Thermoplastic Starch-Based Blends with Improved Thermal and Thermomechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Processing
2.3. Characterization
2.4. Statistical Analysis
3. Result
3.1. Analysis of Functional Groups
3.2. Glass Transition and Crystallinity
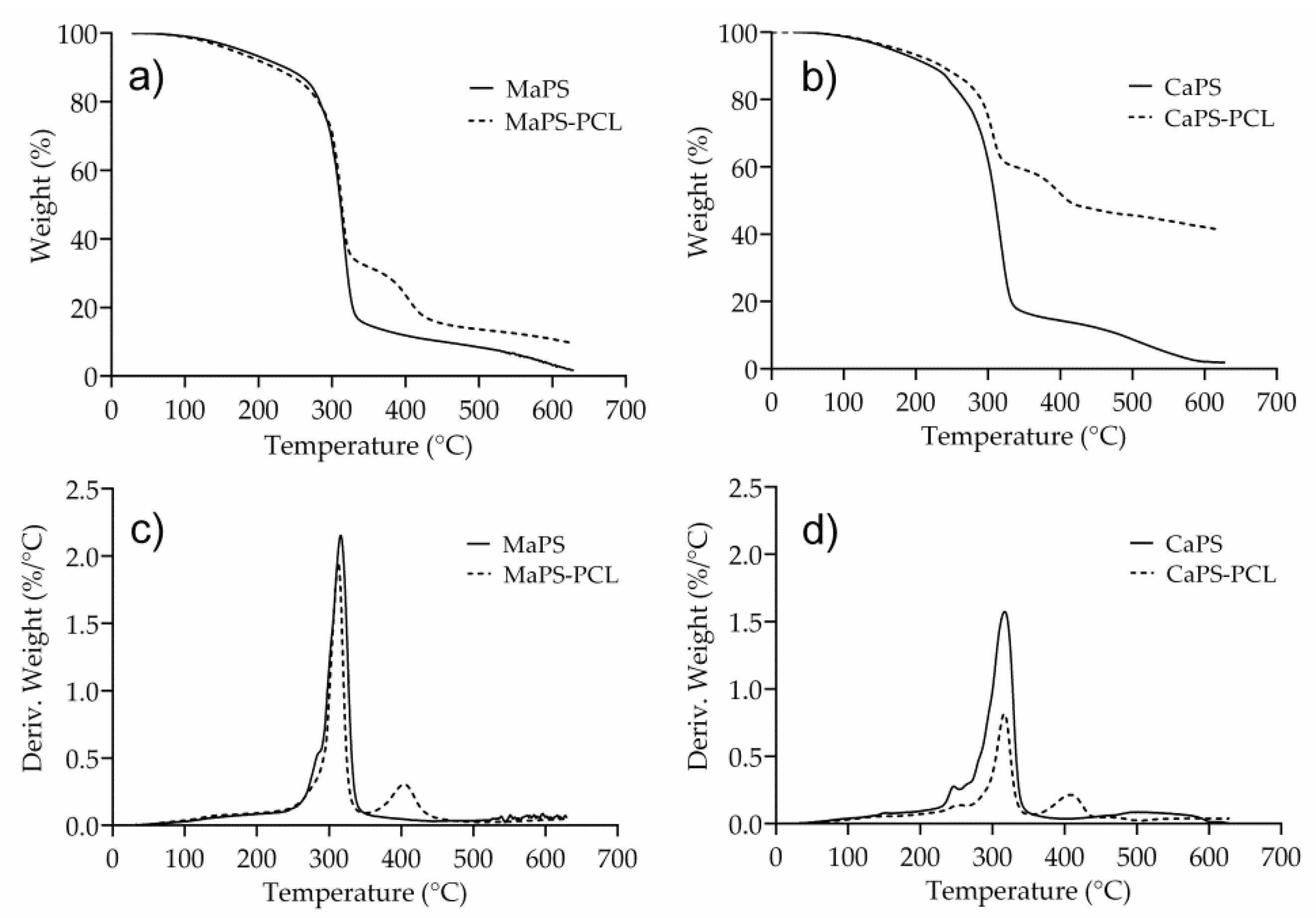
3.3. Thermal Stability
