Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies
Abstract
:1. Introduction
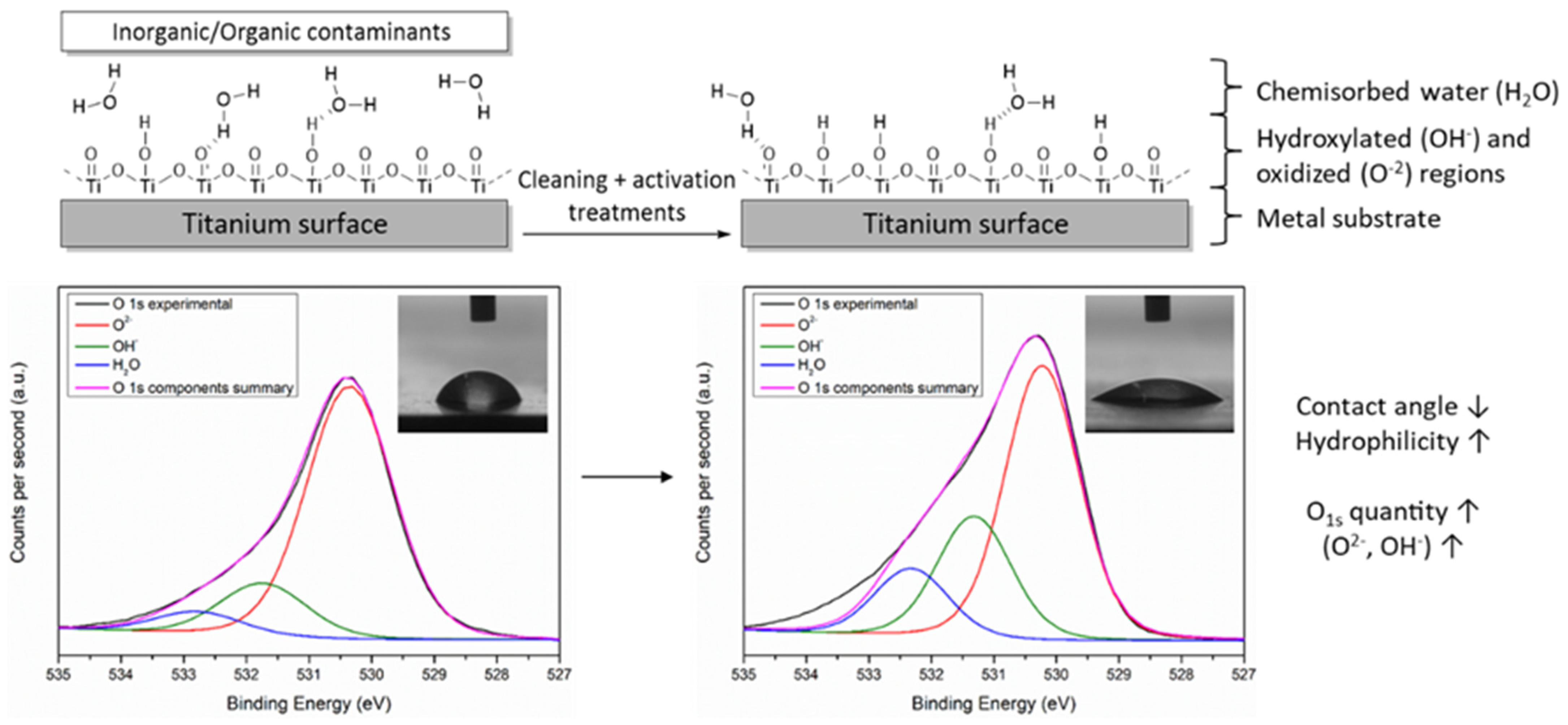
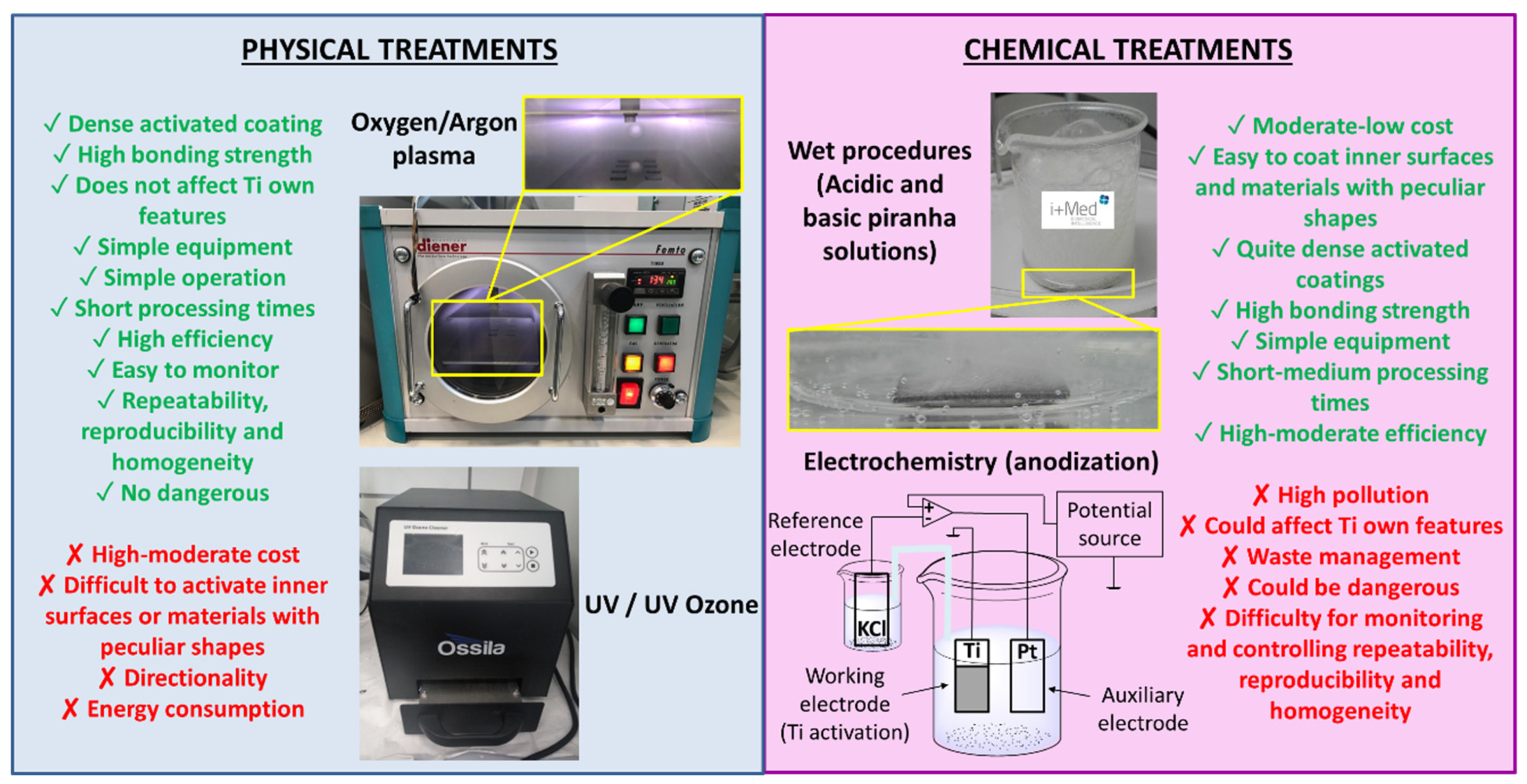
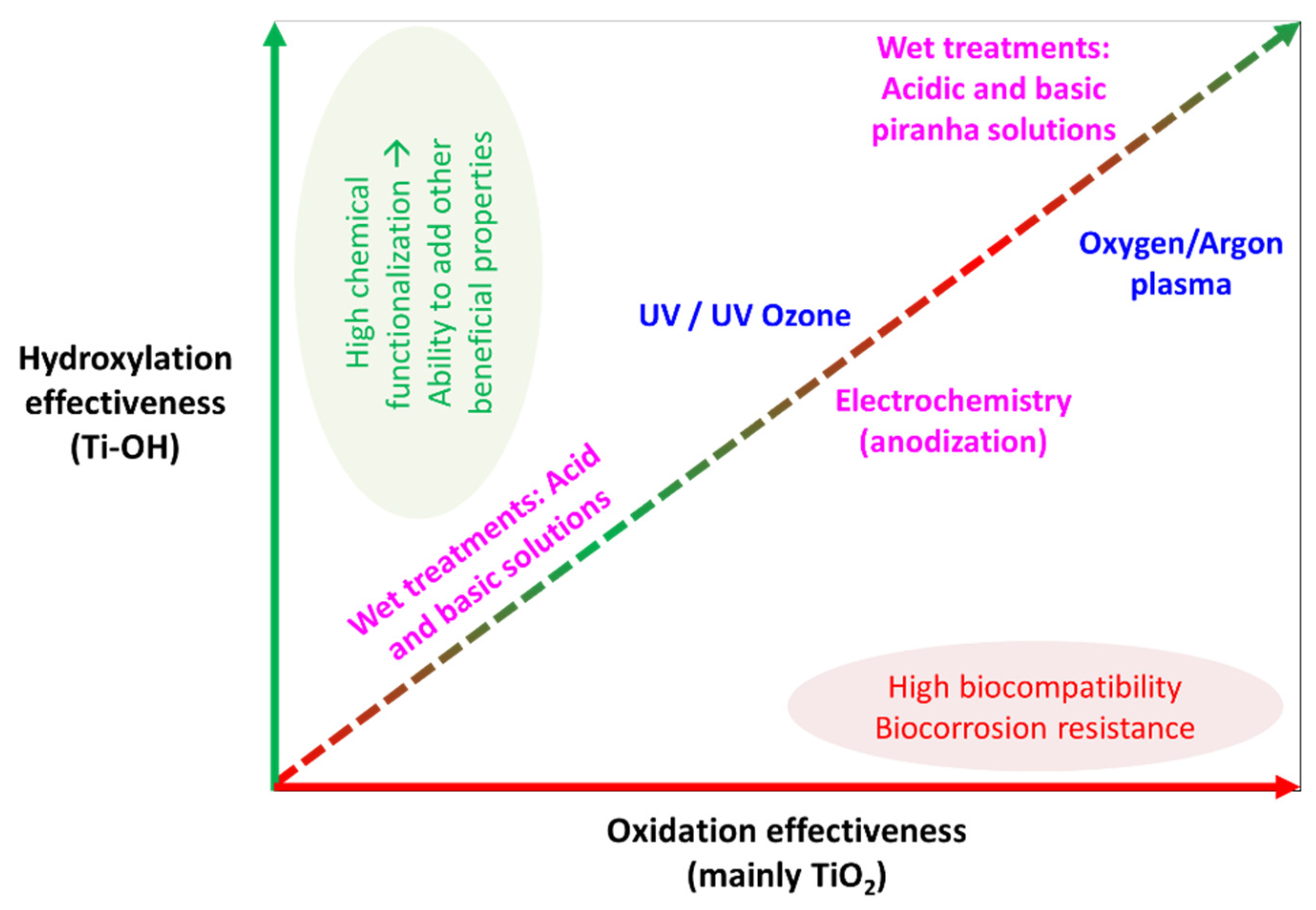
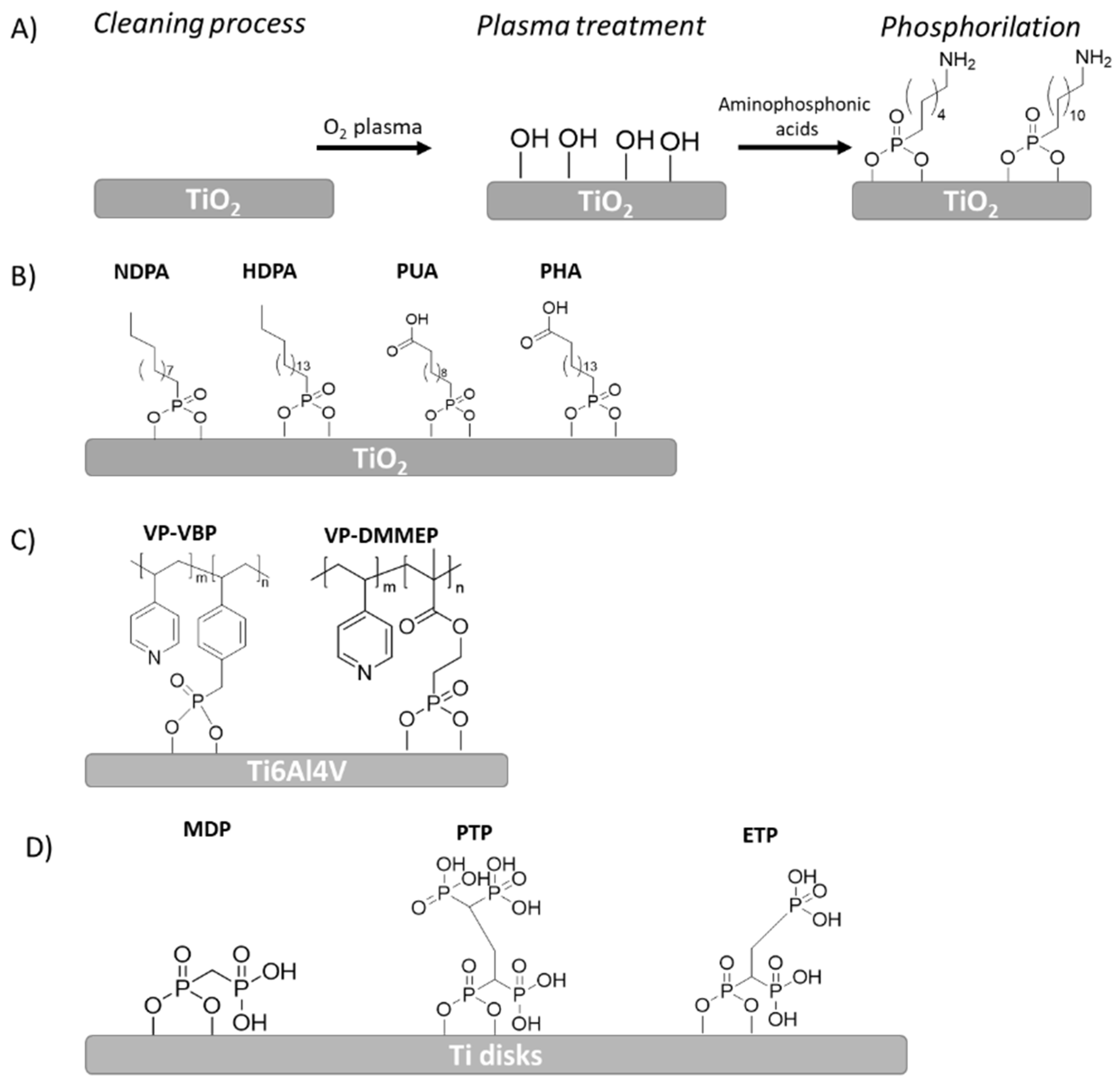
2. Pre-Activation of Ti Surface
3. Self-Assembled Monolayers (SAMs)
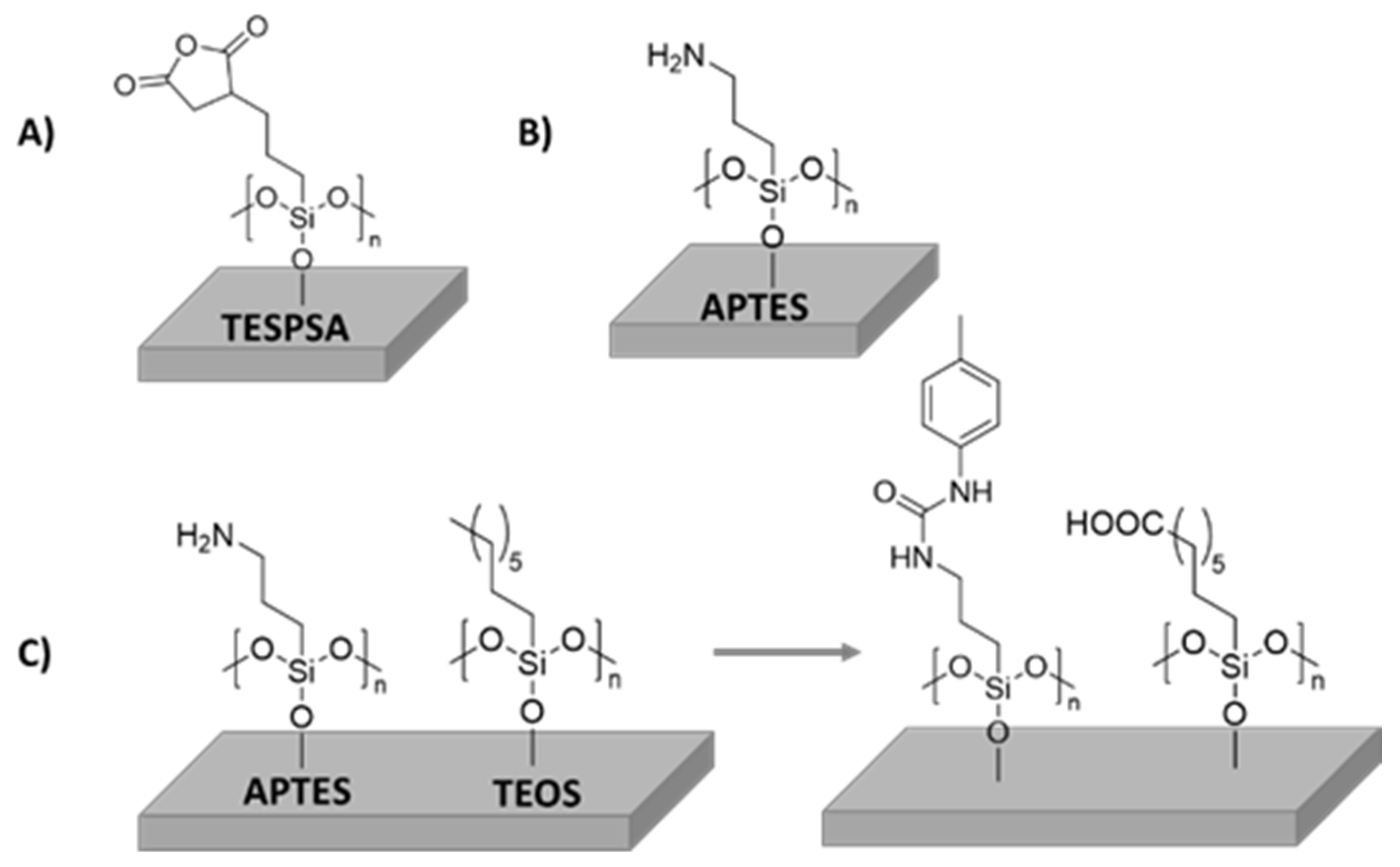
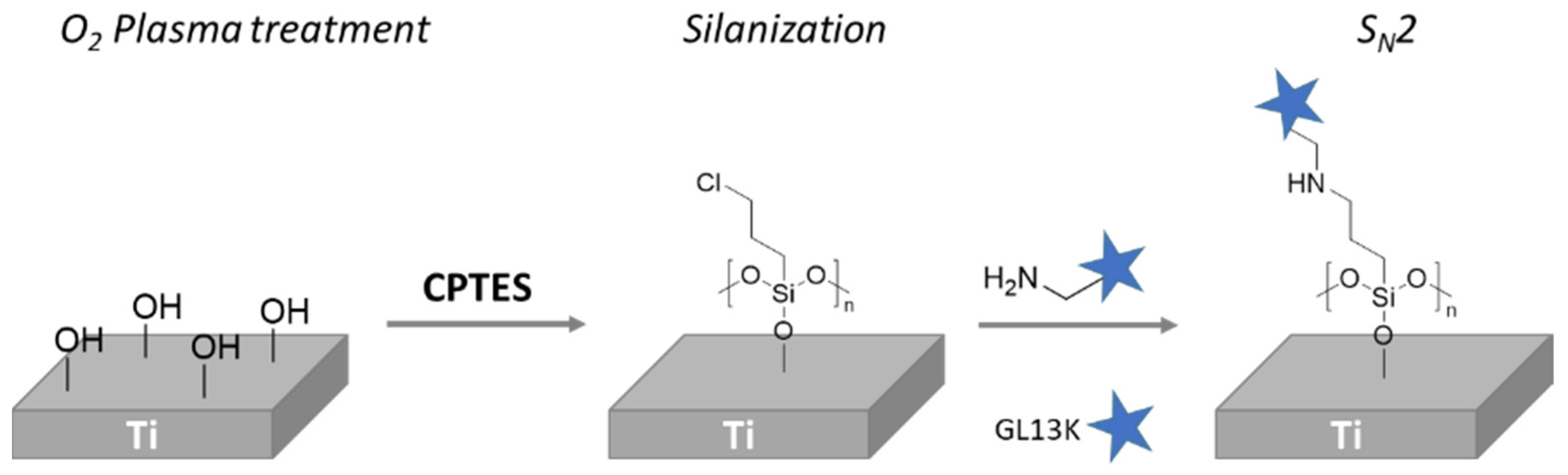
3.1. Silanes
3.2. Phosphonates
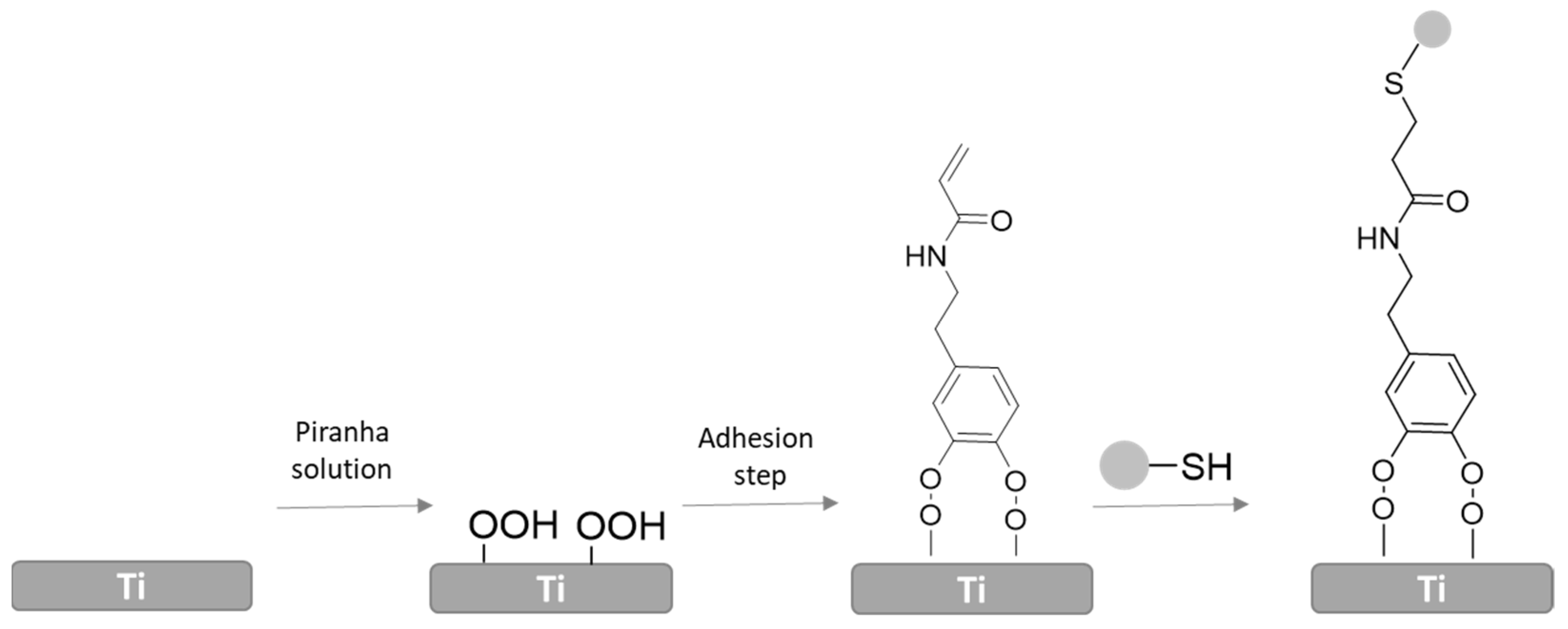
3.3. Catechols
4. Active Layer
4.1. Immobilization
4.1.1. Nucleophilic Substitution Reactions
4.1.2. Click Chemistry
4.2. Release-Based Polymeric Coatings
