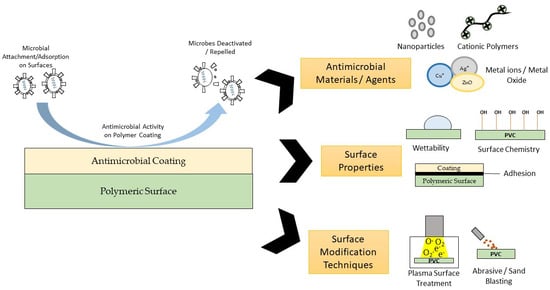
Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review
Abstract
:1. Introduction
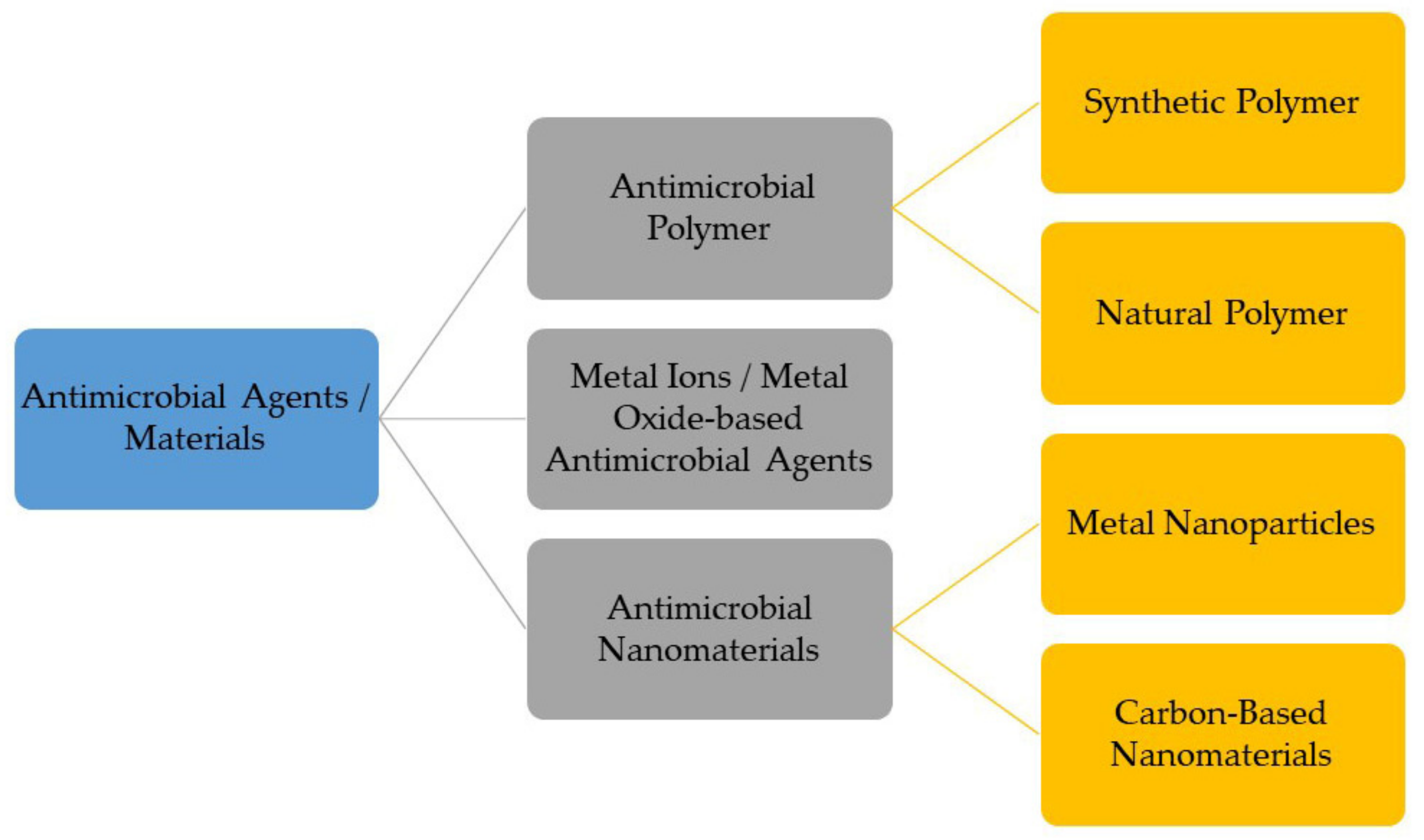
2. Classes of Antimicrobial Materials for Antimicrobial Coating
2.1. Antimicrobial Polymers
2.2. Metal Ions/Metal Oxide-Based Antimicrobial Agents
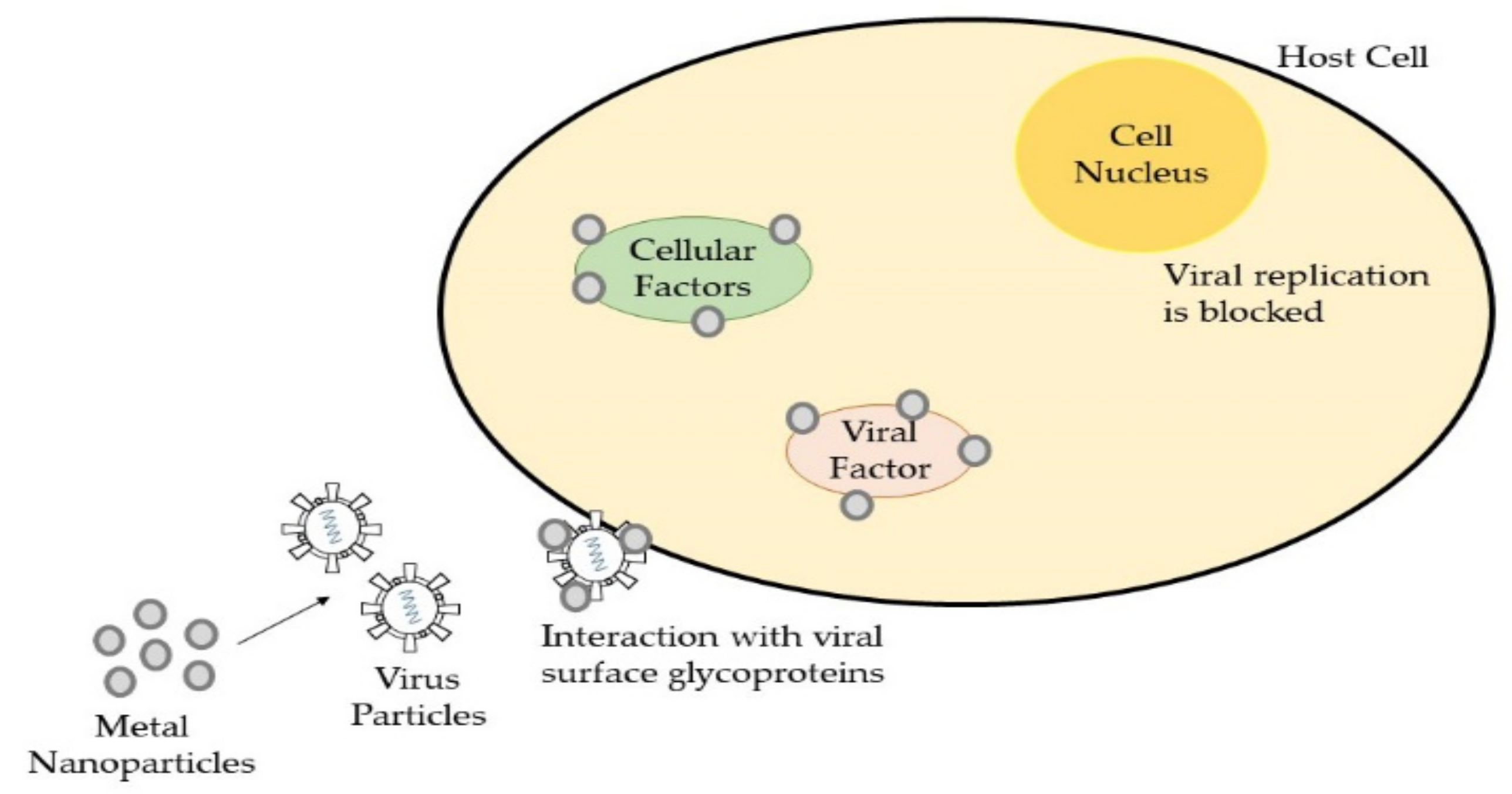
2.3. Antimicrobial Nanomaterials
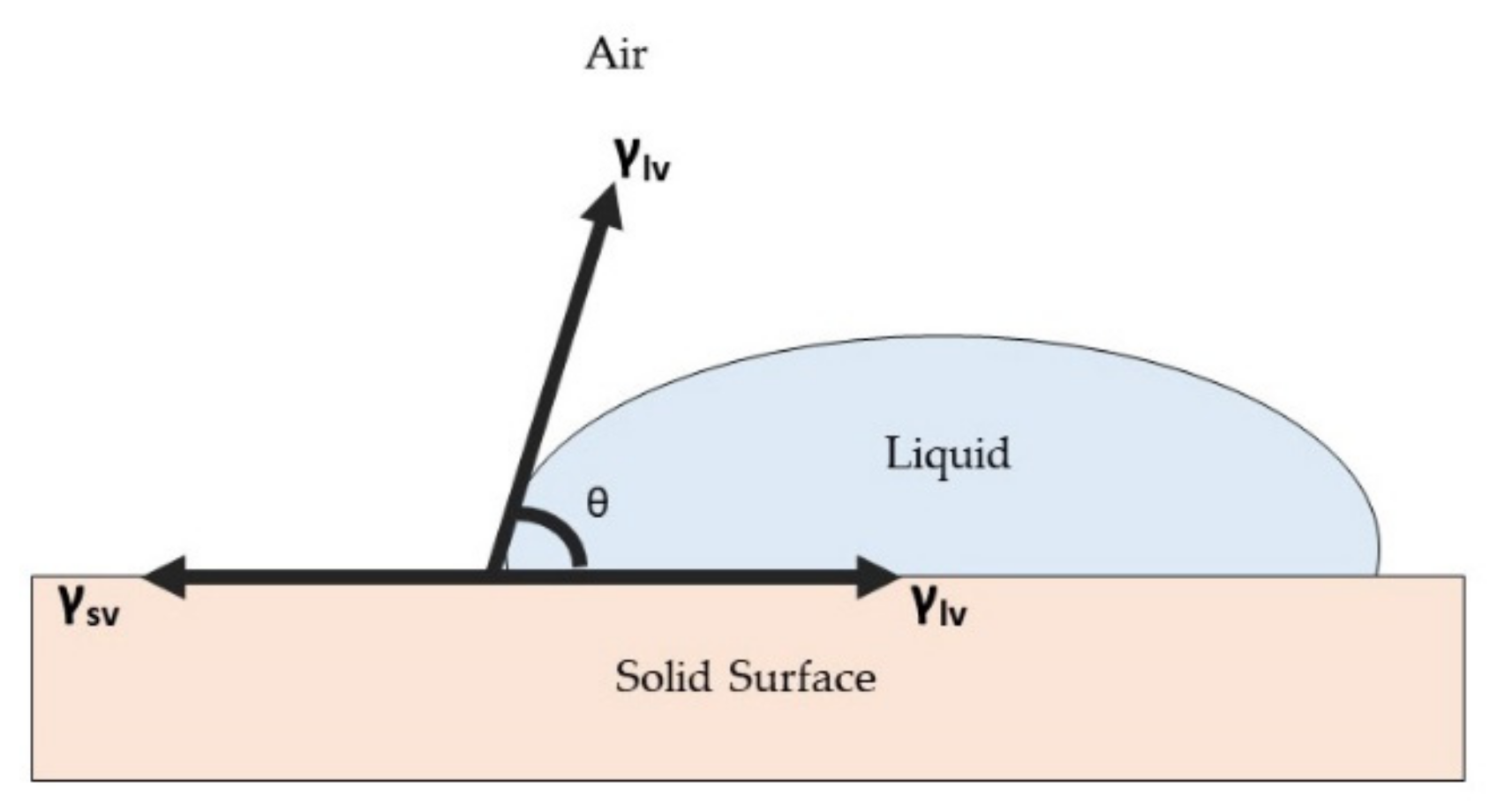
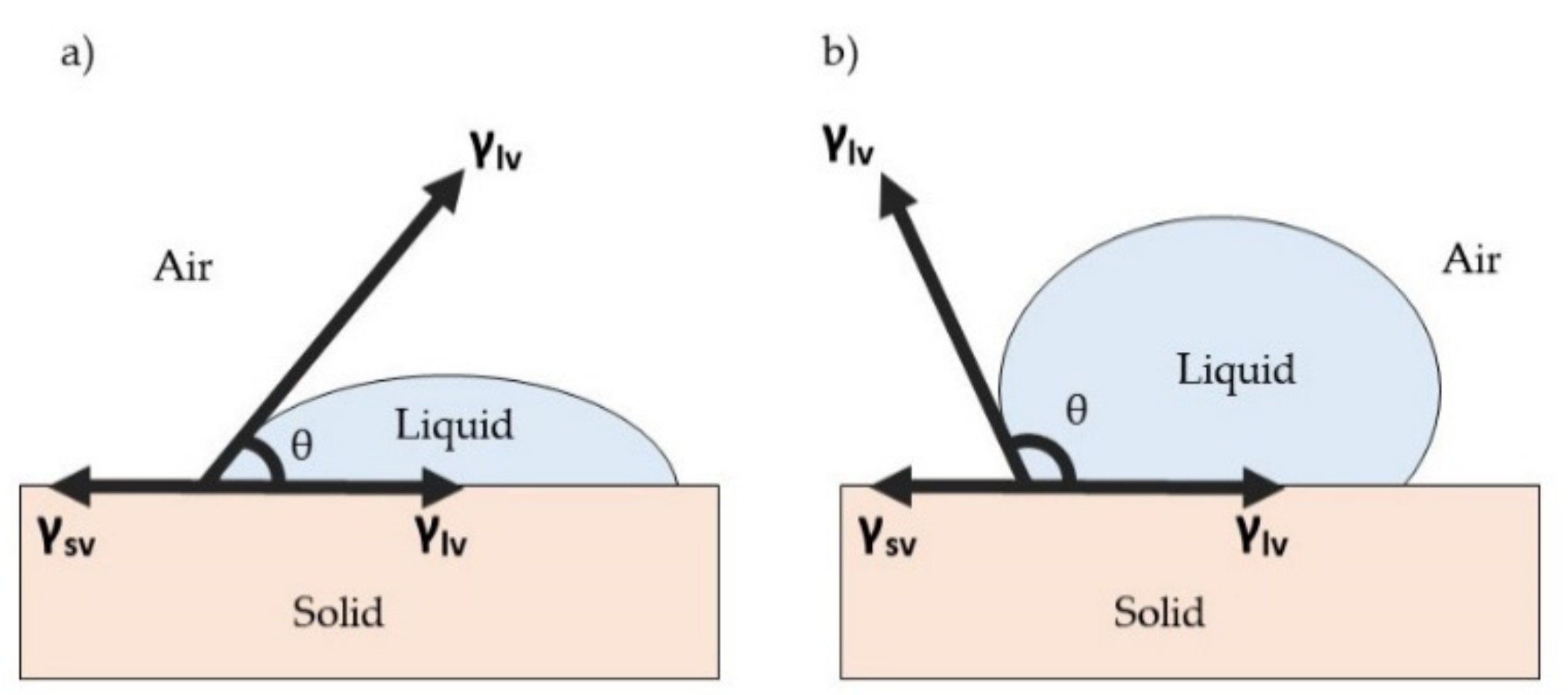
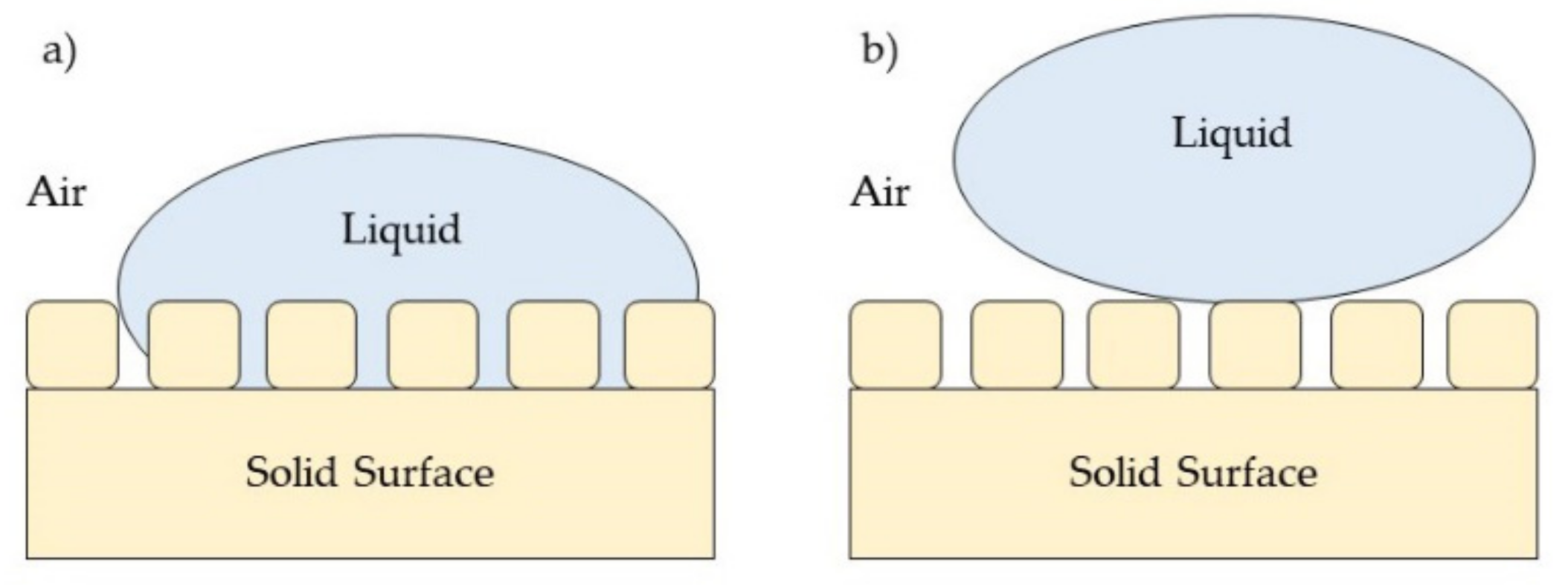
3. Surface Properties for Antimicrobial Surface Coating
3.1. Surface Wettability Properties
3.2. Surface Mechanical Properties
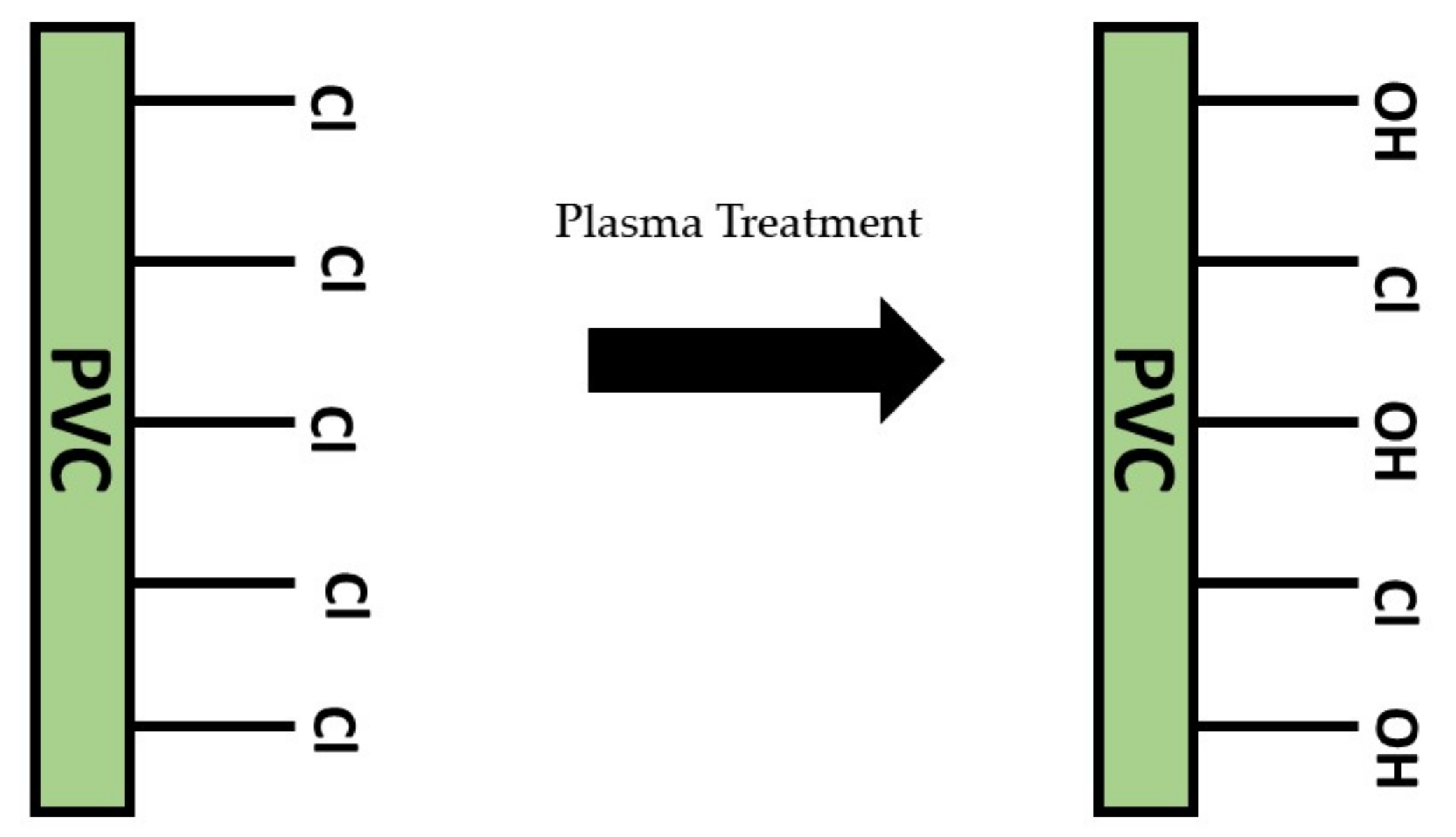
3.3. Surface Chemistry Properties
4. Surface Modification Techniques to Improve Surface Properties
5. Current Antimicrobial Coating to Combat COVID-19
6. Summary and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial nanomaterials and coatings: Current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Akuzov, D.; Franca, L.; Grunwald, I.; Vladkova, T. Sharply reduced biofilm formation from Cobetia marina and in black sea water on modified siloxane coatings. Coatings 2018, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.P.; Song, X.F.; Cui, L.Y.; Qi, Y.H. Synthesis of polydimethylsiloxane-modified polyurethane and the structure and properties of its antifouling coatings. Coatings 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Abad, A.; Ocio, M.J.; Lagarón, J.M.; Sánchez, G. Evaluation of silver-infused polylactide films for inactivation of Salmonella and feline calicivirus in vitro and on fresh-cut vegetables. Int. J. Food Microbiol. 2013, 162, 89–94. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT Food Sci. Technol. 2017, 76, 172–180. [Google Scholar] [CrossRef]
- Candir, E.; Ozdemir, A.E.; Aksoy, M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018, 235, 235–243. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Daud, N.M.; Bahri, I.F.S.; Malek, N.A.N.N.; Hermawan, H.; Saidin, S. Immobilization of antibacterial chlorhexidine on stainless steel using crosslinking polydopamine film: Towards infection resistant medical devices. Colloids Surf. B Biointerfaces 2016, 145, 130–139. [Google Scholar] [CrossRef]
- Jang, H.; Choi, H.; Jeong, H.; Han, S.; Chung, D.; Lee, H. Thermally Crosslinked Biocompatible Hydrophilic Polyvinylpyrrolidone Coatings on Polypropylene with Enhanced Mechanical and Adhesion Properties. Macromol. Res. 2018, 26, 151–156. [Google Scholar] [CrossRef]
- Druvari, D.; Koromilas, N.D.; Bekiari, V.; Bokias, G.; Kallitsis, J.K. Polymeric antimicrobial coatings based on quaternary ammonium compounds. Coatings 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, Z.; Wang, G.; Lau, J.Y.-N.; Zhang, K.; Li, W. COVID-19 in early 2021: Current status and looking forward. Signal. Transduct. Target. Ther. 2021, 6, 114. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, W. Fast-spreading SARS-CoV-2 variants: Challenges to and new design strategies of COVID-19 vaccines. Signal. Transduct. Target. Ther. 2021, 6, 1–6. [Google Scholar] [CrossRef]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschi, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Cuggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Walecka-Zacharska, E.; Kwiecińska-Piróg, J.; Radtke, L.; Gospodarek-Komkowska, E.; Skowron, K. SARS-CoV-2 in the environment—Non-droplet spreading routes. Sci. Total Environ. 2021, 770, 85–94. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, S.S.; Sanders, D.; Wade, J.; Parkin, I.; Carmalt, C.; Smith, A.; Allan, E. Antimicrobial surfaces: A need for stewardship? PLoS Pathog. 2020, 16, 10. [Google Scholar] [CrossRef]
- Sun, Z.; Ostrikov, K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Akbari, M.K.; Yadav, R.; Al-Tamimi, K.A.; Naebe, M. Fight against COVID-19: The case of antiviral surfaces. APL Mater. 2021, 9, 031112. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Klugman, K.P. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob. Health 2020, 12, e1453–e1454. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Cruz, L.A.; Velazco-Medel, M.A.; Cruz-Gómez, A.; Bucio, E. Antimicrobial Polymers. In Advanced Antimicrobial Materials and Applications; Inamuddin, M.I.A., Prasad, R., Eds.; Springer: Singapore, 2021; pp. 1–42. [Google Scholar]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 12, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Shi, C.; Xi, Y.; Zhao, P.; He, H. Fabrication of cationic polymer surface through plasma polymerization and layer-by-layer assembly. Mater. Manuf. Process. 2020, 35, 221–229. [Google Scholar] [CrossRef]
- Li, Y.; Pi, Q.; You, H.H.; Li, J.Q.; Wang, P.C.; Yang, X.; Wu, Y. A smart multi-functional coating based on anti-pathogen micelles tethered with copper nanoparticles: Via a biosynthesis method using l-vitamin C. RSC Adv. 2018, 8, 18272–18283. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, T.R.; Patil, A.; Raza, B.G.; Reurink, D.; van den Hengel, S.K.; Rutjes, S.A.; de Roda Husman, A.M.; Roesink, H.D.W.; de Vos, W.M. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membr. Sci. 2019, 570–571, 494–503. [Google Scholar] [CrossRef]
- Suganya, A.; Shanmugavelayutham, G.; Rodríguez, C.S. Study on plasma pre-functionalized PVC film grafted with TiO2/PVP to improve blood compatible and antibacterial properties. J. Phys. D Appl. Phys. 2017, 50, 145402. [Google Scholar] [CrossRef]
- Haldar, J.; Chen, J.; Tumpey, T.M.; Gubareva, L.V.; Klibanov, M.A. Hydrophobic polycationic coatings inactivate wild-type and zanamivir- and/or oseltamivir-resistant human and avian influenza viruses. Biotechnol. Lett. 2008, 30, 475–479. [Google Scholar] [CrossRef]
- Cerkez, I.; Kocer, H.B.; Worley, D.S.; Broughton, R.M.; Huang, T.S. N-halamine copolymers for biocidal coatings. React. Funct. Polym. 2012, 72, 673–679. [Google Scholar] [CrossRef]