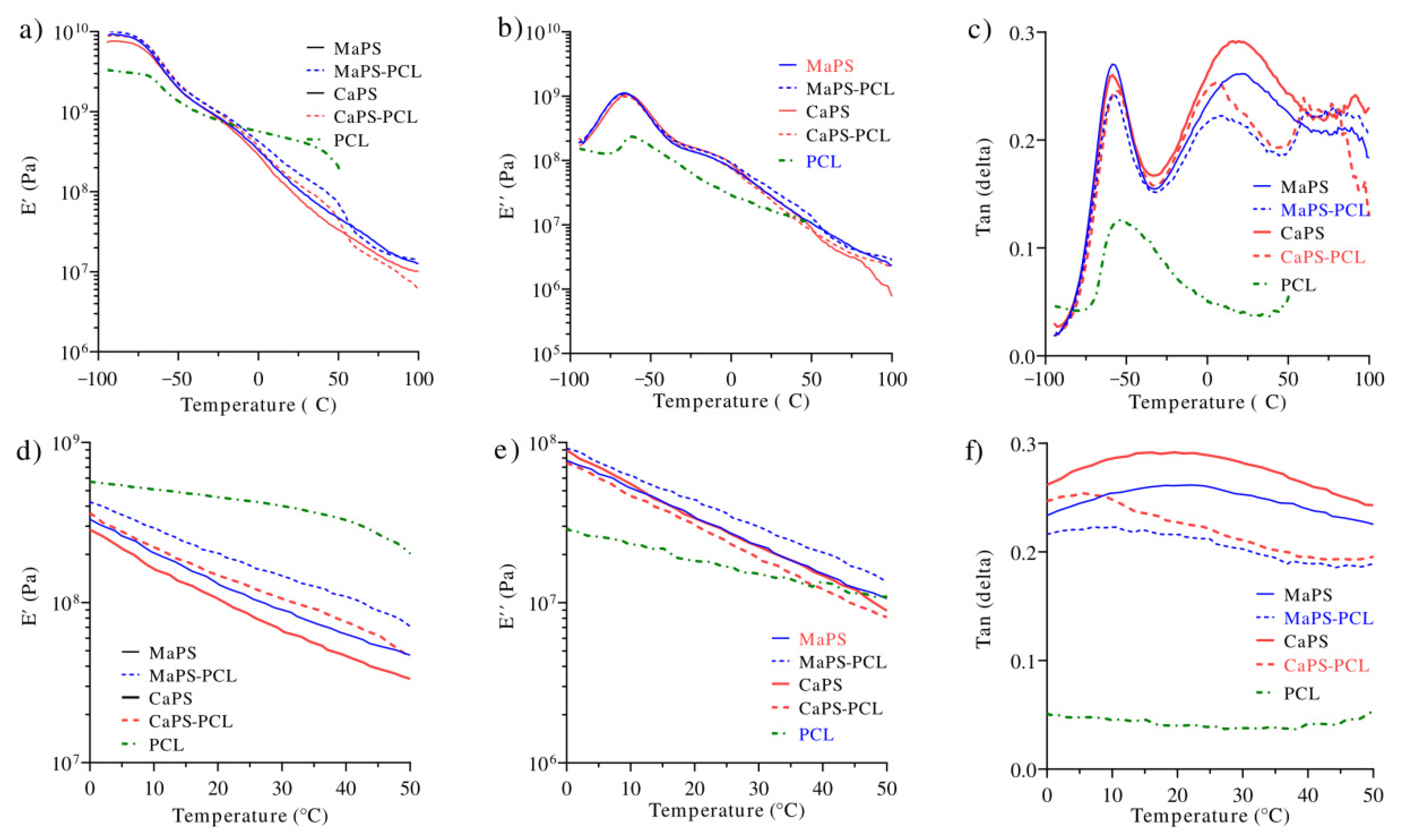
3.4. Dynamic Mechanical Analysis
3.5. Mechanical Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heinrichs, S. Compromiso Mundial Para Reducir Los Plásticos de un Solo Uso. Available online: https://news.un.org/es/story/2019/03/1452961 (accessed on 15 March 2019).
