Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide Nanoparticles
2.2. Spectroscopic Methods
2.3. In Vitro Evaluation
2.3.1. Cell Culture and Treatment
2.3.2. Cell Morphology and Cytotoxicity Assessment
- a.
- Microscopic examination of cell morphology. The morphology of tumor and normal breast cells was analyzed by optical microscopy after 24 and 48 h of incubation with the NPs and DOX suspensions. Different fields of cells were examined under an Olympus IX73 microscope (Olympus, Tokyo, Japan) equipped with a Hamamatsu ORCA-03G camera (A3472-06, Hamamatsu, Japan), and phase-contrast images were acquired using CellSens Dimension software (v1.11, Olympus).
- b.
- Live/Dead staining. The cells were seeded in 24 well plates at a density of 3 × 104 cells/mL and left to adhere overnight. After 24 and 48 h of treatment with NPs and DOX suspensions, the culture media was removed and replaced with a mix of calcein-AM and ethidium homodimer-1 solution following the manufacturer instructions of “LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells” (L3224, Invitrogen, Carlsbad, CA). The live (labeled in green) and dead (labeled in red) cells were analyzed under an Olympus IX73 fluorescence microscope (Olympus, Tokyo, Japan) equipped with a Hamamatsu ORCA-03G camera (A3472-06, Hamamatsu, Japan). The images were acquired using fluorescein isothiocyanate (FITC) and tri-rhodamine-isothiocyanate (TRITC) filters and CellSens Dimension software (v1.11, Olympus).