- Ahi, Z.B.; Renkler, N.Z.; Seker, M.G.; Tuzlakoglu, K. Biodegradable Polymer Films with a Natural Antibacterial Extract as Novel Periodontal Barrier Membranes. Int. J. Biomater. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.M.; Hsu, B.B.; Rautaray, D.; Haldar, J.; Chen, J.; Klibanov, A.M. Hydrophobic polycationic coatings disinfect poliovirus and rotavirus solutions. Biotechnol. Bioeng. 2011, 108, 720–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldar, J.; An, D.; de Cienfuegos, L.A.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, E.; Azzopardi, K.; Taing, H.; Graichen, F.; Jeffery, J.; Mayadunne, R.; Wickramaratna, M.; O’Shea, M.; Nijagal, B.; Watkinson, R.; et al. Efficacy of antimicrobial polymer coatings in an animal model of bacterial infection associated with foreign body implants. J. Antimicrob. Chemother. 2010, 65, 974–980. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Heo, J.; Lee, S.E.; Shin, J.W.; Chang, M.; Hong, J. Polysaccharide-based superhydrophilic coatings with antibacterial and anti-inflammatory agent-delivering capabilities for ophthalmic applications. J. Ind. Eng. Chem. 2018, 68, 229–237. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Antimicrobial Activity of Chitosan-Based Films Enriched with Green Tea Extracts on Murine Norovirus, Escherichia coli, and Listeria innocua. Int. J. Food Sci. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaund, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef] [Green Version]
- El-Fawal, G. Preparation, characterization and antibacterial activity of biodegradable films prepared from carrageenan. J. Food Sci. Technol. 2014, 51, 2234–2239. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Ding, D.; Shao, H.; Peng, Q.; Huang, Y. Antibacterial Activity and Physical Properties of Fish Gelatin-Chitosan Edible Films Supplemented with D-Limonene. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.L.; Namivandi-Zangeneh, R.; Drozdek, S.; Wilk, K.; Boyer, C.; Wong, E.H.H.; Bradshaw-Hajek, B.H.; Krasowska, M.; Beattie, D.A. Incorporation and antimicrobial activity of nisin Z within carrageenan/chitosan multilayers. Sci. Rep. 2021, 11, 1690. [Google Scholar] [CrossRef]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A.G. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63 Pt 9, 1167–1173. [Google Scholar] [CrossRef]
- Clark, S.R.; Lee, K.Y.; Lee, H.; Khetan, J.; Kim, H.C.; Choi, Y.H.; Shin, K.; Won, Y.Y. Determining the effects of PEI adsorption on the permeability of 1,2-dipalmitoylphosphatidylcholine/bis(monoacylglycero)phosphate membranes under osmotic stress. Acta Biomater. 2018, 65, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kwolek, U.; Jamróz, D.; Janiczek, M.; Nowakowska, M.W.; Kepczynski, M. Interactions of Polyethylenimines with Zwitterionic and Anionic Lipid Membranes. Langmuir 2016, 32, 5004–5018. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Vlierberghe, S.V.; Kaplan, D.L.; Chiellini, E.; Blitterswijk, C.V.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Česoniene, L.; Daubaras, R.; Leskauskaite, D.; Zabulione, D. Chitosan coating of red kiwifruit (Actinidia melanandra) for extending of the shelf life. Int. J. Biol. Macromol. 2016, 85, 355–360. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Development of antiviral and bacteriostatic chitosan-based food packaging material with grape seed extract for murine norovirus, Escherichia coli and Listeria innocua control. Food Sci. Nutr. 2020, 8, 6174–6181. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
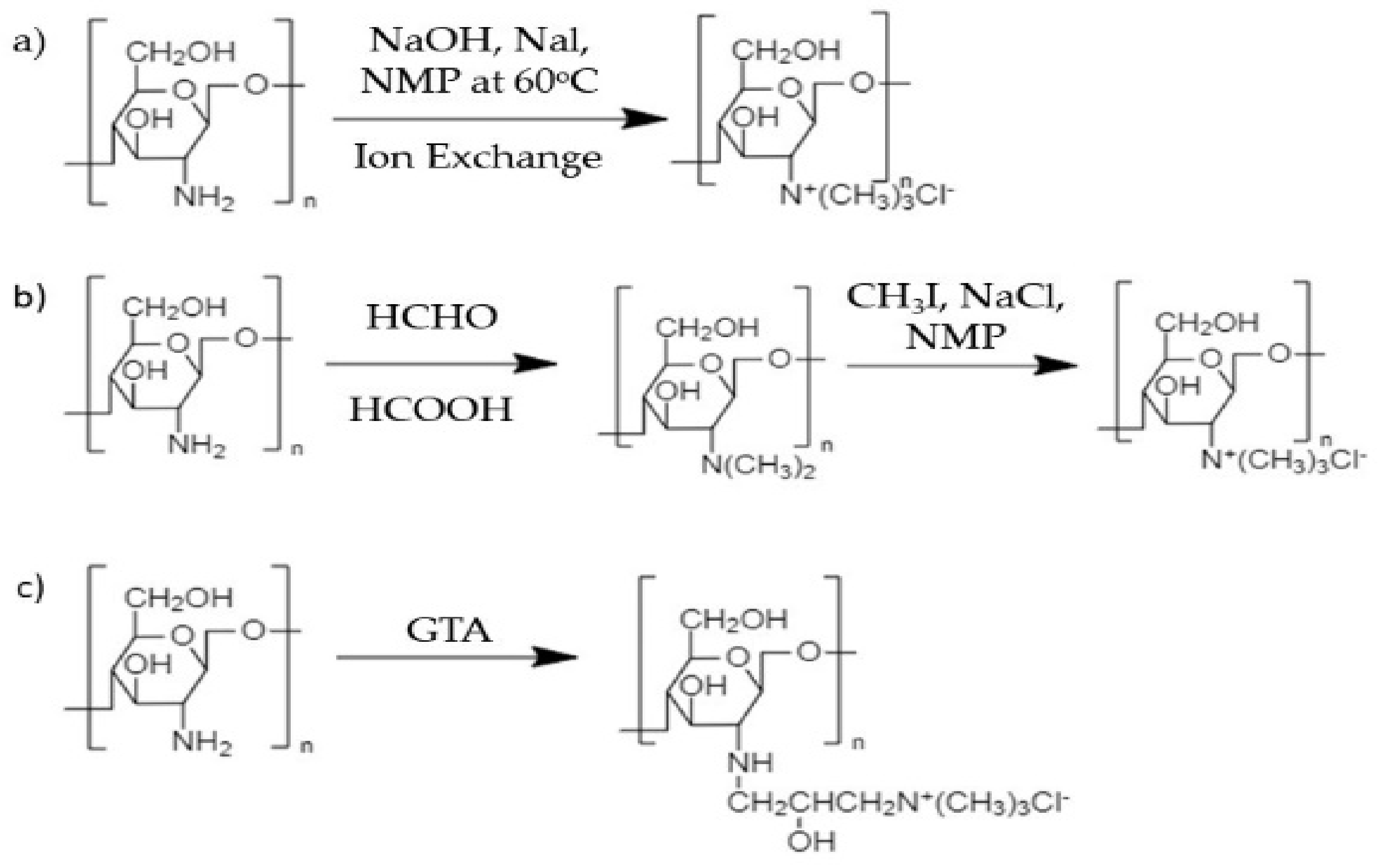
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N, N, N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Wang, G.; Yin, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of N, N, N-trimethyl chitosan salts. Int. J. Biol. Macromol. 2018, 118, 9–14. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.; Assis, O.B. Assis. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. Coli and S. aureus growth. Rev. Bras. Farm. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N, N, N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr. Polym. 2010, 81, 931–936. [Google Scholar] [CrossRef]
- Mushtaq, S.; Ahmad, N.M.; Mahmood, A.; Iqbal, M. Antibacterial amphiphilic copolymers of dimethylamino ethyl methacrylate and methyl methacrylate to control biofilm adhesion for antifouling applications. Polymers 2021, 13, 216. [Google Scholar] [CrossRef]
- Peng, C.; Vishwakarma, A.; Mankoci, S.; Barton, A.H.; Joy, A. Structure-Activity Study of Antibacterial Poly(ester urethane)s with Uniform Distribution of Hydrophobic and Cationic Groups. Biomacromolecules 2019, 20, 1675–1682. [Google Scholar] [CrossRef]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; López-Fabal, F.; Fernández-García, M. Hemolytic and antimicrobial activities of a series of cationic amphiphilic copolymers comprised of same centered comonomers with thiazole moieties and polyethylene glycol derivatives. Polymers 2020, 12, 972. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Hou, Z.; Kang, E.T.; Chan-Park, M.B. Increasing bacterial affinity and cytocompatibility with four-arm star glycopolymers and antimicrobial α-polylysine. Polym. Chem. 2017, 8, 3364–3373. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Barman, S.; Mukherjee, R.; Haldar, J. Amphiphilic Cationic Macromolecules Highly Effective Against Multi-Drug Resistant Gram-Positive Bacteria and Fungi with No Detectable Resistance. Front. Bioeng. Biotechnol. 2020, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z.; Hou, Y.; Wu, C.; Fan, Y.; Liu, L.; et al. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Gîfu, I.C.; Maxim, M.E.; Cinteza, L.O.; Poap, M.; Aricove, L.; Leonties, A.R.; Anastasescu, M.; Anghel, D.F.; Ianchis, R.; Ninciuleanu, C.M.; et al. Antimicrobial activities of hydrophobically modified poly(acrylate) films and their complexes with different chain length cationic surfactants. Coatings 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K.S.M. Biosurfactants: A Covid-19 Perspective. Front. Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- Hsieh, I.N.; de Luna, X.; White, M.R.; Hartshorn, K.L. The role and molecular mechanism of action of surfactant protein D in innate host defense against influenza A virus. Front. Immunol. 2018, 9, 1368. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhou, C.; Zhang, Z.; Roland, J.D.; Lee, B.P. Antimicrobial property of halogenated catechols. Chem. Eng. J. 2020, 403, 126340. [Google Scholar] [CrossRef]
- Loh, B.; Vozzolo, L.; Mok, B.J.; Lee, C.C.; Fitzmaurice, R.J.; Caddick, S.; Fassati, A. Inhibition of hiv-1 replication by isoxazolidine and isoxazole sulfonamides. Chem. Biol. Drug Des. 2010, 75, 461–474. [Google Scholar] [CrossRef]
- Stefanska, J.; Nowicka, G.; Struga, M.; Szulczyk, D.; Koziol, A.E.; Augustynowicz-Kopec, E.; Napiorkowska, A.; Bielenica, A.; Filipowski, W.; Filipowska, A.; et al. Antimicrobial and anti-biofilm activity of thiourea derivatives incorporating a 2-aminothiazole scaffold. Chem. Pharm. Bull. 2015, 63, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, J.; Huang, Y.; Wang, R.; Zhang, L.; Qiao, K.; Li, L.; Liu, C.; Ouyang, Y.; Xu, W.; et al. Design, Synthesis, and Biological Evaluation of 1-[(2-Benzyloxyl/alkoxyl)methyl]-5-halo-6-aryluracils as Potent HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors with an Improved Drug Resistance Profile. J. Med. Chem. 2012, 55, 2242–2250. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-halamine polymers and coatings: A review of their synthesis, characterization, and applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Polymeric antimicrobial N-halamine epoxides. ACS Appl. Mater. Interfaces 2011, 3, 2845–2850. [Google Scholar] [CrossRef]