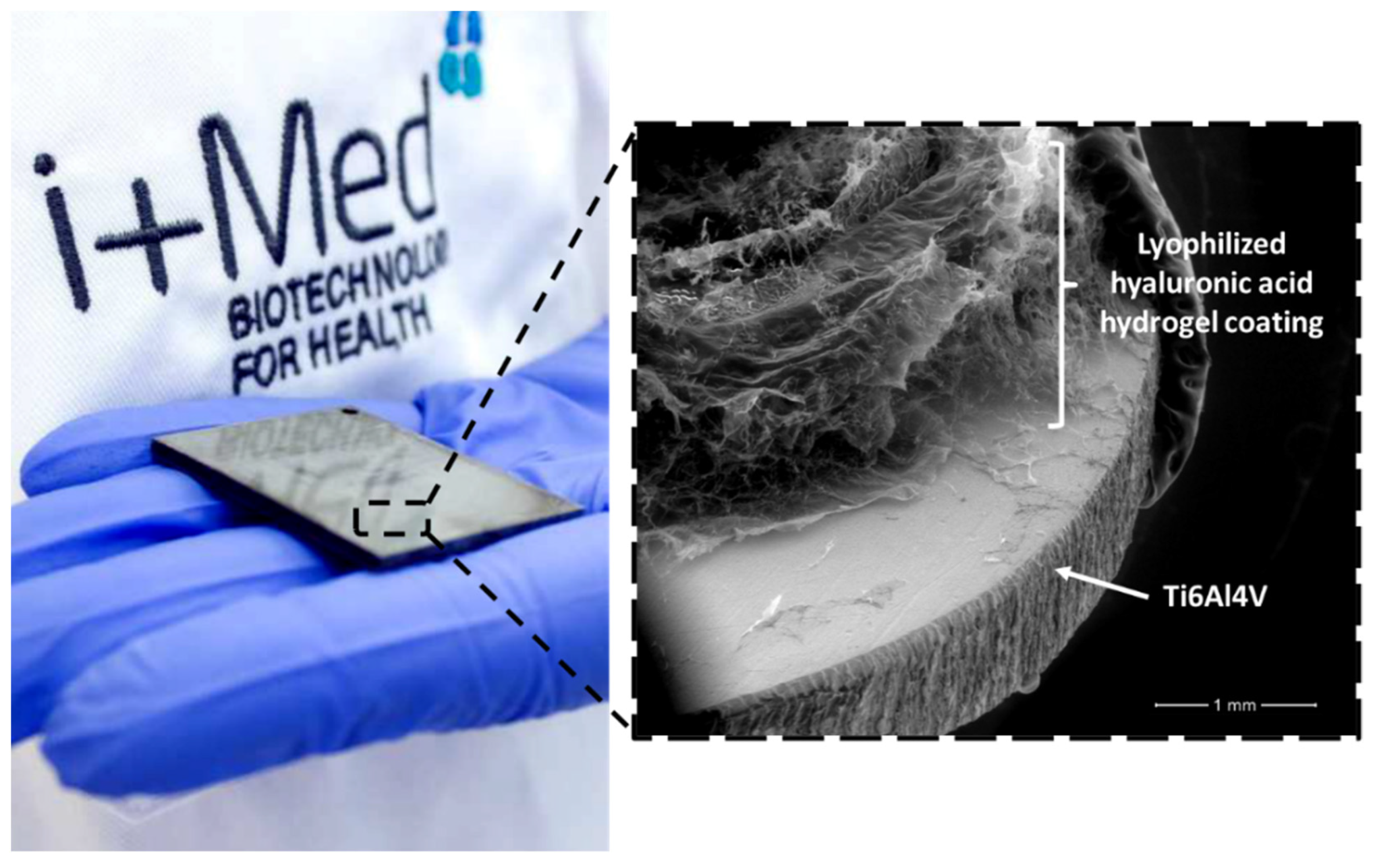
4.2.1. Hydrogel Coatings
4.2.2. Multilayer Coatings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishfaq, K.; Rehman, M.; Khan, A.R.; Wang, Y. A review on the performance characteristics, applications, challenges and possible solutions in electron beam melted Ti-based orthopaedic and orthodontic implants. Rapid Prototyp. J. 2021. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Nicholson, W.J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcan, M.; Hämmerle, C. Titanium as a reconstruction and implant material in dentistry: Advantages and pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef] [Green Version]