- c.
- MTT cell viability test. The cells were seeded in 96 well plates at a density of 3 × 104 cells/mL in 200 µL culture media. After exposure to DOX (25, 50, 100, 250 ng/mL) and NPs (3.125, 6.25, 12.5, 31.25 µg/mL) for 24 and 48 h, culture media was removed and replaced with 100 µL of 1 mg/mL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) for 2 h at 37 °C. The product formed (purple formazan) was solubilized with 150 µL isopropanol, and the absorbance of the samples was measured at 595 nm at a Flex Station 3 microplate reader (Molecular Devices, San Jose, CA, USA).
- d.
- Lactate dehydrogenase (LDH) assay. The activity of LDH released in cell culture media as a result of cell membrane permeabilization was measured using the “Cytotoxicity Detection Kit (LDH)” (cat. no. 11644793001, Roche, Basel, Switzerland) in a 96 well plate. After exposure for 24 and 48 h to IONPs and DOX suspensions, 50 µL culture media of treated and control cells were incubated with 50 µL mix reaction (catalyst and dye solution) from the kit for 15 min at room temperature, in the dark. The absorbance of the samples was read at 490 nm using a microplate reader.
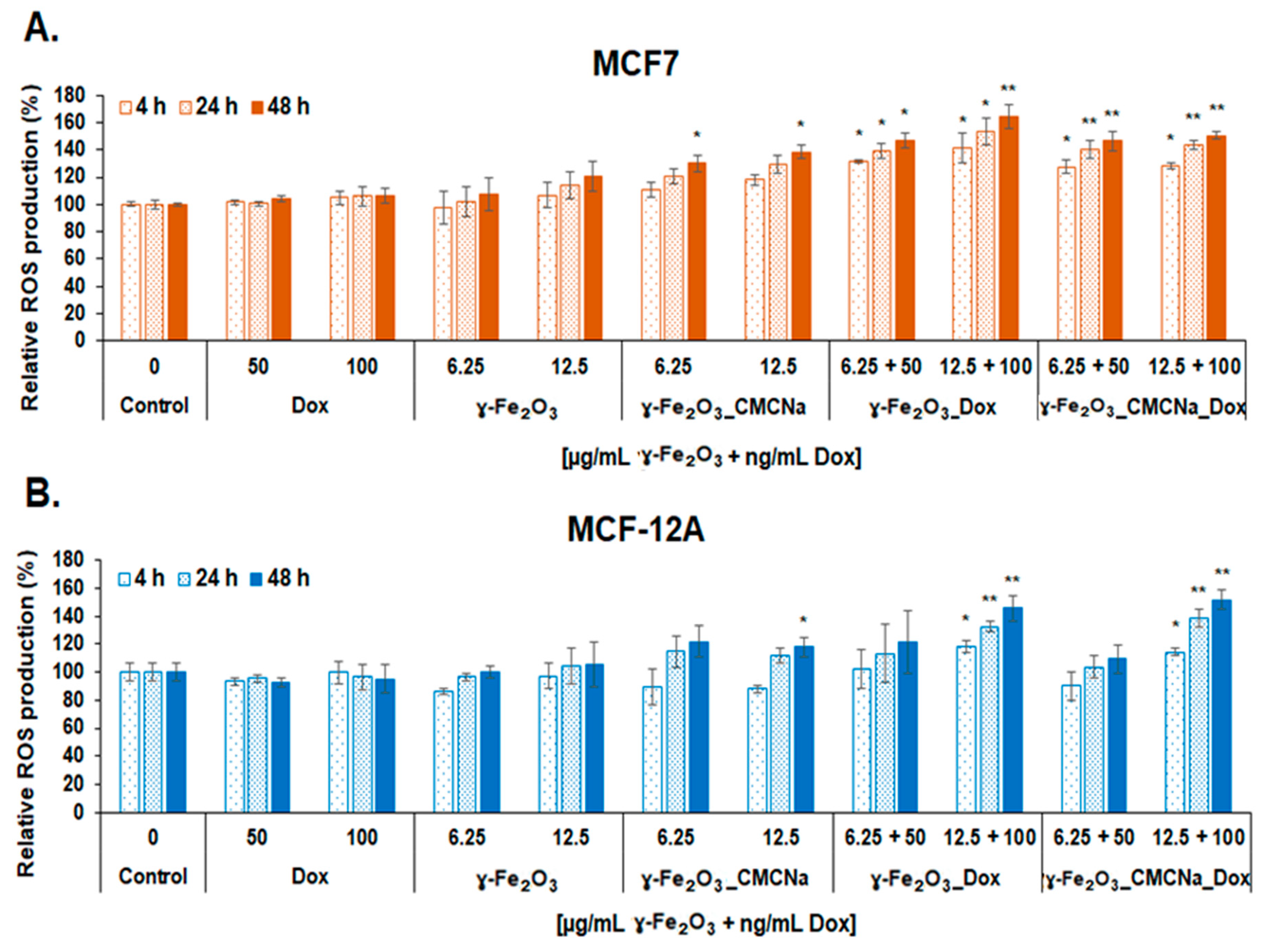
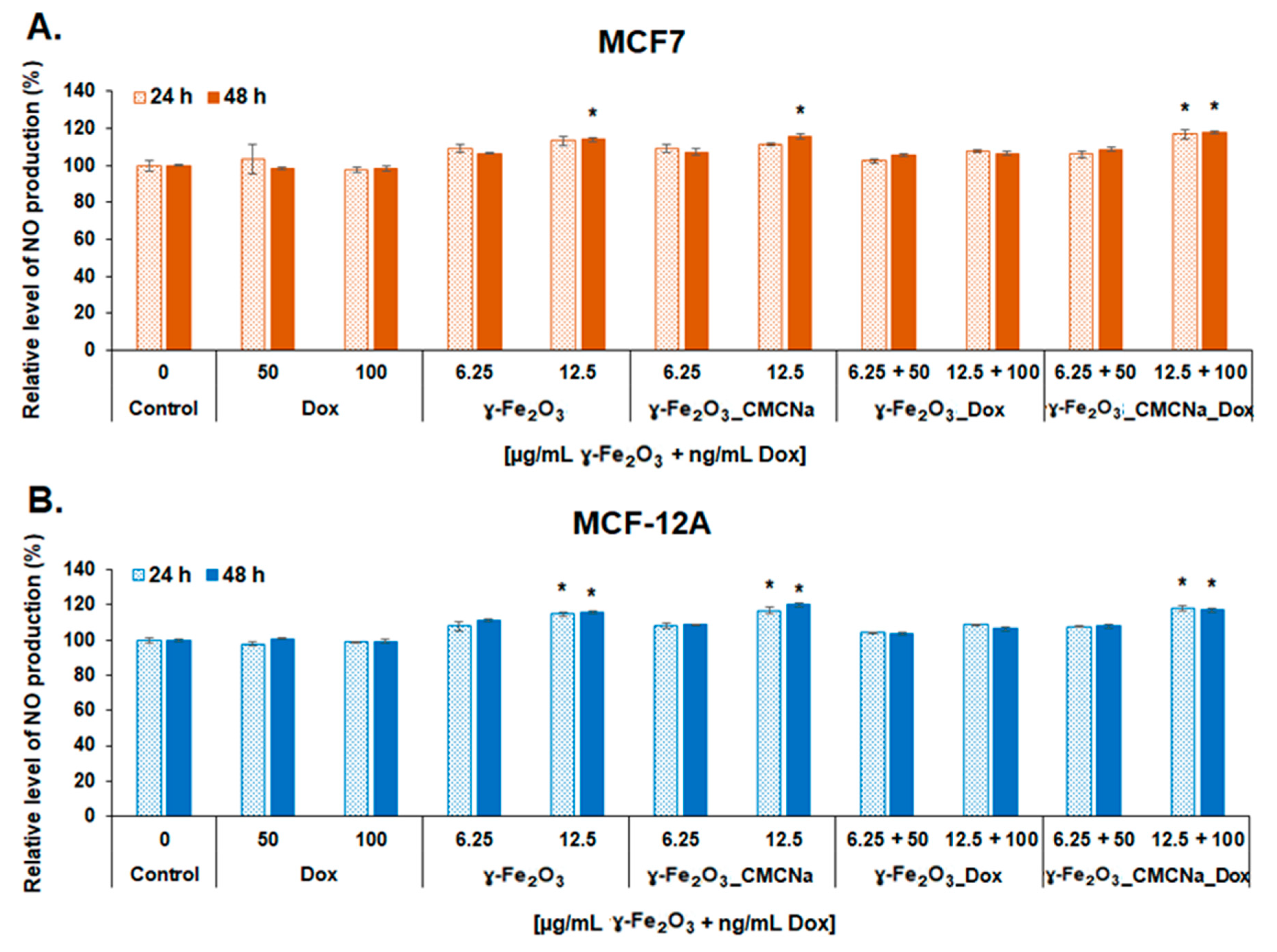
2.3.3. Measurement of Reactive Oxygen Species (ROS) and Nitric Oxide (NO) Production
- a.