- Demir, B.; Broughton, R.M.; Qiao, M.; Huang, T.S.; Worley, S.D. N-halamine biocidal materials with superior antimicrobial efficacies for wound dressings. Molecules 2017, 22, 1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef]
- Warnes, S.L.; Summersgill, E.N.; Keevil, C.W. Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity. Appl. Environ. Microbiol. 2015, 81, 1085–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human coronavirus 229E remains infectious on common touch surface material. smBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [Green Version]
- Soni, D.; Bafana, A.; Gandhi, D.; Sivanesan, S.; Pandey, A.R. Stress response of Pseudomonas species to silver nanoparticles at the molecular level. Environ. Toxicol. Chem. 2014, 33, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Samanovic, M.I.; Ding, C.; Thiele, D.J.; Darwin, K.H. Copper in microbial pathogenesis: Meddling with the metal. Cell Host Microbe 2012, 11, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.P.; Cornejo, J.A.; Sumner, J.J.; Schuler, A.J.; Atanassov, P.; Ista, K.L. Conjugated gold nanoparticles as a tool for probing the bacterial cell envelope: The case of Shewanella oneidensis MR-1. Biointerphases 2016, 11, 011003. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xie, G.; Zhang, W.; Liang, M.; Liu, B.; Xiang, H. Label-free sandwich type of immunosensor for hepatitis C virus core antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchim. Acta 2012, 178, 331–340. [Google Scholar] [CrossRef]
- Antoine, T.E.; Hadigal, S.R.; Yakoub, A.M.; Mishra, Y.K.; Bhattacharya, P.; Haddad, C.; Valyi-Nagy, T.; Adelung, R.; Prabhakar, B.S.; Shukla, D. Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital. J. Immunol. 2016, 196, 4566–4575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roner, M.R.; Carraher, C.E.; Shahi, K.; Barot, G. Antiviral activity of metal-containing polymers-organotin and cisplatin-like polymers. Materials 2011, 4, 991–1012. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C 2018, 89, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Ao, Z.; Girilal, M.; Chen, L.; Ziao, X.; Kalaichelvan, P.T.; Yao, X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: A new approach to inhibit HIV- and HSV-transmitted infection. Int. J. Nanomed. 2012, 7, 5007–5018. [Google Scholar]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, M.S. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monmaturapoj, N.; Sri-on, A.; Klinsukhon, W.; Boonnak, K.; Prahsarn, C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater. Sci. Eng. C 2018, 92, 96–102. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Ko, Y.S.; Lee, S.J.; Lee, C.; Woo, K.; Ko, G.P. Inactivation of influenza A virus via exposure to silver nanoparticle-decorated silica hybrid composites. Environ. Sci. Pollut. 2018, 25, 27021–27030. [Google Scholar] [CrossRef]
- Hodek, J.; Zajícová, V.; Lovětinská-Šlamborová, I.; Stibor, I.; Müllerová, J.; Weber, J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-González, N.; Goncalves, L.V.; Condezo, G.N.; Martin, C.S.; Rubiano, M.; Fallis, I.; Rubino, J.R.; Ijaz, M.K.; Maillard, J.Y.; De Pablo, P.J. Virucidal Action Mechanism of Alcohol and Divalent Cations Against Human Adenovirus. Front. Mol. Biosci. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Joshy, K.S.; Susan, M.A.; Snigdha, S.; Nandakumar, K.; Laly, A.P.; Sabu, T. Encapsulation of zidovudine in PF-68 coated alginate conjugate nanoparticles for anti-HIV drug delivery. Int. J. Biol. Macromol. 2018, 107, 929–937. [Google Scholar] [CrossRef]
- Belgamwar, A.; Khan, S.; Yeole, P. Intranasal chitosan-g-HPβCD nanoparticles of efavirenz for the CNS targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 374–386. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, M.E.; Del Carlo, M.; Hussein, H.A.; Salah, T.A.; El-Deeb, A.H.; Emara, M.M.; Pezzoni, G.; Compagnone, D. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J. Nanobiotechnol. 2018, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, M.; Lima, D.; Viana, A.G.; Fujiwara, S.T.; Pessôa, C.A.; Etto, R.M.; Wohnrath, K. A sensitive label-free impedimetric DNA biosensor based on silsesquioxane-functionalized gold nanoparticles for Zika Virus detection. Biosens. Bioelectron. 2019, 141, 111351. [Google Scholar] [CrossRef]
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2020, 199, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 2015, 23, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botelho, M.C.; Fernandes, M.M.; Souza, J.M.; Dias, N.; Sousa, A.M.; Teixeira, J.A.; Fangueiro, R.; Zille, A. New textile for personal protective equipment—plasma chitosan/silver nanoparticles nylon fabric. Fibers 2021, 9, 3. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Ruslan, N.S.; Mohtar, N.; Rahiman, S.S.F.; Gazzali, A.M. The Influence of Preparation Factors on Physical Characteristics of Chitosan Nanoparticles. J. Phys. Sci. 2020, 31, 47–60. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Guan, Z.; Shu, Y.; Ma, Y.; Wan, J. Factors affecting the physicochemical properties of the modified core/shell NH2-SiO2@NZVI nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 18–26. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Umai, D.; Vikranth, A.; Meenambiga, S.S. A study on the green synthesis of silver nanoparticles from Olea europaea and its activity against oral pathogens. Mater. Today Proc. 2020, 44, 3647–3651. [Google Scholar] [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Muhsinah, A.B.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of lampranthus coccineus and malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [Green Version]