- Muhammad Shamsuddin, I. Bioplastics as Better Alternative to Petroplastics and Their Role in National Sustainability: A Review. Adv. Biosci. Bioeng. 2017, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Zambrano, M.C.; Pawlak, J.J.; Venditti, R.A. Effect of lignocellulosic fiber composition on the aquatic biodegradation of wood pulps and the isolated cellulose, hemicellulose and lignin components: Kinetic modelling of the biodegradation process. Cellulose 2021, 28, 2863–2877. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Polish J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Dordevic, D.; Necasova, L.; Antonic, B.; Jancikova, S.; Tremlová, B. Plastic cutlery alternative: Case study with biodegradable spoons. Foods 2021, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Le Guen, M.J.; Tucker, N.; Parker, K. Thermo-mechanical, morphological and water absorption properties of thermoplastic starch/cellulose composite foams reinforced with PLA. Cellulose 2019, 26, 4463–4478. [Google Scholar] [CrossRef]
- Souza, R.C.R.; Andrade, C.T. Processing and properties of thermoplastic starch and its blends with sodium alginate. J. Appl. Polym. Sci. 2001, 81, 412–420. [Google Scholar] [CrossRef]
- Hernández-Medina, M.; Torruco-Uco, J.G.; Chel-Guerrero, L.; Betancur-Ancona, D. Physical-chemical characterization of starch from cultivated tubers in Yucatan, Mexico. Cienc. Tecnol. Aliment. 2008, 28, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, A.C. Starches: Characterization, Properties, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- García, N.; Aranguren, M.; Dufresne, A.; Goyanes, S. Efecto de la concentración de amilopectina en la respuesta fisicomecánica de films de almidón. In Proceedings of the Congreso SAM/CONAMET 2007, San Nicolás, Buenos Aires, Argentina, 4–7 September 2007. [Google Scholar]
- Rivadeneira-Velasco, K.E.; Utreras-Silva, C.A.; Díaz-Barrios, A.; Sommer-Márquez, A.E.; Tafur, J.P.; Michell, R.M. Green nanocomposites based on thermoplastic starch: A review. Polymers 2021, 13, 3227. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in starch plastics: Consequences for material properties. Trends Biotechnol. 1997, 15, 208–213. [Google Scholar] [CrossRef]