- DCF-DA intracellular ROS detection. The MCF7 and MCF-12A cells were seeded in 96-well black sterile plates, clear bottom (165305, Thermo Scientific Nunc, Rochester, NY, USA), at a density of 3 × 104 cells/mL. After cell adhesion, culture media was removed, and the cells were incubated with 100 µL of 50 µM H2DCFDA solution (2’,7’-dichlorodihydrofluorescein diacetate; D6883, Sigma-Aldrich, St. Louis, MO, USA) prepared in HBSS (Hank’s Balanced Salt Solution) for 1 h at 37 °C for entering cells. Further, the non-internalized H2DCFDA was removed, and the cells were treated with 6.25 and 12.5 µg/mL γ-Fe2O3 nanoparticles and, respectively, 50 and 100 ng/mL DOX. Upon generation of ROS, a fluorescent compound 2’,7’-dichlorofluorescein (DCF) was formed and read at 485 nm ex./520 nm em. after 4, 24 and 48 h of treatment. The level of ROS in treated samples was calculated in relation to the control sample and expressed in percentages.
- b.
- In vitro NO assay. The level of NO released in culture media was measured to estimate the inflammatory potential of the suspensions on tumor and normal breast cells using Griess method. After the exposure of MCF7 and MCF-12A cells to 6.25 and 12.5 µg/mL NPs and, respectively, 50 and 100 ng/mL DOX for 24 and 48 h, 80 µL of culture media were mixed with 80 µL Griess reagent. The absorbance of the samples was measured at 540 nm. The NO concentration of the samples was determined using a NaNO2 standard curve (0–100 µM) and expressed as percentages relative to control.
2.3.4. Statistical Analysis
3. Results and Discussions
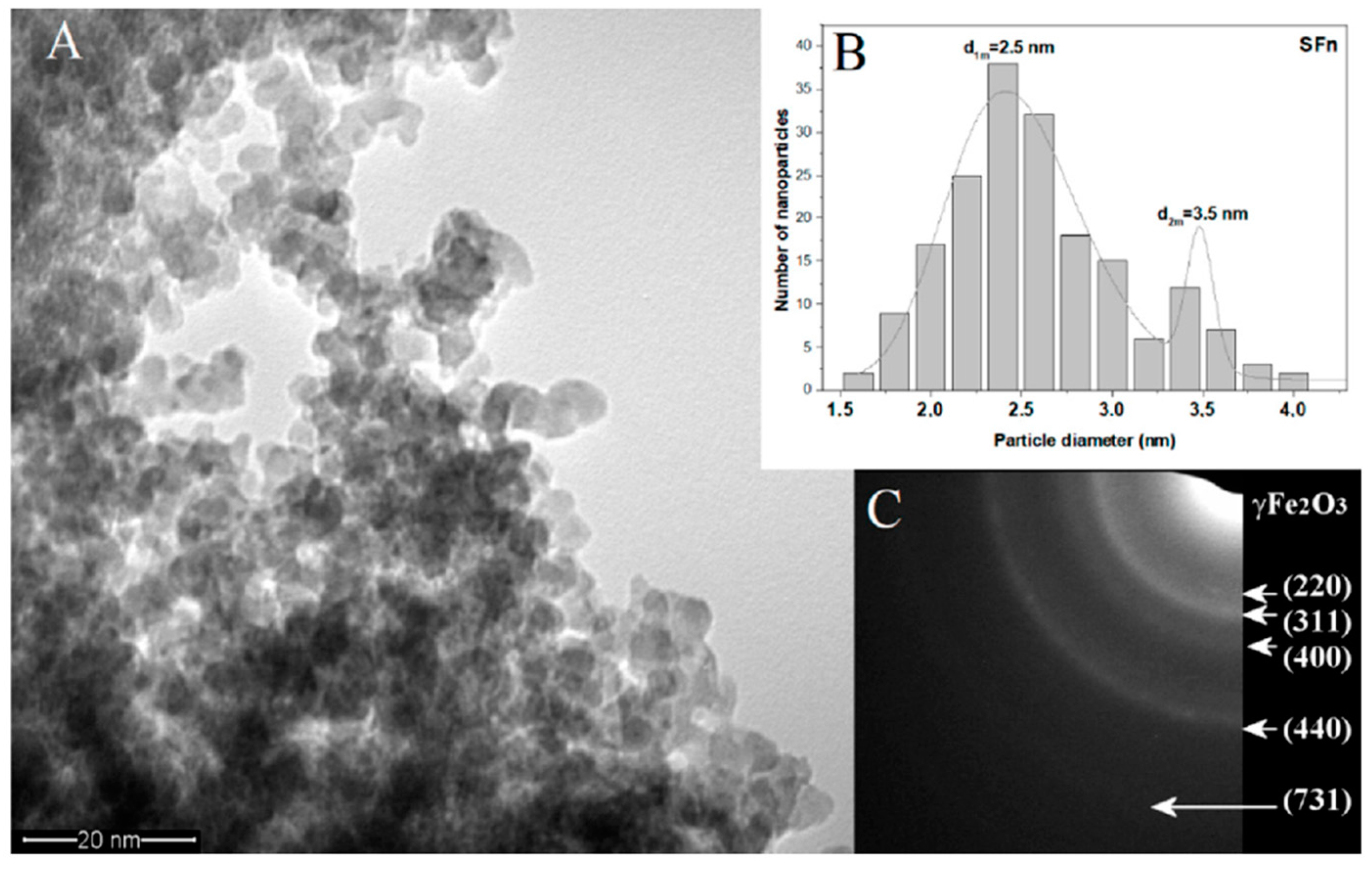
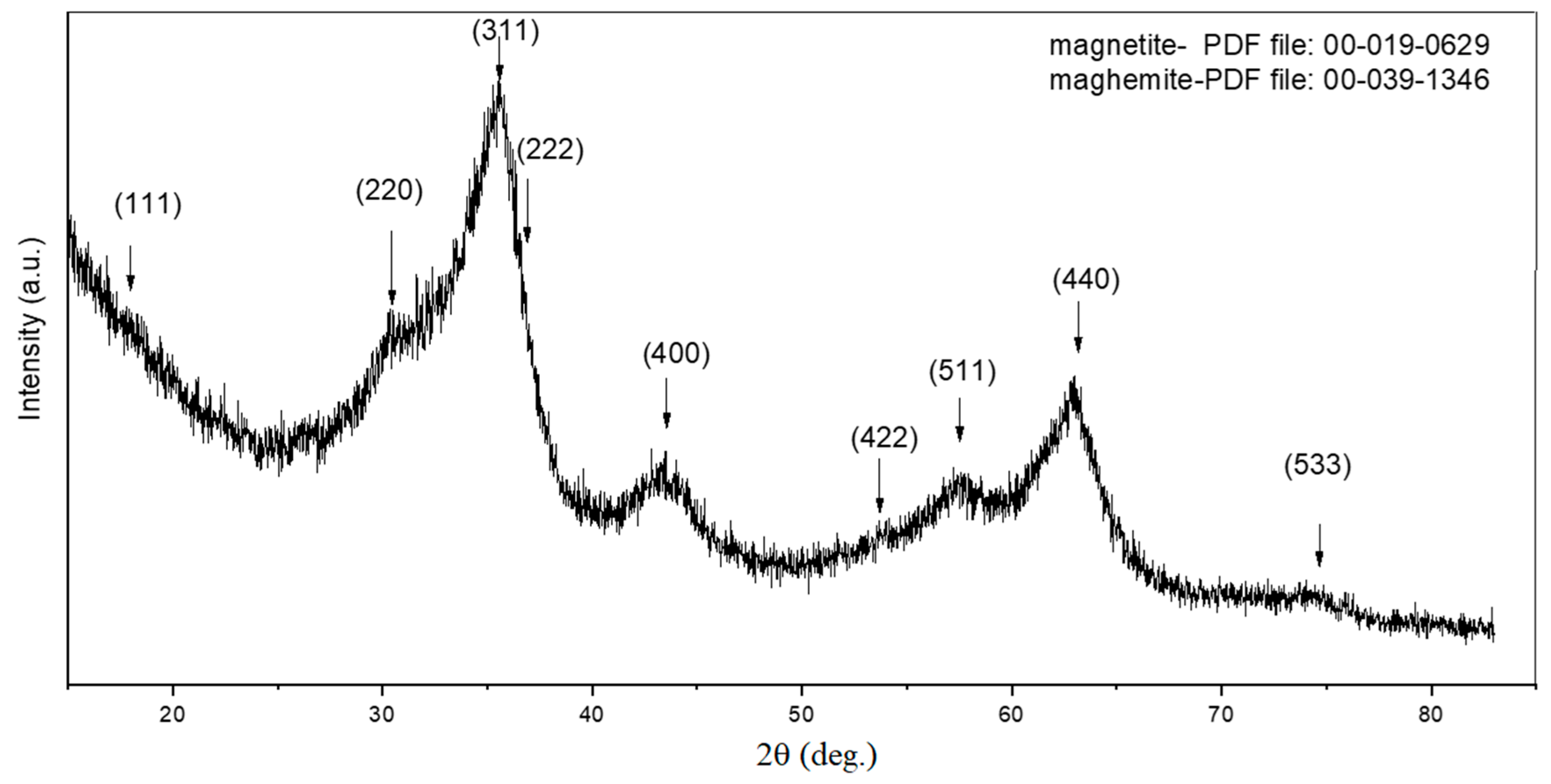
3.1. Characterization of Synthesized γ-Fe2O3 NPs