- Elbeshehy, E.K.F.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacilluss, nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [Green Version]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qamar, H.; Rehman, S.; Chauhan, D.K.; Tiwari, A.K.; Upmanyu, V. Green synthesis, characterization and antimicrobial activity of copper oxide nanomaterial derived from Momordica charantia. Int. J. Nanomed. 2020, 15, 2541–2553. [Google Scholar] [CrossRef] [Green Version]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Green synthesis and characterization of Manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity. J. Appl. Pharm. Sci. 2015, 5, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Kumar, R.; Dalal, U.; Tomar, S.; Reddy, S.N. Green Synth. nanometal impregnated biomass—Antiviral potential. Mater. Sci. Eng. C 2020, 112, 110934. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Jalal, M.; Ali, S.G.; Pal, R.; Musarrat, J. Green synthesis of Al2O3 nanoparticles and their bactericidal potential against clinical isolates of multi-drug resistant Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2015, 31, 153–164. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Huang, K.Y.; Chao, T.L.; Kao, H.C.; Pang, Y.H.; Lu, L.; Chiu, C.L.; Huang, H.C.; Cheng, T.J.R.; Fang, J.M.; et al. Nanoparticle composite TPNT1 is effective against SARS-CoV-2 and influenza viruses. Sci. Rep. 2021, 11, 1–13. [Google Scholar]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Hejazi, J.; Khaksar, R. Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mater. Sci. Eng. C 2016, 58, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Priya, A.; Arun, A.; Hait, S.; Chowdhury, A. Antibacterial and natural room-light driven photocatalytic activities of CuO nanorods. Mater. Chem. Phys. 2019, 226, 106–112. [Google Scholar] [CrossRef]
- Huy, T.Q.; Thanh, N.T.H.; Thuy, N.T.; Chung, P.V.; Hung, P.N.; Le, A.T.; Hanh, N.T. HCytotoxicity and antiviral activity of electrochemical—Synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Silva, T.; Pokhrel, L.R.; Dubey, B.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model-predicted and observed toxicity. Sci. Total Environ. 2014, 468–469, 968–976. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, H.W. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size—And shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.H.; Hong, J.; McGuffie, M.; Yeom, B.; Vanepps, J.S.; Kotov, A.N. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano 2015, 9, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Objects 2020, 24, 100620. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Dong, X.; Moyer, M.M.; Yang, F.; Sun, Y.P.; Yang, L. Carbon Dots’ Antiviral Functions Against Noroviruses. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Martinez, Z.S.; Castro, E.; Seong, C.S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.C.; Chang-Jian, C.W.; Chen, H.C.; Chuang, K.S.; Zheng, J.H.; Hsiao, Y.S.; Lee, K.C.; Huang, J.H. Robust multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion, and anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Chen, Z.; Chen, G.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. Robust translucent superhydrophobic PDMS/PMMA facile one-step spray for self-cleaning and efficient emulsion separation. Chem. Eng. J. 2017, 330, 26–35. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Rosa, V.; Ho, D.; Sabino-Silva, R.; Siqueira, W.L.; Silikas, N. Fighting viruses with materials science: Prospects for antivirus surfaces, drug delivery systems and artificial intelligence. Dent. Mater. 2021, 37, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, A.R.H. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Gates, I. Apparent Contact Angle around the Periphery of a Liquid Drop on Roughened Surfaces. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Almasi, L.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of antimicrobial biopolymer films containing essential oil-loaded microemulsions or nanoemulsions. Food Hydrocoll. 2021, 117, 106733. [Google Scholar] [CrossRef]
- Hosseini, M.; Chin, A.W.H.; Behzadinasab, S.; Poon, L.L.M.; Ducker, W.A. Cupric Oxide Coating That Rapidly Reduces Infection by SARS-CoV-2 via Solids. ACS Appl. Mater. Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards laser-textured antibacterial surfaces. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Siddiquie, R.Y.; Gaddam, A.; Agrawal, A.; Dimov, S.S.; Joshi, S.S. Anti-Biofouling Properties of Femtosecond Laser-Induced Submicron Topographies on Elastomeric Surfaces. Langmuir 2020, 36, 5349–5358. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, A.W. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Sarimai; Ratnawulan; Yulkifli; Fauzi, A. Fabrication of superhydrophobic CuO/polystyrene nanocomposite coating with variation concentration. J. Phys. Conf. Ser. 2019, 1185, 012014. [Google Scholar]
- Zhong, H.; Zhu, Z.; You, P.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.Y.; Li, G. Plasmonic and Superhydrophobic Self-Decontaminating N95 Respirators. ACS Nano 2020, 14, 8846–8854. [Google Scholar] [CrossRef] [PubMed]
- Milionis, A.; Tripathy, A.; Donati, M.; Sharma, C.S.; Pan, F.; Maniura-Weber, K.; Ren, Q.; Poulikakos, D. Water-Based Scalable Methods for Self-Cleaning Antibacterial ZnO-Nanostructured Surfaces. Ind. Eng. Chem. Res. 2020, 59, 14323–14333. [Google Scholar] [CrossRef]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant. Dent. 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Rungraeng, N.; Song, W.; Jun, S. Superhydrophobic and superhydrophilic nanocomposite coatings for preventing Escherichia coli K-12 adhesion on food contact surface. J. Food Eng. 2014, 131, 135–141. [Google Scholar] [CrossRef]
- Chang, C.H.; Tien, C.C.; Hsueh, H.C.; Sung, L. A macroscopically nondestructive method for characterizing surface mechanical properties of polymeric coatings under accelerated weathering. J. Coat. Technol. Res. 2018, 15, 913–922. [Google Scholar] [CrossRef]
- Wan, H.; Song, D.; Li, X.; Zhang, D.; Gao, J.; Du, C. Failure mechanisms of the coating/metal interface in waterborne coatings: The effect of bonding. Materials 2017, 10, 397. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, M.; Saleh, M.N.; Poulis, J.A. Improving the adhesion strength of polymers: Effect of surface treatments. J. Adhes. Sci. Technol. 2020, 34, 1853–1870. [Google Scholar] [CrossRef]
- Rocha, R.C.; Galdino, A.G.D.; da Silva, S.N.; Machado, M.L.P. Surface, microstructural, and adhesion strength investigations of a bioactive hydroxyapatite-titanium oxide ceramic coating applied to Ti-6Al-4V alloys by plasma thermal spraying. Mater. Res. 2018, 21, 11–14. [Google Scholar] [CrossRef]
- Grubova, I.; Priamushko, T.; Surmeneva, M.; Korneva, O.; Epple, M.; Prymak, O.; Surmenev, R. Sand-blasting treatment as a way to improve the adhesion strength of hydroxyapatite coating on titanium implant. J. Phys. Conf. Ser. 2017, 830, 012109. [Google Scholar] [CrossRef] [Green Version]