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of thermoplastic blends: Starch-polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Zhang, Z.; Ye, C.; Chen, Y.; Wei, P.; Wang, Y.; Xia, Y. Thermoplastic starch plasticized by polymeric ionic liquid. Eur. Polym. J. 2021, 148, 110367. [Google Scholar] [CrossRef]
- Abera, G.; Woldeyes, B.; Demash, H.D.; Miyake, G. The effect of plasticizers on thermoplastic starch films developed from the indigenous Ethiopian tuber crop Anchote (Coccinia abyssinica) starch. Int. J. Biol. Macromol. 2020, 155, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Giroto, A.S.; Garcia, R.H.S.; Colnago, L.A.; Klamczynski, A.; Glenn, G.M.; Ribeiro, C. Role of urea and melamine as synergic co-plasticizers for starch composites for fertilizer application. Int. J. Biol. Macromol. 2020, 144, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Effect of plasticizers on physical, thermal, and tensile properties of thermoplastic films based on Dioscorea hispida starch. Int. J. Biol. Macromol. 2021, 185, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, P.; Behzad, T.; Sadeghi, M. Investigation of cross-linked PVA/starch biocomposites reinforced by cellulose nanofibrils isolated from aspen wood sawdust. Cellulose 2017, 24, 3323–3339. [Google Scholar] [CrossRef]
- Lerma-Canto, A.; Gomez-Caturla, J.; Herrero-Herrero, M.; Garcia-Garcia, D.; Fombuena, V. Development of polylactic acid thermoplastic starch formulations using maleinized hemp oil as biobased plasticizer. Polymers 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Balla, B.; Bartos, A.; Kun, D.; Csiszár, E.; Móczó, J.; Fekete, E. Improving mechanical and water sorption properties of thermoplastic starch by incorporating chitosan filler. Polym. Test. 2021, 101, 107278. [Google Scholar] [CrossRef]
- Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the tensile and tear properties of thermoplastic starch/dolomite biocomposite film through sonication process. Polymers 2021, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Carmona, V.B.; De Campos, A.; Marconcini, J.M.; Mattoso, L.H.C. Kinetics of thermal degradation applied to biocomposites with TPS, PCL and sisal fibers by non-isothermal procedures. J. Therm. Anal. Calorim. 2014, 115, 153–160. [Google Scholar] [CrossRef]
- Dubois, P.; Krishnan, M.; Narayan, R. Aliphatic polyester-grafted starch-like polysaccharides by ring-opening polymerization. Polymer 1999, 40, 3091–3100. [Google Scholar] [CrossRef]