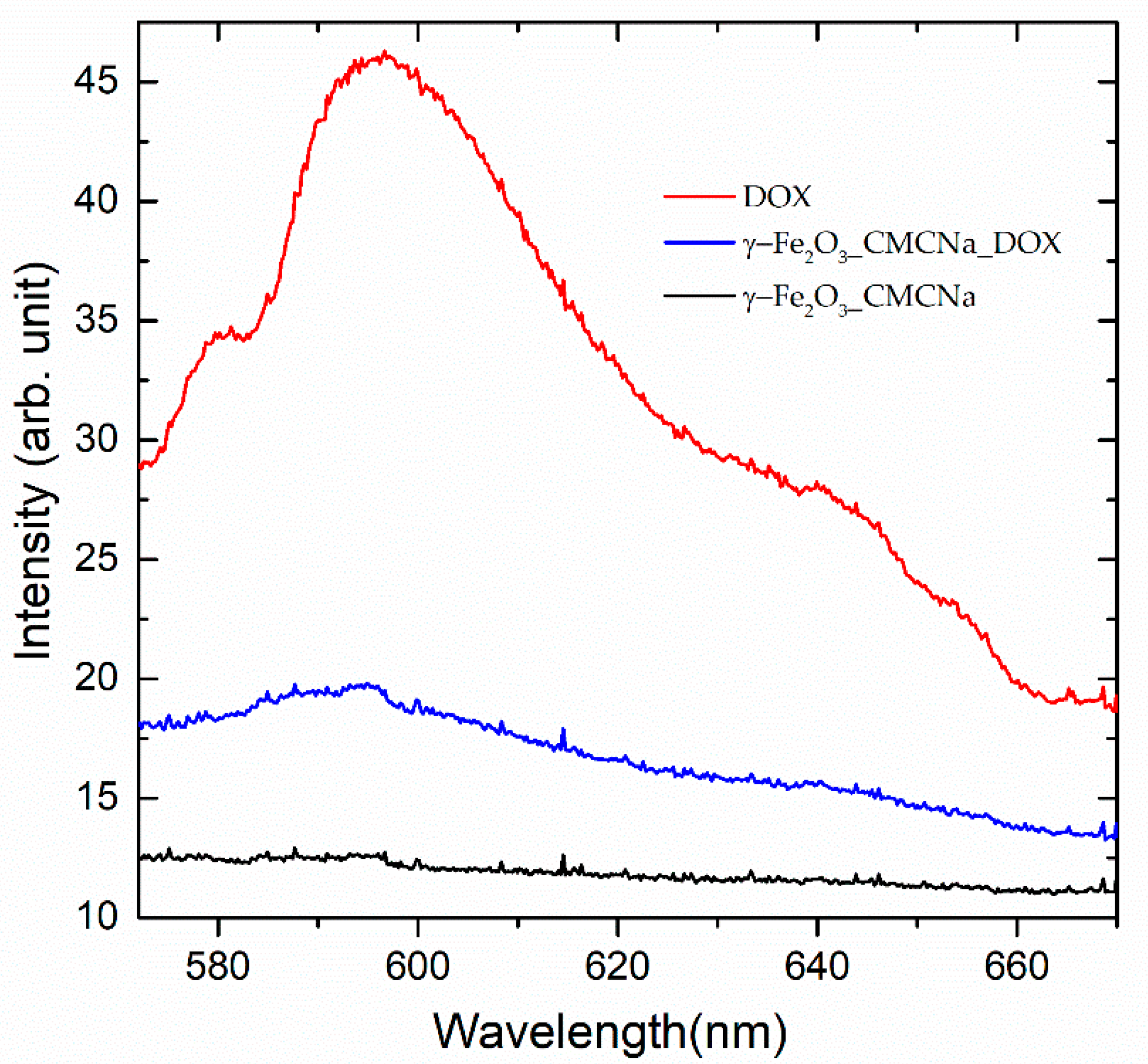
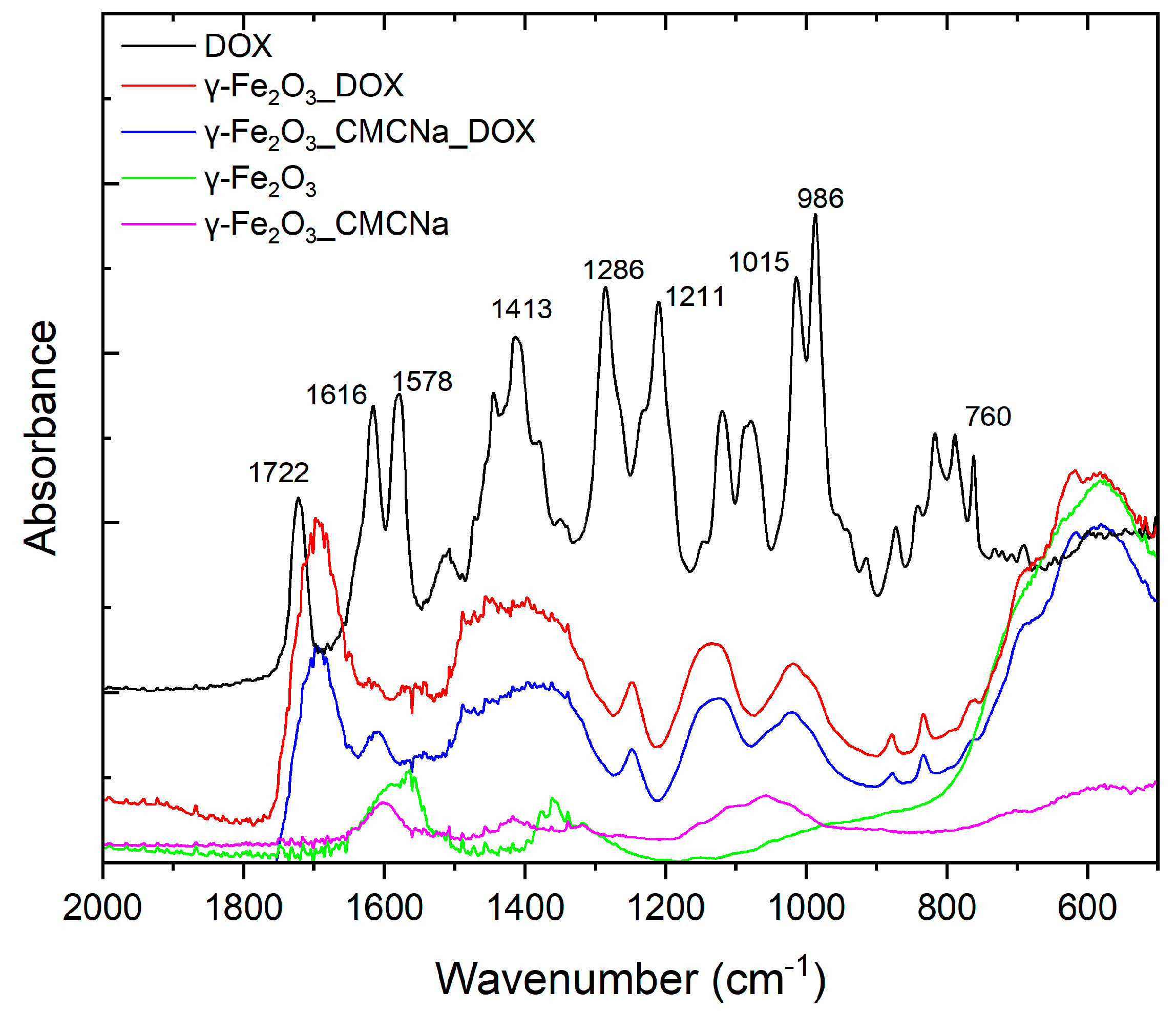
3.2. Spectroscopic Analysis
3.3. In Vitro Biological Evaluation
3.3.1. Anticancer Cell Efficiency
3.3.2. Oxidative and Inflammatory Potential
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Libson, S.; Lippman, M. A review of clinical aspects of breast cancer. Int. Rev. Psychiatry 2014, 26, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Preventing Cancer. Available online: http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 25 November 2020).