- Reggente, M.; Masson, P.; Dollinger, C.; Palkowsk, H.; Zafeiratos, S.; Jacomine, L.; Passeri, D.; Rossi, M.; Engin Vrana, N.; Pourroy, G.; et al. Novel Alkali Activation of Titanium Substrates to Grow Thick and Covalently Bound PMMA Layers. ACS Appl. Mater. Interfaces 2018, 10, 5967–5977. [Google Scholar] [CrossRef]
- Kim, C.; Kendall, M.R.; Miller, M.A.; Long, C.L.; Larson, P.R.; Humphrey, M.B.; Madden, A.S.; Tas, A.C. Comparison of titanium soaked in 5 M NaOH or 5 M KOH solutions. Mater. Sci. Eng. C 2013, 33, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanib, N.H.; Hamzah, F.; Omar, Z.; Subuki, I. Surface Characterization on Alkali-Heat-Treatment on Titanium Alloy. Malays. J. Anal. Sci. 2016, 20, 1429–1436. [Google Scholar]
- Podgorski, L.; de Meijer, M.; Lanvin, D.J. Influence of coating formulation on its mechanical properties and cracking resistance. Coatings 2017, 7, 163. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Sebastiani, M.; Mughal, M.Z.; Daniel, R.; Bemporad, E. Influence of the silver content on mechanical properties of Ti-Cu-Ag thin films. Nanomaterials 2021, 11, 435. [Google Scholar] [CrossRef]
- Madian, N.G.; Mohamed, N. Enhancement of the dynamic mechanical properties of chitosan thin films by crosslinking with greenly synthesized silver nanoparticles. J. Mater. Res. Technol. 2020, 9, 12970–12975. [Google Scholar] [CrossRef]
- Nie, Y.; Ma, S.; Tian, M.; Zhang, Q.; Huang, J.; Cao, M.; Li, Y.; Sun, L.; Pan, J.; Wang, Y.; et al. Superhydrophobic silane-based surface coatings on metal surface with nanoparticles hybridization to enhance anticorrosion efficiency, wearing resistance and antimicrobial ability. Surf. Coat. Technol. 2021, 410, 126966. [Google Scholar] [CrossRef]
- Kolewe, K.W.; Peyton, S.R.; Schiffman, J.D. Fewer Bacteria Adhere to Softer Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 19562–19569. [Google Scholar] [CrossRef]
- Kolewe, K.W.; Zhu, J.; Mako, N.R.; Nonnenmann, S.S.; Schiffman, J.D. Bacterial Adhesion Is Affected by the Thickness and Stiffness of Poly(ethylene glycol) Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 2275–2281. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Ren, D. Stiffness of cross-linked poly(dimethylsiloxane) affects bacterial adhesion and antibiotic susceptibility of attached cells. Langmuir 2014, 30, 10354–10362. [Google Scholar] [CrossRef]
- Song, F.; Wang, H.; Sauer, K.; Ren, D. Cyclic-di-GMP and oprF are involved in the response of Pseudomonas aeruginosa to substrate material stiffness during attachment on polydimethylsiloxane (PDMS). Front. Microbiol. 2018, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro-and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.; Stewart, D.S.; Nikolov, A.D.; Wasan, D.T.; Wang, R.; Yan, R.; Shieh, Y.C. Differential MS2 interaction with food contact surfaces determined by atomic force microscopy and virus recovery. Appl. Environ. Microbiol. 2017, 83, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.T.T.; Tarabara, V.V. Virus deposition onto polyelectrolyte-coated surfaces: A study with bacteriophage MS2. J. Colloid Interface Sci. 2019, 540, 155–166. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Chen, B.; Edirisinghe, M. Surface interactions and viability of coronaviruses: Surface interactions and viability of coronaviruses. J. R. Soc. Interface 2021, 18, 174. [Google Scholar] [CrossRef]
- Donskyi, I.S.; Azab, W.; Cuellar-Camacho, J.L.; Guday, G.; Lippitz, A.; Unger, W.E.S.; Osterrieder, K.; Adeli, M.; Haag, R. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale 2019, 11, 15804–15809. [Google Scholar] [CrossRef] [PubMed]
- Michen, B.; Graule, T. Isoelectric points of viruses. J. Appl. Microbiol. 2010, 109, 388–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joonaki, E.; Hassanpouryouzband, A.; Heldt, C.L.; Areo, O. Surface Chemistry Can Unlock Drivers of Surface Stability of SARS-CoV-2 in a Variety of Environmental Conditions. Chem 2010, 6, 2135–2146. [Google Scholar] [CrossRef]
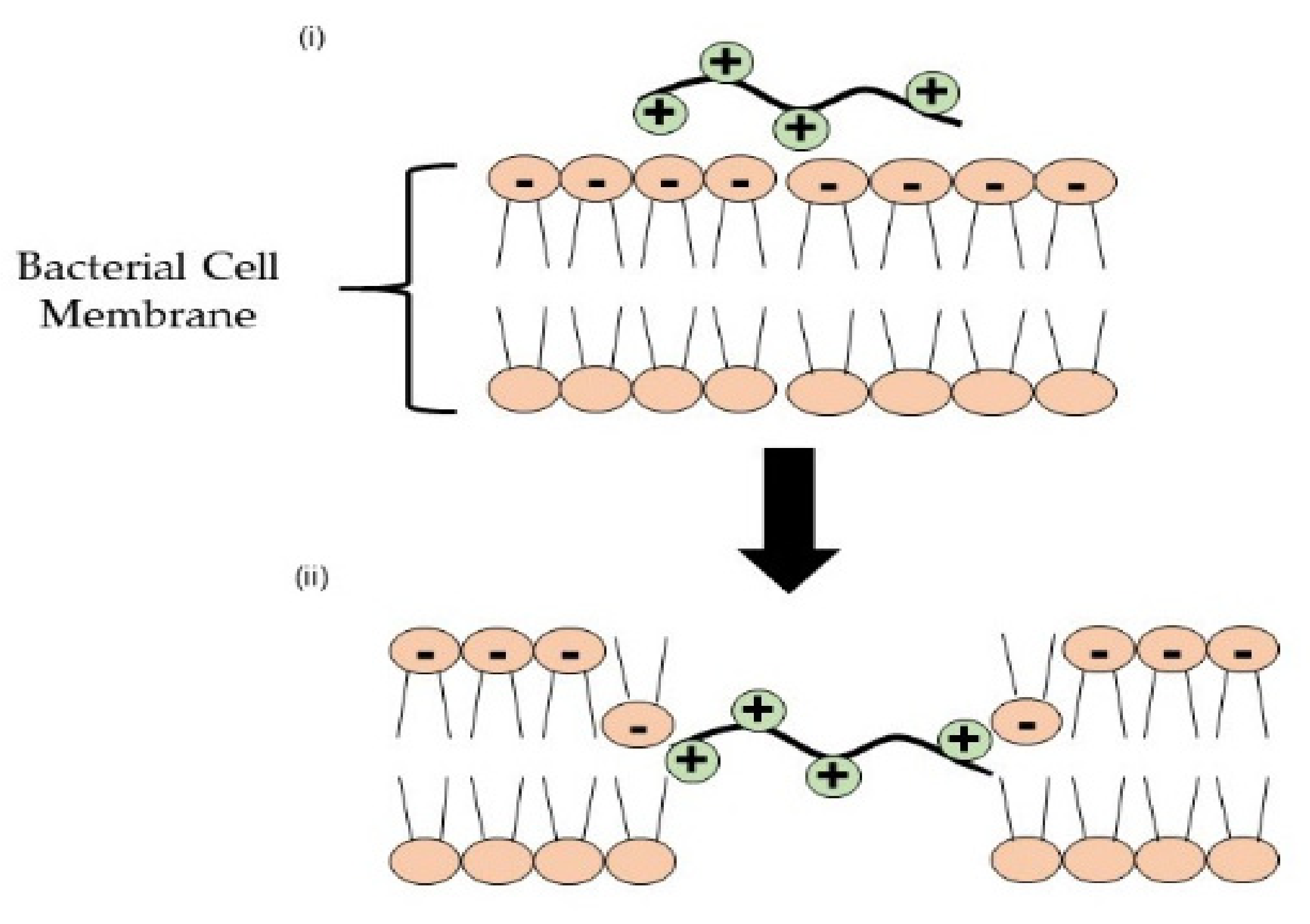
- Alfei, S.; Schito, A.M. Positively charged polymers as promising devices against multidrug resistant gram-negative bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Nap, R.J.; Božič, A.L.; Szleifer, I.; Podgornik, R. The role of solution conditions in the bacteriophage pp7 capsid charge regulation. Biophys. J. 2014, 107, 1970–1979. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, R.T.; Robles, D.; Raza, B.; van den Hengel, S.; Rutjes, S.A.; de Roda Husman, A.M.; de Grooth, J.; de Vos, W.M.; Roesink, H.D.W. Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 33–41. [Google Scholar] [CrossRef]
- Poverenov, E.; Shemesh, M.; Gulino, A.; Cristaldi, D.A.; Zakin, V.; Yefremov, T.; Granit, R. Durable contact active antimicrobial materials formed by a one-step covalent modification of polyvinyl alcohol, cellulose and glass surfaces. Colloids Surf. B Biointerfaces 2013, 112, 356–361. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Science 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Petterson, T.; Illergård, J.; Ek, M.; Wågberg, L. Influence of Cellulose Charge on Bacteria Adhesion and Viability to PVAm/CNF/PVAm-Modified Cellulose Model Surfaces. Biomacromolecules 2019, 20, 2075–2083. [Google Scholar] [CrossRef]
- Guo, S.; Kwek, M.Y.; Toh, Z.Q.; Pranantyo, D.; Kang, E.T.; Loh, X.J.; Zhu, X.; Jańczewski, D.; Neoh, K.G. Tailoring Polyelectrolyte Architecture to Promote Cell Growth and Inhibit Bacterial Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 7882–7891. [Google Scholar] [CrossRef]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Heinonen, S.; Nikkanen, J.P.; Laakso, J.; Raulio, M.; Priha, O.; Levänen, E. Bacterial growth on a superhydrophobic surface containing silver nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012064. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yasukawa, A.; Yonezawa, S. Enhanced dispersion stability and fluidity of rutile TiO2 particles using surface fluorination. Mater. Today Proc. 2018, 20, 311–319. [Google Scholar] [CrossRef]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymeric Scaffolds for Tissue Engineering Applications. Regen. Eng. Transl. Med. 2018, 4, 75–91. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofova, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical Applications of Plasma Treatments: Current State and Perspectives. Appl. Microbiol. Biotechno. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Xue, Z. Effect of microwave irradiation on the physical properties and structures of cotton fabric. Eng. Fibers Fabr. 2018, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, H.; Sivakumar, G.; Kasi, P.; Jaganathan, S.K.; Supriyanto, E. Microwave-assisted surface modification of metallocene polyethylene for improving blood compatibility. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Riveiro, A.; Maçon, A.L.B.; del Val, J.; Comesaña, R.; Pou, J. Laser surface texturing of polymers for biomedical applications. Front. Phys. 2018, 6, 16. [Google Scholar] [CrossRef]