- Carmona, V.B.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Properties of a Biodegradable Ternary Blend of Thermoplastic Starch (TPS), Poly(ε-Caprolactone) (PCL) and Poly(Lactic Acid) (PLA). J. Polym. Environ. 2015, 23, 83–89. [Google Scholar] [CrossRef]
- Piñeros-Guerrero, N.; Piñeros-Castro, Y.; Ortega-Toro, R. Active biodegradable films based on thermoplastic starch and poly (ε-caprolactone): Technological application of antioxidant extracts from rice husk. Rev. Mex. Ing. Quim. 2020, 19, 1095–1101. [Google Scholar] [CrossRef]
- Hernandez, M.; Herminsul, J. Effect of the incorporation of polycaprolactone (PCL) on the retrogradation of binary blends with cassava thermoplastic starch (TPS). Polymers 2021, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Carballo, Z.B.; Duarte-Aranda, S.; Canché-Escamilla, G. Properties and Biodegradability of Thermoplastic Starch Obtained from Granular Starches Grafted with Polycaprolactone. Int. J. Polym. Sci. 2017, 2017, 3975692. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ϵ-caprolactone. comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Vertuccio, L.; Gorrasi, G.; Sorrentino, A.; Vittoria, V. Nano clay reinforced PCL/starch blends obtained by high energy ball milling. Carbohydr. Polym. 2009, 75, 172–179. [Google Scholar] [CrossRef]
- Moreno, O.; Cárdenas, J.; Atarés, L.; Chiralt, A. Influence of starch oxidation on the functionality of starch-gelatin based active films. Carbohydr. Polym. 2017, 178, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zullo, R.; Iannace, S. The effects of different starch sources and plasticizers on film blowing of thermoplastic starch: Correlation among process, elongational properties and macromolecular structure. Carbohydr. Polym. 2009, 77, 376–383. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Barrios-Guzmán, A.J.; García-Enriquez, S.; Rivera-Prado, J.D.J.; Manríquez-González, R. Chemical and Mechanical Evaluation of Bio-composites Based on Thermoplastic Starch and Wood Particles Prepared by Thermal Compression. BioResources 2014, 9, 2960–2974. [Google Scholar] [CrossRef]
- Mina, J.; Valadez, A.; Toledano, T. Physico-chemical studied of thermoplastic starch (TPS) and polycaprolactone (PCL). Biotecnol. Sect. Agropecu. Agroind. 2013, 11, 31–40. [Google Scholar]