- Su, Y.L.; Hu, S.H. Functional Nanoparticles for Tumor Penetration of Therapeutics. Pharmaceutics 2018, 10, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, J.; Nohria, A. Cardiac Toxicity from Breast Cancer Treatment: Can We Avoid This? Curr. Oncol. Rep. 2018, 20, 61. [Google Scholar] [CrossRef]
- Conte, P.F.; Gennari, A.; Landucci, E.; Orlandini, C. Role of Epirubicin in Advanced Breast Cancer. Clin. Breast Cancer 2000, 1, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. IJMS 2018, 19, 3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Can. Res. Ther. 2014, 10, 853. [Google Scholar] [CrossRef]
- Shafei, A.; El-Bakly, W.; Sobhy, A.; Wagdy, O.; Reda, A.; Aboelenin, O.; Marzouk, A.; El Habak, K.; Mostafa, R.; Ali, M.A.; et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. [Google Scholar] [CrossRef]
- Anghel, N.; Herman, H.; Balta, C.; Rosu, M.; Stan, M.; Nita, D.; Ivan, A.; Galajda, Z.; Ardelean, A.; Dinischiotu, A.; et al. Acute cardiotoxicity induced by doxorubicin in right ventricle is associated with increase of oxidative stress and apoptosis in rats. Histol. Histopathol. 2017, 33, 11932. [Google Scholar] [CrossRef]
- Hanusova, V.; Skalova, L.; Kralova, V.; Matouskova, P. Potential Anti-cancer Drugs Commonly Used for Other Indications. CCDT 2015, 15, 35–52. [Google Scholar] [CrossRef]
- Lloyd-Parry, O.; Downing, C.; Aleisaei, E.; Jones, C.; Coward, K. Nanomedicine applications in women’s health: State of the art. IJN 2018, 13, 1963–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 2017, 14, 123–136. [Google Scholar] [CrossRef]
- Shabestari Khiabani, S.; Farshbaf, M.; Akbarzadeh, A.; Davaran, S. Magnetic nanoparticles: Preparation methods, applications in cancer diagnosis and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 6–17. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Martinkova, P.; Brtnicky, M.; Kynicky, J.; Pohanka, M. Iron Oxide Nanoparticles: Innovative Tool in Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2018, 7, 1700932. [Google Scholar] [CrossRef]
- Huber, D. Synthesis, Properties, and Applications of Iron Nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. NSA 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Xie, J.; Gao, H.; Yang, M.; Zhang, F.; Liu, G.; Lin, X.; Wang, A.; Eden, H.S.; Lee, S.; et al. HSA Coated Iron Oxide Nanoparticles as Drug Delivery Vehicles for Cancer Therapy. Mol. Pharm. 2011, 8, 1669–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.K.; Jeong, Y.Y.; Park, J.; Park, S.; Kim, J.W.; Min, J.J.; Kim, K.; Jon, S. Drug-Loaded Superparamagnetic Iron Oxide Nanoparticles for Combined Cancer Imaging and Therapy In Vivo. Angew. Chem. Int. Ed. 2008, 47, 5362–5365. [Google Scholar] [CrossRef]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [Green Version]
- Plichta, Z.; Kozak, Y.; Panchuk, R.; Sokolova, V.; Epple, M.; Kobylinska, L.; Jendelová, P.; Horák, D. Cytotoxicity of doxorubicin-conjugated poly[N-(2-hydroxypropyl)methacrylamide]-modified γ-Fe2O3 nanoparticles towards human tumor cells. Beilstein J. Nanotechnol. 2018, 9, 2533–2545. [Google Scholar] [CrossRef] [Green Version]
- Plichta, Z.; Horák, D.; Mareková, D.; Turnovcová, K.; Kaiser, R.; Jendelová, P. Poly[N-(2-hydroxypropyl)methacrylamide]-Modified Magnetic γ-F2O3 Nanoparticles Conjugated with Doxorubicin for Glioblastoma Treatment. ChemMedChem 2020, 15, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, R.; Wang, D.; Feng, L.; Cui, K. Synthesis of hollow maghemite (-Fe2O3) particles for magnetic field and pH-responsive drug delivery and lung cancer treatment. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Bomatí-Miguel, O.; Zhao, X.Q.; Martelli, S.; Di Nunzio, P.E.; Veintemillas-Verdaguer, S. Modeling of the laser pyrolysis process by means of the aerosol theory: Case of iron nanoparticles. J. Appl. Phys. 2010, 107, 014906. [Google Scholar] [CrossRef]