- Coathup, M.J.; Blunn, G.W.; Mirhosseini, N.; Erskine, K.; Liu, Z.; Garrord, D.R.; Li, L. Controlled Laser Texturing of Titanium Results in Reliable Osteointegration. J. Orthop. Res. 2017, 35, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, X.; Maclean, M.; Qin, Y.; Duxbury, M.; Ding, F. A single-step fabrication approach for development of antimicrobial surfaces. J. Mater. Process. Technol. 2019, 271, 249–260. [Google Scholar] [CrossRef]
- Shin, J.; Liu, X.; Chikthimmah, N.; Lee, Y.S. Polymer surface modification using UV treatment for attachment of natamycin and the potential applications for conventional food cling wrap (LDPE). Appl. Surf. Sci. 2016, 386, 276–284. [Google Scholar] [CrossRef]
- Yasuda, K.; Okazaki, Y.; Abe, Y.; Tsuga, K. Effective UV/Ozone irradiation method for decontamination of hydroxyapatite surfaces. Heliyon 2017, 3, e00372. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Matthews, D.T.A.; Igartua, A.; Rodriguez-Vidal, E.; Fortes, J.C.; de Viteri, V.S.; Pagano, F.; Wadman, B.; Wiklund, E.D.; et al. Selection of micro-fabrication techniques on stainless steel sheet for skin friction. Friction 2016, 4, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Rudawska, A.; Danczak, I.; Müller, M.; Valasek, P. The effect of sandblasting on surface properties for adhesion. Int. J. Adhes. Adhes. 2016, 70, 176–190. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, M.; Park, S.; Kim, Y.; Lee, E.; Im, S.G. A Versatile Surface Modification Method via Vapor-phase Deposited Functional Polymer Films for Biomedical Device Applications. Biotechnol. Bioprocess. Eng. 2021, 26, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Small, M.; Faglie, A.; Craig, A.J.; Pieper, M.; Narcisse, V.E.F.; Neuenschwander, P.F.; Chou, S.F. Nanostructure-enabled and macromolecule-grafted surfaces for biomedical applications. Micromachines 2018, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zopper, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.J.; Myung, S.W.; Lee, C.S.; Choi, H.S. Comparison of the surface characteristics of polypropylene films treated by Ar and mixed gas (Ar/O2) atmospheric pressure plasma. J. Colloid Interface Sci. 2006, 295, 409–416. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Morais, D.S.; Ávila, B.; Lopes, C.; Rodrigues, M.A.; Vaz, F.; Machado, A.V.; Fernandes, M.H.; Guedes, R.M.; Lopes, M.A. Surface functionalization of polypropylene (PP) by chitosan immobilization to enhance human fibroblasts viability. Polym. Test. 2020, 86, 106507. [Google Scholar] [CrossRef]
- Pedrosa, P.; Fiedler, P.; Lopes, C.; Alves, E.; Barradas, N.P.; Haueisen, J.; Machado, A.V.; Fonseca, C.; Vaz, F. Ag:TiN-Coated Polyurethane for Dry Biopotential Electrodes: From Polymer Plasma Interface Activation to the First EEG Measurements. Plasma Process. Polym. 2016, 13, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.T.; Mirzadeh, H. Effect of oxygen plasma treatment on surface charge and wettability of PVC blood bag-In vitro assay. Radiat. Phys. Chem. 2007, 76, 1011–1016. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, P.K.; Ji, J.; Zhang, Y.; Liu, X.; Fu, R.K.Y.; Ha, P.C.T.; Yan, Q. Plasma surface modification of poly vinyl chloride for improvement of antibacterial properties. Biomaterials 2006, 27, 44–51. [Google Scholar] [CrossRef]
- Juárez-Moreno, J.A.; Ávila-Ortega, A.; Oliva, A.I.; Avilés, F.; Cauich-Rodríguez, J.V. Effect of wettability and surface roughness on the adhesion properties of collagen on PDMS films treated by capacitively coupled oxygen plasma. Appl. Surf. Sci. 2015, 349, 763–773. [Google Scholar] [CrossRef]
- Mandolfino, C.; Lertora, E.; Gambaro, C. Effect of Cold Plasma Treatment on Surface Roughness and Bonding Strength of Polymeric Substrates. Key Eng. Mater. 2014, 611–612, 1484–1493. [Google Scholar] [CrossRef]
- Keshel, S.H.; Azhdadi, S.N.K.; Asefnejad, A.; Sadraeian, M.; Montazeri, M.; Biazar, E. The relationship between cellular adhesion and surface roughness for polyurethane modified by microwave plasma radiation. Int. J. Nanomed. 2011, 6, 641–647. [Google Scholar]
- Czylkowski, D.; Hrycak, B.; Sikora, A.; Moczała-Dusanowska, M.; Dors, M.; Jasiński, M. Surface modification of polycarbonate by an atmospheric pressure argon microwave plasma sheet. Materials 2019, 12, 2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabedini, S.M.; Rahimi, H.; Hamedifar, S.; Mohseni, S.M. Microwave irradiation of polypropylene surface: A study on wettability and adhesion. Int. J. Adhes. Adhes. 2004, 24, 163–170. [Google Scholar] [CrossRef]
- Sikora, A.; Czylkowski, D.; Hrycak, B.; Moczala-Dusanowska, M.; Łapiński, M.; Dors, M.; Jasiński, M. Surface modification of PMMA polymer and its composites with PC61BM fullerene derivative using an atmospheric pressure microwave argon plasma sheet. Sci. Rep. 2021, 11, 1–18. [Google Scholar]
- Rukosuyev, M.V.; Lee, J.; Cho, S.J.; Lim, G.; Jun, M.B. One-step fabrication of superhydrophobic hierarchical structures by femtosecond laser ablation. Appl. Surf. Sci. 2014, 313, 411–417. [Google Scholar] [CrossRef]
- Chen, C.; Enrico, A.; Pettersson, T.; Ek, M.; Herland, A.; Niklaus, F.; Stemme, G.; Wågberg, L. Bactericidal surfaces prepared by femtosecond laser patterning and layer-by-layer polyelectrolyte coating. J. Colloid Interface Sci. 2020, 575, 286–297. [Google Scholar] [CrossRef]
- Oyane, A.; Nakamura, M.; Sakamaki, I.; Shimizu, Y.; Miyata, S.; Miyaji, H. Laser-assisted wet coating of calcium phosphate for surface-functionalization of PEEK. PLoS ONE 2018, 13, e0206524. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhang, S.; Liu, W.; Zhu, X.; Chen, X.; Chen, X. Improving the flame retardancy of PET fabric by photo-induced grafting. Polym. Degrad. Stab. 2010, 95, 1934–1942. [Google Scholar] [CrossRef]
- Özçam, A.E.; Efimenko, K.; Genzer, J. Effect of ultraviolet/ozone treatment on the surface and bulk properties of poly(dimethyl siloxane) and poly(vinylmethyl siloxane) networks. Polymer 2014, 55, 3107–3119. [Google Scholar] [CrossRef]
- Yao, Y.T.; Liu, S.; Swain, M.V.; Zhang, X.P.; Zhao, K.; Jian, Y.T. Effects of acid-alkali treatment on bioactivity and osteoinduction of porous titanium: An in vitro study. Mater. Sci. Eng. C 2019, 94, 200–210. [Google Scholar] [CrossRef]