- Cesteros Iturbe, L.C. Aplicaciones de la FTIR al estudio de las interacciones polímero-polímero. Rev. Iberoam. Polímeros 2004, 5, 111–132. [Google Scholar]
- Campos, A.; Teodoro, K.B.R.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Wood, D.F.; Williams, T.G.; Mattoso, L.H.C. Properties of thermoplastic starch and TPS/polycaprolactone blend reinforced with sisal whiskers using extrusion processing. Polym. Eng. Sci. 2013, 53, 800–808. [Google Scholar] [CrossRef]
- Correa, A.C.; Carmona, V.B.; Simão, J.A.; Capparelli Mattoso, L.H.; Marconcini, J.M. Biodegradable blends of urea plasticized thermoplastic starch (UTPS) and poly(ε-caprolactone) (PCL): Morphological, rheological, thermal and mechanical properties. Carbohydr. Polym. 2017, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. Characterization of amylose and amylopectin fractions separated from potato, banana, corn, and cassava starches. Int. J. Biol. Macromol. 2019, 132, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ninago, M.D.; López, O.V.; Lencina, M.M.S.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villar, M.A. Enhancement of thermoplastic starch final properties by blending with poly(ε-caprolactone). Carbohydr. Polym. 2015, 134, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, A.; Terrié, C.; Agoulon, A.; Leblanc, N.; Youssef, B. Thermoplastic starch and poly(ε-caprolactone) blends: Morphology and mechanical properties as a function of relative humidity. J. Polym. Res. 2013, 20, 229. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch-Staerke 2013, 65, 61–72. [Google Scholar] [CrossRef]
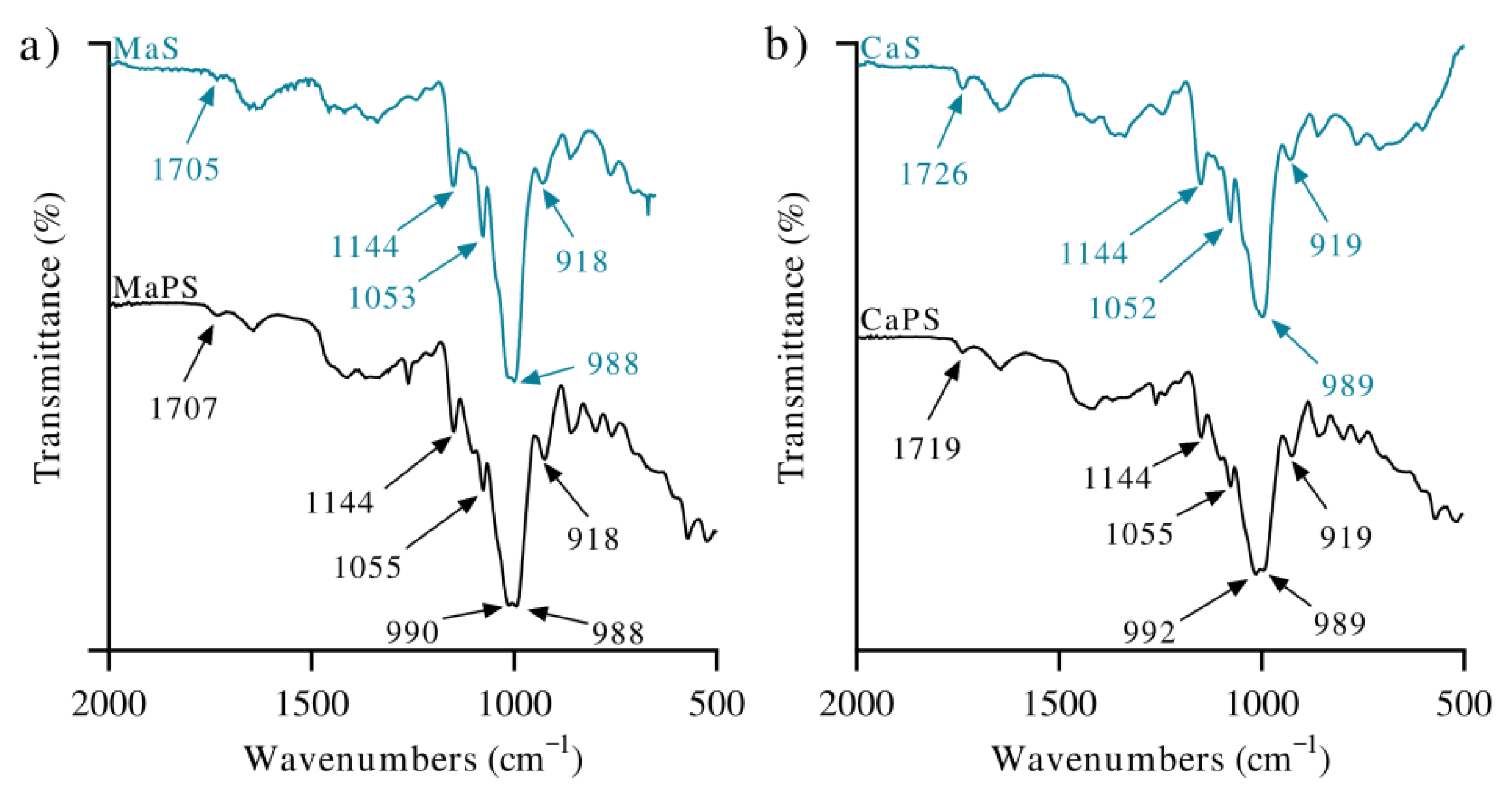
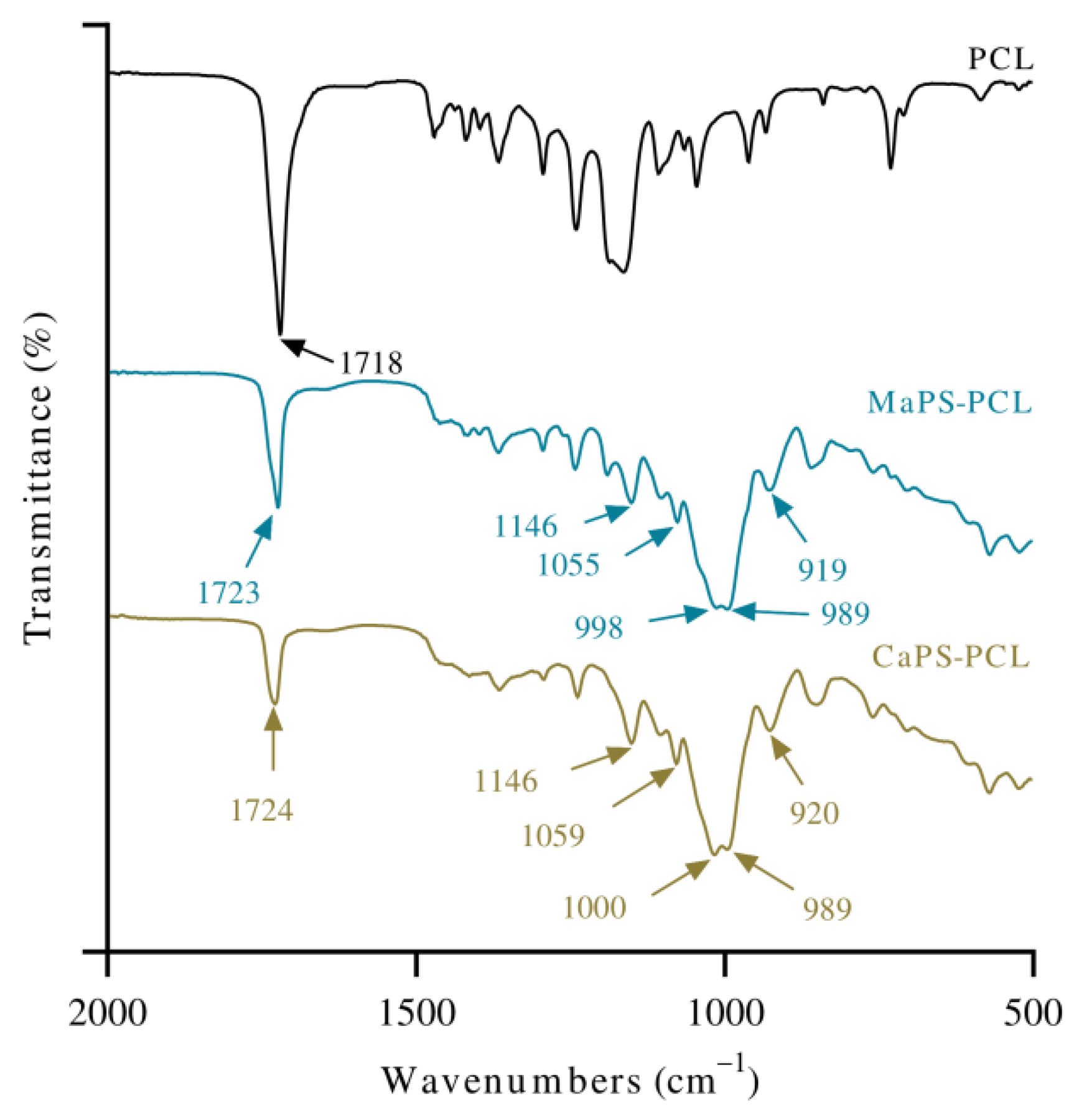


| Bonding | Wavenumbers (cm−1) | |||||
|---|---|---|---|---|---|---|
| Experimental Values | Mina et al. [33] | PCL | MaPS | CaPS | ||
| MaPS–PCL | CaPS–PCL | |||||
| O–H stretching | 3270 | 3270 | 3331 | 3288 | 3270 | |
| Asymmetric/symmetric CH2/CH3 | 2929/2872 | 2930/2870 | 2945/2866 | 2942/2866 | 2913/2887 | 2920/2887 |
| C=O Stretching (PCL) | 1723 | 1724 | 1724 | 1718 | ||
| Asymmetric stretching C–O–C (PCL) | 1242 | 1242 | 1242 | 1242 | 1242 | |
| Stretching of Glycosidic C–O–C | 1055/1146 | 1059/1146 | 1043/1029 | 1055/1144 | 1055/1144 | |
| Water absorption | 1622 | 1621 | 1618 | 1625 | ||