- Morjan, I.; Alexandrescu, R.; Dumitrache, F.; Birjega, R.; Fleaca, C.; Soare, I.; Luculescu, C.R.; Filoti, G.; Kuncer, V.; Vekas, L.; et al. Iron oxide-based nanoparticles with different mean sizes obtained by the laser pyrolysis: Structural and magnetic properties. J. Nanosci. Nanotechnol. 2010, 10, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Greculeasa, S.G.; Palade, P.; Schinteie, G.; Leca, A.; Dumitrache, F.; Lungu, I.; Prodan, G.; Kuncser, A.; Kuncser, V. Tuning structural and magnetic properties of Fe oxide nanoparticles by specific hydrogenation treatments. Sci. Rep. 2020, 10, 17174. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, N.S.H.; Parvin, P.; Ghasemi, F.; Atyabi, F. Fluorescence properties of several chemotherapy drugs: Doxorubicin, paclitaxel and bleomycin. Biomed. Opt. Express 2016, 7, 2400. [Google Scholar] [CrossRef] [Green Version]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Rana, S.; Gallo, A.; Srivastava, R.S.; Misra, R.D.K. On the suitability of nanocrystalline ferrites as a magnetic carrier for drug delivery: Functionalization, conjugation and drug release kinetics. Acta Biomater. 2007, 3, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Su, S.; Liou, B.; Lin, C.; Lee, K. Sulbactam-enhanced cytotoxicity of doxorubicin in breast cancer cells. Cancer Cell Int. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denard, B.; Lee, C.; Ye, J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife 2012, 1, e00090. [Google Scholar] [CrossRef]
- Eom, Y.W.; Kim, M.A.; Park, S.S.; Goo, M.J.; Kwon, H.J.; Sohn, S.; Kim, W.H.; Yoon, G.; Choi, K.S. Two distinct modes of cell death induced by doxorubicin: Apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene 2005, 24, 4765–4777. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Kwon, H.K.; Lee, J.H.; Gui, X.; Achek, A.; Kim, J.H.; Choi, S. Doxorubicin-induced necrosis is mediated by poly-(ADP-ribose) polymerase 1 (PARP1) but is independent of p53. Sci. Rep. 2015, 5, 15798. [Google Scholar] [CrossRef] [Green Version]
- Nestal de Moraes, G.; Vasconcelos, F.C.; Delbue, D.; Mognol, G.P.; Sternberg, C.; Viola, J.P.B.; Maia, R.C. Doxorubicin induces cell death in breast cancer cells regardless of Survivin and XIAP expression levels. Eur. J. Cell Biol. 2013, 92, 247–256. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, S.; Bhatkar, D.; Sharma, N.K. A novel method to detect intracellular metabolite alterations in MCF-7 cells by doxorubicin induced cell death. bioRxiv 2019, 812255. [Google Scholar] [CrossRef]
- Kievit, F.M.; Wang, F.Y.; Fang, C.; Mok, H.; Wang, K.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J. Control. Release 2011, 152, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef] [Green Version]
- Asensio-López, M.C.; Soler, F.; Pascual-Figal, D.; Fernández-Belda, F.; Lax, A. Doxorubicin-induced oxidative stress: The protective effect of nicorandil on HL-1 cardiomyocytes. PLoS ONE 2017, 12, e0172803. [Google Scholar] [CrossRef] [Green Version]






| Sample | D-C2H4/Fe(CO)5 | D-Synth. Air | D-Ar conf | D-Ar windows | p | plaser |
|---|---|---|---|---|---|---|
| m.u. | sccm | sccm | sccm | sccm | mbar | W |
| γ-Fe2O3 | 100/20 | 100 | 2000 | 300 | 300 | 55/53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, I.I.; Nistorescu, S.; Badea, M.A.; Petre, A.-M.; Udrea, A.-M.; Banici, A.-M.; Fleacă, C.; Andronescu, E.; Dinischiotu, A.; Dumitrache, F.; et al. Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells. Polymers 2020, 12, 2799. https://doi.org/10.3390/polym12122799
Lungu II, Nistorescu S, Badea MA, Petre A-M, Udrea A-M, Banici A-M, Fleacă C, Andronescu E, Dinischiotu A, Dumitrache F, et al. Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells. Polymers. 2020; 12(12):2799. https://doi.org/10.3390/polym12122799
Chicago/Turabian StyleLungu, Iulia Ioana, Simona Nistorescu, Mădălina Andreea Badea, Andreea-Mihaela Petre, Ana-Maria Udrea, Ana-Maria Banici, Claudiu Fleacă, Ecaterina Andronescu, Anca Dinischiotu, Florian Dumitrache, and et al. 2020. "Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells" Polymers 12, no. 12: 2799. https://doi.org/10.3390/polym12122799
APA StyleLungu, I. I., Nistorescu, S., Badea, M. A., Petre, A.-M., Udrea, A.-M., Banici, A.-M., Fleacă, C., Andronescu, E., Dinischiotu, A., Dumitrache, F., Staicu, A., & Balaș, M. (2020). Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells. Polymers, 12(12), 2799. https://doi.org/10.3390/polym12122799