- Losic, D. Advancing of titanium medical implants by surface engineering: Recent progress and challenges. Expert Opin. Drug Deliv. 2021, 18, 1355–1378. [Google Scholar] [CrossRef]
- Cai, K.; Rechtenbach, A.; Hao, J.; Jandt, K.D. Polysaccharide-protein surface modification of titanium via a layer-by-layer technique: Characterization and cell behaviour aspects. Biomaterials 2005, 26, 5960–5971. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Ko, C.L. Roughened titanium surfaces with silane and further RGD peptide modification in vitro. Mater. Sci. Eng. C 2013, 33, 2713–2722. [Google Scholar] [CrossRef]
- Branemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- López-Valverde, N.; Macedo-De-Sousa, B.; López-Valverde, A.; Ramírez, J.M. Effectiveness of antibacterial surfaces in osseointegration of titanium dental implants: A systematic review. Antibiotics 2021, 10, 360. [Google Scholar] [CrossRef]
- López-Valverde, A.; López-Valverde, N.; Flores-Fraile, J. The unknown process osseointegration. Biology 2020, 9, 168. [Google Scholar] [CrossRef]
- Johnsen, S.P.; Sørensen, H.T.; Pedersen, A.B.; Lucht, U.; Søballe, K.; Overgaard, S. Patient-related predictors of implant failure after primary total