| Glycosidic bond, C–O stretching | 989 | 989 | 988 | 989 | ||
| After plasticization | 998 | 1000 | 990 | 992 | ||
| Sample | Tm (°C) | ΔHm (J g−1) | Tc (°C) | ΔHc (J/g) | CI (%) |
|---|---|---|---|---|---|
| CaPS–PCL | 61.0 | 10.0 | - | - | 38.0 |
| MaPS–PCL | 61.5 | 16.5 | - | - | 62.0 |
| PCL | 16.6 | 39.7 | 65.4 | 233 | 28.5 |
| Material | σmax (MPa) | Elongation (%) | Cases | Media | Standard Error | Homogeneous Groups |
|---|---|---|---|---|---|---|
| MaPS | 0.210 ± 0.019 | 63 | 6 | 0.1642 | 0.010219 | X |
| CaPS | 0.164 ± 0.005 | 90 | 6 | 0.2101 | 0.010219 | X |
| MaPS–PCL | 0.168 ± 0.003 | 60 | 6 | 0.1680 | 0.010219 | X |
| CaPS–PCL | 0.227 ± 0.040 | 70 | 6 | 0.2272 | 0.010219 | X |
| Contrast | Significance | Difference | +/− Limits |
|---|---|---|---|
| MaPS–MaPS–PCL | * | 0.0421 | 0.0301 |
| MaPS–CaPS | * | 0.0458 | 0.0301 |
| MaPS–CaPS–PCL | −0.0171 | 0.0301 | |
| MaPS–CaPS | 0.0037 | 0.0301 | |
| MaPS-PLC–CaPS–PCL | * | −0.0592 | 0.0301 |
| CaPS–MaPS–PCL | * | −0.0629 | 0.0301 |
| Material | Sample Size | Average |
|---|---|---|
| MaPS | 6 | 17.16 |
| MaPS–PCL | 6 | 8.50 |
| CaPS | 6 | 6.50 |
| CaPS–PCL | 6 | 17.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Monje, A.; Alonso-Romero, S.; Zitzumbo-Guzmán, R.; Estrada-Moreno, I.A.; Zaragoza-Contreras, E.A. Thermoplastic Starch-Based Blends with Improved Thermal and Thermomechanical Properties. Polymers 2021, 13, 4263. https://doi.org/10.3390/polym13234263
Estrada-Monje A, Alonso-Romero S, Zitzumbo-Guzmán R, Estrada-Moreno IA, Zaragoza-Contreras EA. Thermoplastic Starch-Based Blends with Improved Thermal and Thermomechanical Properties. Polymers. 2021; 13(23):4263. https://doi.org/10.3390/polym13234263
Chicago/Turabian StyleEstrada-Monje, Anayansi, Sergio Alonso-Romero, Roberto Zitzumbo-Guzmán, Iván Alziri Estrada-Moreno, and Erasto Armando Zaragoza-Contreras. 2021. "Thermoplastic Starch-Based Blends with Improved Thermal and Thermomechanical Properties" Polymers 13, no. 23: 4263. https://doi.org/10.3390/polym13234263
APA StyleEstrada-Monje, A., Alonso-Romero, S., Zitzumbo-Guzmán, R., Estrada-Moreno, I. A., & Zaragoza-Contreras, E. A. (2021). Thermoplastic Starch-Based Blends with Improved Thermal and Thermomechanical Properties. Polymers, 13(23), 4263. https://doi.org/10.3390/polym13234263