- Tham, C.Y.; Hamid, Z.A.A.; Ahmad, Z.; Ismail, H. Surface modification of poly (lactic acid) (PLA) via alkaline hydrolysis degradation. Adv. Mater. Res. 2014, 970, 324–327. [Google Scholar] [CrossRef]
- Su, N.; Yue, L.; Liao, Y.; Liu, W.; Zhang, H.; Li, X.; Shen, J. The effect of various sandblasting conditions on surface changes of dental zirconia and shear bond strength between zirconia core and indirect composite resin. J. Adv. Prosthodont. 2015, 7, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, M.; Niinomi, M.; Tsutsumi, H.; Saito, K.; Goto, T. Calcium phosphate coating of biomedical titanium alloys using metal—Organic chemical vapour deposition Calcium phosphate coating of biomedical titanium alloys using metal—Organic chemical vapour deposition. Mater. Technol. 2015, 7857, B8–B12. [Google Scholar] [CrossRef]
- Martin, T.P.; Kooi, S.E.; Chang, S.H.; Sedransk, K.L.; Gleason, K. Initiated chemical vapor deposition of antimicrobial polymer coatings. Biomaterials 2007, 28, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Eldin, M.S.M.; Tamer, T.M.; Saied, M.A.A.; Soliman, E.A.; Madi, N.K.; Ragab, I.; Fadel, I. Click Grafting of Chitosan onto PVC Surfaces for Biomedical Applications. Adv. Polym. Technol. 2018, 37, 38–49. [Google Scholar] [CrossRef]
- Mantlo, E.K.; Paessler, S.; Seregin, A.; Mitchell, A. Luminore CopperTouch Surface Coating Effectively Inactivates SARS-CoV-2, Ebola Virus, and Marburg Virus In Vitro. Antimicrob. Agents Chemother. 2021, 65, e01390. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View 2020, 1, e16. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Oertle, J.; Warren, D.; Prato, D. Quercetin: A Promising Flavonoid with a Dynamic Ability to Treat Various Diseases, Infections, and Cancers. J. Cancer Ther. 2016, 7, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Cristescu, R.; Narayan, R.J.; Chrisey, D.B. Novel antimicrobial surfaces to defeat COVID-19 transmission. MRS Adv. 2020, 2019, 2839–2851. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations. Nat. Prod. Commun. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging covered with antiviral and antibacterial coatings based on zno nanoparticles supplemented with geraniol and carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Goswami, M.; Yadav, A.K.; Chauhan, V.; Singh, N.; Kumar, S.; Das, A.; Yadav, V.; Mandal, A.; Tiwari, J.K.; Siddiqui, H.; et al. Facile Development of Graphene-Based Air Filters Mounted on 3D Printed Mask for COVID-19. J. Sci. Adv. Mater. Devices 2021, 6, 404–417. [Google Scholar] [CrossRef]
- Martí, M.; Tuňón-Molina, A.; Aachmann, F.L.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, A. Protective face mask filter capable of inactivating SARS-CoV-2, and methicillin-resistant staphylococcus aureus and staphylococcus epidermidis. Polymers 2021, 13, 207. [Google Scholar] [CrossRef]
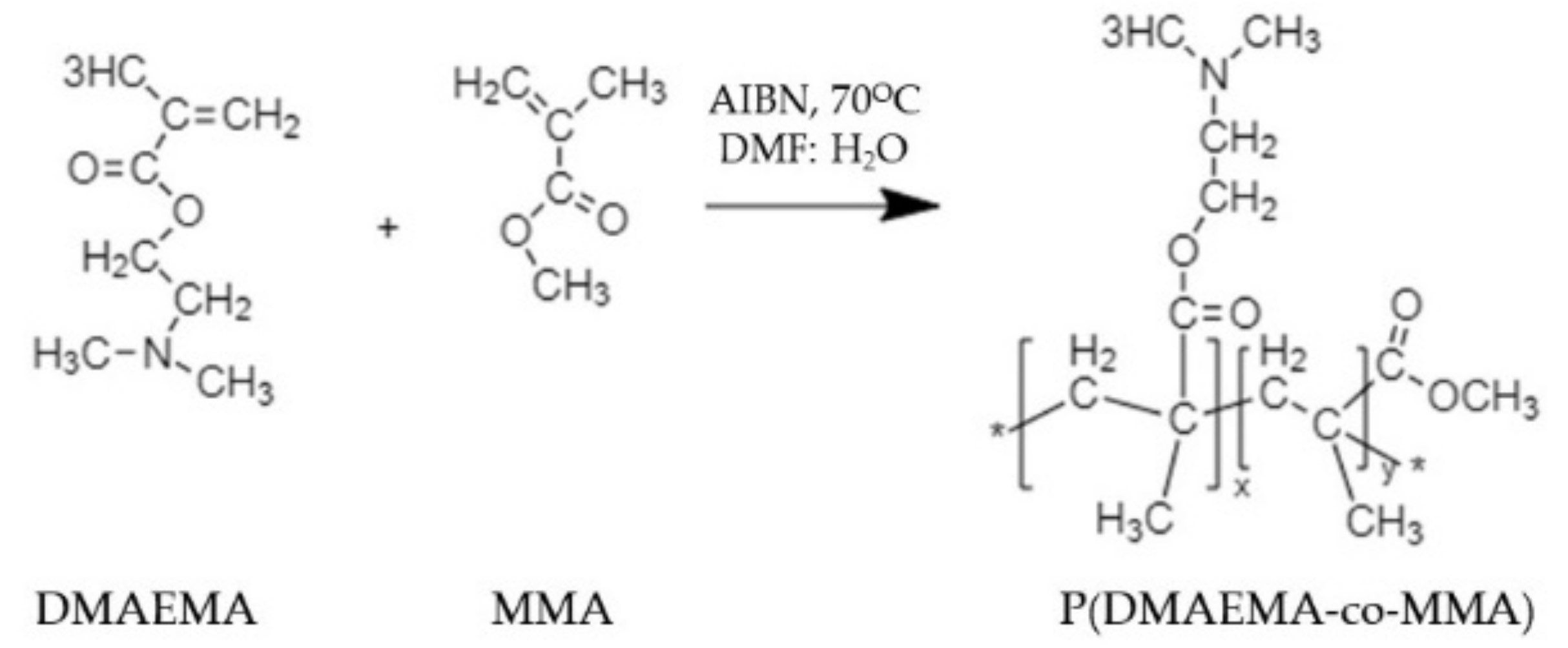
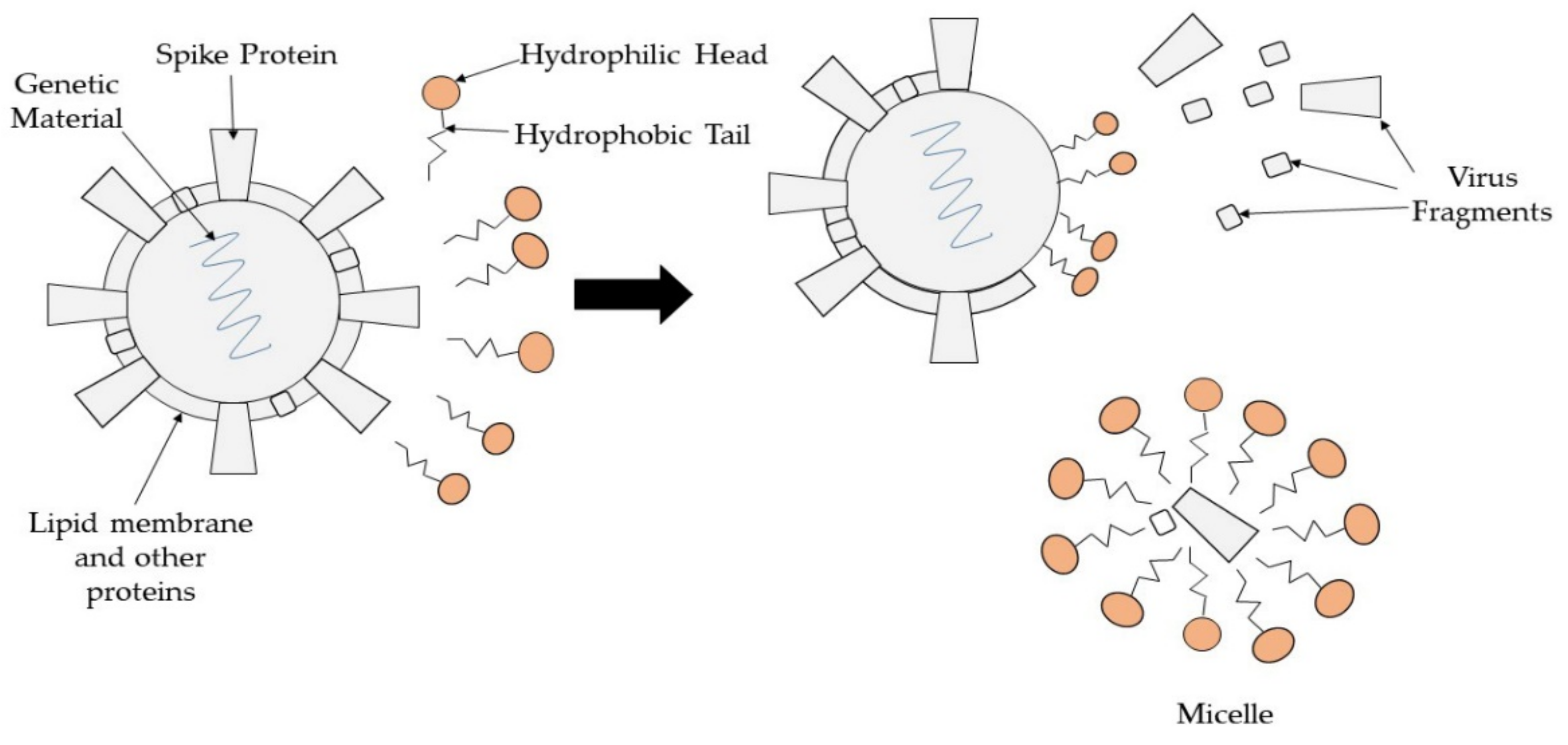
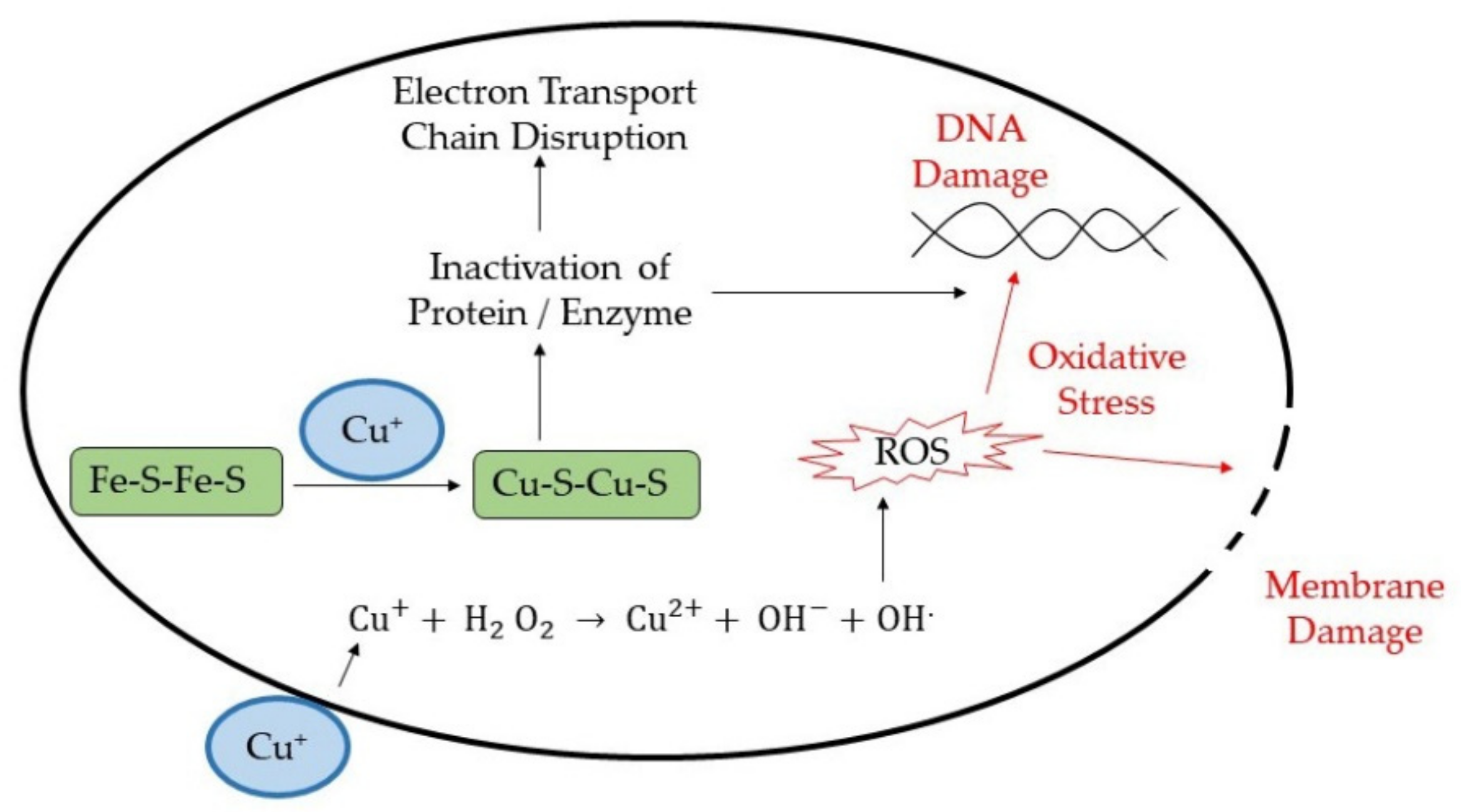
| Coating Materials | Coating Techniques | Microbes | Antimicrobial Activity | Application | Ref. |
|---|---|---|---|---|---|
| Synthetic Coating Materials | |||||
| Poly(allylamine)-poly(sodium 4-styrenesulfonate)/poly(allylamine hydrochloride) multilayer | Plasma polymerization and layer-by-layer assembly | Staphylococcus aureus and Escherichia coli | 77.78 ± 1.72% 95.15 ± 2.40% The antibacterial capability is measured based on antibacterial ratio | Titanium implant antibacterial coating | [27] |
| An amphiphilic polymer made up of polyoxypropylene (poly(propylene oxide)) flanked with two hydrophilic chains of polyoxyethylene (poly(ethylene oxide)), embedded with chlorine dioxide, copper, and L-vitamin C | Not mentioned | Influenza A (H1N1), methicillin-resistant Staphylococcus aureus (MRSA) and Acinebacter baumannii | No virus plaque observed After 1 min of contact, viral protein envelope is damaged Over 99.9% of antimicrobial activity after 5 min of contact time | Antipathogenic coating for additional measure | [28] |
| PEI-silver nanoparticle and copper nanoparticle membrane | Covalent linking via layer-by-layer | MS2 bacteriophage | 4.5 to 5 log reduction | Membrane filter for drinking water | [29] |
| Polyvinylpyyrolidone/titanium dioxide | Simple dip coating | Escherichia coli | The width of inhibition zone ranges from 4.5 to 8 mm | Medical device coating with improved blood compatibility and antimicrobial activity | [30] |
| N,N-dodecyl, methyl-PEI | Physical painting using cotton swab | Influenza A wild-type and resistant type (H3N2) and avian influenza A wild-type and resistant type (H4N2) | 100% biocidal efficiency for all tested viruses | Antiviral surface painting | [31] |
| Poly(hydantoinylacrylamide-co-3-(trimethyoxysilyl)propyl methacrylate) (HASL) | Covalent binding with cellulose cotton fabric | Staphylococcus aureus and Escherichia coli | About 6 log reduction in all tested microbes | Not mentioned | [32] |
| Poly(hydantoinylacrylamide-co-glycidyl methacrylate) (HAGM) | Covalent binding with cellulose cotton fabric | ||||
| Poly(hydantoinylacrylamide-co-2-hydroxyethyl methacrylate) (HAOH) | Cross-linking via an agent to cellulose cotton fabric | ||||
| Poly(L-lactide)/poly (ε-caprolactone)/propolis | Solvent casting | Staphylococcus aureus | Inhibition zone diameter ranges from 13 to 17 nm | Guided tissue regeneration application | [33] |
| N,N-hexyl, methyl-PEI | Covalent attachment | Poliovirus | 100% virucidal activity | Aqueous solution disinfection | [34] |
| N,N-dodecyl, methyl-PEI | Physical painting | Influenza virus, Staphylococcus aureus, and Escherichia coli | 100% virucidal and bactericidal activity | Not mentioned | [35] |
| Polyester/polyurethane/levofloxacin | Hot-press polymer immobilization | Staphylococcus aureus | No viable bacteria found on coated substrate | Antimicrobial implant coating application | [36] |
| Natural Coating Materials | |||||
| Carboxymethylcellulose/chitosan multilayer | Chemical cross-linked layer-by-layer assembly | Staphylococcus aureus and Pseudomonas aeruginosa | 74% reduction at 24 h 83% reduction at 72 h | Superhydrophilic coating for ophthalmic applications | [37] |
| Carrageenan/green tea extract | Simple dip coating | Murine norovirus (MNV-1) and hepatitis A virus (HAV) | Below detection limit at any condition Lower than 3 log reduction | Antiviral edible coating for fruits | [38] |
| Chitosan/green tea extract film coating | Solution casting onto polypropylene film | Murine norovirus (MNV-1) | 1.6 to 4.5 logs PFU/mL reduction after 24 h incubation | Active food packaging | [39] |