hip replacement in the initial, short- and long-term: A nationwide Danish folow-up study including 36 984 patients. J. Bone Jt. Surg.-Ser. B 2006, 88, 1303–1308. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Park, K.H.; Jung, N.; Shim, J.S.; Moon, H.S.; Kim, H.J.; Oh, S.H.; Kim, Y.Y.; Ku, S.Y.; Park, Y.B. In vivo study for clinical application of dental stem cell therapy incorporated with dental titanium implants. Materials 2021, 14, 381. [Google Scholar] [CrossRef]
- Park, J.W.; Kurashima, K.; Tustusmi, Y.; An, C.H.; Suh, J.Y.; Doi, H.; Nomura, N.; Noda, K.; Hanawa, T. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly(ethylene glycol) in rabbit cancellous bone. Acta Biomater. 2011, 7, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Ravikumar, K.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A.; Karandikar, B.; Gibbins, B.L. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2008, 87, 455–460. [Google Scholar] [CrossRef]
- Benčina, M.; Mavrič, T.; Junkar, I.; Bajt, A.; Krajnović, A.; Lakota, K.; Žigon, P.; Sodin-Šemrl, S.; Kralj-Iglič, V.; Iglič, A. The Importance of Antibacterial Surfaces in Biomedical Applications. Adv. Biomembr. Lipid Self-Assem. 2018, 28, 115–165. