and Fiberboards, Determination of Swelling in Thickness after Immersion; European Committee for Standardization: Brussels, Belgium, 1993.
- ASTM International. ASTM D570-98 (2010) e1-Standard Test Method for Water Absorption of Plastics; ASTM International: West Conshohocken, PA, USA, 2010.
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic performance of ZnO nanomaterials for self-sensitized degradation of malachite green dye under solar light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Hussein, M.Z.B.; Taufiq-Yap, Y.H. Synthesis and characterization of ZnO nanostructures using palm olein as bio template. Chem. Cent. J. 2013, 7, 1–10. [Google Scholar] [CrossRef]
- Lepot, N.; Van Bael, M.K.; Van den Rul, H.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Synthesis of ZnO nanorods from aqueous solution. Mater. Lett. 2007, 61, 2624–2627. [Google Scholar] [CrossRef]
- Alwan, S.H.; Alshamsi, H.A.H.; Jasim, L.S. Rhodamine B removal on A-rGO/cobalt oxide nanoparticles composite by adsorption from contaminated water. J. Mol. Struct. 2008, 1161, 356–365. [Google Scholar] [CrossRef]
- Gul, W.; Alrobei, H.; Shah, S.R.A.; Khan, A. Effect of Iron Oxide Nanoparticles on the Physical Properties of Medium Density Fiberboard. Polymers 2020, 12, 2911. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, F.A.; Demirata, B.; Apak, R. Adsorptive removal of methylene blue from simulated dyeing wastewater with melamine-formaldehyde-urea resin. J. Appl. Polym. Sci. 2009, 112, 3442–3448. [Google Scholar] [CrossRef]
- Sun, X.H.; Li, C.P.; Wong, W.K.; Wong, N.B.; Lee, C.S.; Lee, S.T.; Teo, B.K. Formation of silicon carbide nanotubes and nanowires via reaction of silicon (from disproportionate of silicon monoxide) with carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 14464–14471. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Shukla, S.K.; Singh, N.B. Zinc oxide-urea formaldehyde nanocomposite film as low-cost adsorbent for removal of Cu (II) from aqueous solution. Adv. Mater. Lett. 2015, 6, 172–178. [Google Scholar] [CrossRef]
- Gul, W.; Alrobei, H.; Shah, S.R.A.; Khan, A.; Hussain, A.; Asiri, A.M.; Kim, J. Effect of Embedment of MWCNTs for Enhancement of Physical and Mechanical Performance of Medium Density Fiberboard. Nanomaterials 2021, 11, 29. [Google Scholar] [CrossRef]





| Composition | ||||
|---|---|---|---|---|
| Materials | ZnO0 | ZnO1 | ZnO2 | ZnO3 |
| UF (gram) | 200 | 200 | 200 | 200 |
| ZnO (wt %) | 0 | 1 | 2 | 3 |
| UF | UF-ZnO | Stretching/Bending Vibrations |
|---|---|---|
| 3312 | 3308 | (–NH2) group |
| 2960 | 2957.7 | Stretching vibrations for C–H |
| 1619 | 1627 | Stretching vibrations of C=O bonds |
| 1521 | 1529 | stretching vibrations of –OH |
| 990 | 993 | –CO bending |
| 843 | 840 | Bending vibrations for C–H bond |
| Groups | Iteration | Sum | Average | Variance | ||
|---|---|---|---|---|---|---|
| ZnO (0.0%) | 3 | 1911 | 637 | 57 | ||
| ZnO (1.0%) | 3 | 1930 | 643.33 | 72.33 | ||
| ZnO (2.0%) | 3 | 1906 | 635.33 | 165.33 | ||
| ZnO (3.0%) | 3 | 1918 | 639.33 | 114.33 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 108.25 | 3 | 36.08 | 0.35 | 0.78 | 4.06 |
| Within Groups | 818 | 8 | 102.25 | |||
| Total | 926.25 | 11 |
| Groups | Iteration | Sum | Average | Variance | ||
|---|---|---|---|---|---|---|
| ZnO (0.0%) | 3 | 76 | 25.33 | 2.33 | ||
| ZnO (1.0%) | 3 | 66.8 | 22.26 | 0.41 | ||
| ZnO (2.0%) | 3 | 53 | 17.66 | 1.42 | ||
| ZnO (3.0%) | 3 | 46.9 | 15.63 | 1.40 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 173.67 | 3 | 57.89 | 41.54 | 3.18 × 10−5 | 4.06 |
| Within Groups | 11.14 | 8 | 1.39 | |||
| Total | 184.82 | 11 |
| Groups | Iteration | Sum | Average | Variance | ||
|---|---|---|---|---|---|---|
| ZnO(0.0%) | 3 | 134 | 44.66 | 17.33 | ||
| ZnO (1.0%) | 3 | 132 | 44 | 9 | ||
| ZnO (2.0%) | 3 | 119.67 | 39.89 | 7.69 | ||
| ZnO (3.0%) | 3 | 87.9 | 29.3 | 34.77 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 453.39 | 3 | 151.13 | 8.78 | 0.006 | 4.06 |
| Within Groups | 137.59 | 8 | 17.19 | |||
| Total | 590.99 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, W.; Shah, S.R.A.; Khan, A.; Pruncu, C.I. Characterization of Zinc Oxide-Urea Formaldehyde Nano Resin and Its Impact on the Physical Performance of Medium-Density Fiberboard. Polymers 2021, 13, 371. https://doi.org/10.3390/polym13030371
Gul W, Shah SRA, Khan A, Pruncu CI. Characterization of Zinc Oxide-Urea Formaldehyde Nano Resin and Its Impact on the Physical Performance of Medium-Density Fiberboard. Polymers. 2021; 13(3):371. https://doi.org/10.3390/polym13030371
Chicago/Turabian StyleGul, Waheed, Syed Riaz Akbar Shah, Afzal Khan, and Catalin I. Pruncu. 2021. "Characterization of Zinc Oxide-Urea Formaldehyde Nano Resin and Its Impact on the Physical Performance of Medium-Density Fiberboard" Polymers 13, no. 3: 371. https://doi.org/10.3390/polym13030371
APA StyleGul, W., Shah, S. R. A., Khan, A., & Pruncu, C. I. (2021). Characterization of Zinc Oxide-Urea Formaldehyde Nano Resin and Its Impact on the Physical Performance of Medium-Density Fiberboard. Polymers, 13(3), 371. https://doi.org/10.3390/polym13030371