| Chitosan | Covalent linking via silanization step | Escherichia coli and Staphylococcus aureus | No viable cells observed after 24 h | Antibacterial surface for biomedical devices | [40] |
| Carrageenan/citric acid | Not mentioned | Staphylococcus aureus, Dickeya chrysanthemi, Escherichia coli, Proteus mirabilis, and Pseudomonas aeruginosa | Inhibition zone diameter for carrageenan film with highest concentration of citric acid ranges from 3.25 ± 0.29 mm to 4.18 ± 0.28 mm | Biodegradable film | [41] |
| Gelatin/chitosan/d-limonene | Solvent casting | Escherichia coli | Film containing highest d-limonene concentration has inhibition zone diameter with 22.0 ± 1.2 mm | Antimicrobial edible film for food packaging | [42] |
| Polyelectrolyte multilayer composed of carrageenan and chitosan embedded with nisin Z | Layer-by-layer coating | Staphylococcus aureus and MRSA | Kill over 90% and 99% of planktonic and biofilm cells, respectively | Antimicrobial multilayer coating | [43] |
| Inorganic Metal Nanoparticle | Synthesis Route | Size and Shape | Antimicrobial Activity | Ref. |
|---|---|---|---|---|
| Ag | Biological synthesis using Olea europea aqueous extract | 11.6–20.7 nm Spherical | Inhibition zone diameter ranged from 14 to 22 mm for Streptococcus mutans and 7 to 13 mm for Candida albicans | [113] |
| Ag | Biological synthesis using Lamptanthus coccineus aqueous and hexane extract Malephora lutea aqueous and hexane extracts | 10.12–27.89 nm Spherical 8.91–14.48 nm Spherical | The antiviral activity was measured based on IC50 1 (µg/mL) For HAV-10 virus: no activity for aqueous extract 11.71 for hexane extract For HSV-1 virus: 520.6 for aqueous extract 36.36 for hexane extract For CoxB4 virus: no activity for aqueous extract 12.74 for hexane extract For HAV-10 virus: no activity for aqueous extract 31.38 for hexane extract For HSV-1 virus: no activity for both aqueous and hexane extract For CoxB4 virus: 46.44 for aqueous extract 29.04 for hexane extract | [114] |
| Ag | Biological synthesis using bacterial enzyme | 77–92 nm Spherical, triangular, and hexagonal | Inhibited the growth of Bean Yellow Mosaic Virus | [115] |
| Silver oxide (AgO) | Biological synthesis using bioactive compounds from Oscillatoria sp. | 14.42–48.97 nm Spherical | 49.23% reduction of HSV-1 reproduction in dilution ranging from 10−1–10−8 | [116] |
| Au | Biological synthesis using bioactive compounds from Spirulina platensis | 15.60–77.13 Octahedral, pentagonal, and triangular | 42.75% reduction of HSV-1 reproduction in dilution ranging from 10−1–10−8 | |
| Copper oxide (CuO) | Biological reduction using Momordica charantia aqueous extract | 61.48 ± 2 nm Rod-shaped | Inhibited Bacillus cereus with 31.66 nm zone of inhibition 80% viability of infected embryo with Newcastle Disease Virus (NDV) was observed by using 100 µg/mL concentration of CuO nanoparticles | [117] |
| Manganese (Mn) | Biological reduction using curcumin ethanolic extract | In the range of 50 nm Spherical | Inhibition zone diameter ranged from 11 to 20 mm for various bacterial species and fungal species | [118] |
| Ag | Biological reduction using Citrus limetta peels | 5 nm Spherical | More than 90% inhibition against chikungunya virus (CHIKV) at different nanoparticle concentrations (0.05 mg/mL, 0.1 mg/mL, and 0.2 mg/mL) | [119] |
| Iron (Fe) | 32 nm Spherical | |||
| ZnO | 12 nm Spherical | |||
| Aluminum oxide (Al2O3) | Biological reduction using Cymbopogan citratus leaf extract | 34.5 nm Spherical | Complete growth inhibition against Pseudomona aeruginosa | [120] |
| Titanium dioxide (TiO2) | Biological reduction using Psidium guajava leaf extract | 32.58 nm Spherical | Maximum inhibition zone diameters achieved were 25 mm and 23 mm for Staphylococcus aureus and Escherichia coli, respectively | [121] |
| Nickel oxide (NiO) | Biological reduction using Eucalyptus globulus plant extract | 10 to 20 nm | Inhibition zone diameter ranged from 13 to 17 mm for various bacteria | [122] |
| Au-Ag-zinc ZnO-chlorine dioxide nanocomposite | Chemical reduction using citric acid | 20–40 nm for AuNP 10–40 nm for AgNP 25–35 nm for ZnO Nanoparticle | Inhibited 93.5–100% of SARS-CoV-2 formation | [123] |
| Au | Chemical reduction using mixture of tetraethoxysilane and triethoxysilane | 1.5–20 nm Spherical | 55–96% inhibition of adenovirus reproduction in MDBK cell culture at various nanoparticle dilutions | [124] |
| ZnO | Chemical synthesis via molten salt method | 39.7 nm Star-like shape | Growth curve of both Bacillus subtilis and Enterobacter aerogenes decreased after 24 h of incubation | [125] |
| CuO | Chemical synthesis using sodium hydroxide | Average diameter is 10 nm Nanorod | 99%, 98%, and 93% growth reduction in Escherichia coli, Shigella flexneri, and Staphylococcus aureus, respectively | [126] |
| Ag | Electrochemical | 7.1 nm Quasi-spherical | Effective concentration was 3.13 ppm against poliovirus | [127] |
| Ag nanocluster with silica composite | Radio frequency co-sputtering process with argon | Less than 200 nm | 100% inhibition against coronavirus | [128] |
| Modification Techniques | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Plasma Surface Treatment |
|
| [193,194] |
| Microwave Radiation |
|
| [195,196,197] |
| Laser Surface Texturing or Patterning |
|
| [198,199,200] |
| Ultraviolet Irradiation Surface Treatment |
|
| [193,201,202] |
| Acid/Alkali Hydrolysis |
|
| [193,203,204] |
| Abrasive Blasting or Sand Blasting |
|
| [199,205] |
| Chemical Vapor Deposition |
|
| [203,206] |
| Click Grafting |
|
| [207,208] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, N.; Rusli, A.; Teramoto, N.; Jaafar, M.; Ku Ishak, K.M.; Shafiq, M.D.; Abdul Hamid, Z.A. Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review. Polymers 2021, 13, 4234. https://doi.org/10.3390/polym13234234
Nasri N, Rusli A, Teramoto N, Jaafar M, Ku Ishak KM, Shafiq MD, Abdul Hamid ZA. Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review. Polymers. 2021; 13(23):4234. https://doi.org/10.3390/polym13234234
Chicago/Turabian StyleNasri, Nazihah, Arjulizan Rusli, Naozumi Teramoto, Mariatti Jaafar, Ku Marsilla Ku Ishak, Mohamad Danial Shafiq, and Zuratul Ain Abdul Hamid. 2021. "Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review" Polymers 13, no. 23: 4234. https://doi.org/10.3390/polym13234234
APA StyleNasri, N., Rusli, A., Teramoto, N., Jaafar, M., Ku Ishak, K. M., Shafiq, M. D., & Abdul Hamid, Z. A. (2021). Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review. Polymers, 13(23), 4234. https://doi.org/10.3390/polym13234234