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Bhatnagar, D.; Leszczak, V.; Popat, K.C. Titania nanostructures: A biomedical perspective. RSC Adv. 2015, 5, 37149–37171. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, H.; Yang, Y.; Huang, J.; Wu, K.; Chen, Z.; Wang, X.; Lin, C.; Lai, Y. Progress in TiO2 nanotube coatings for biomedical applications: A review. J. Mater. Chem. B 2018, 6, 1862–1886. [Google Scholar] [CrossRef]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Hanawa, T. A comprehensive review of techniques for biofunctionalization of titanium. J. Periodontal Implant Sci. 2011, 41, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Shibata, Y.; Tanimoto, Y. A review of improved fixation methods for dental implants. Part I: Surface optimization for rapid osseointegration. J. Prosthodont. Res. 2015, 59, 20–33. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, Y.C. Contact Angle Relaxation and Long-Lasting Hydrophilicity of Sputtered Anatase TiO 2 Thin Films by Novel Quantitative XPS Analysis. Langmuir 2019, 36, 2066–2077. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Karpagavalli, R.; Zhou, A.; Chellamuthu, P.; Nguyen, K. Corrosion behavior and biocompatibility of nanostructured TiO 2 film on Ti6Al4V. J. Biomed. Mater. Res. Part A 2007, 83A, 1087–1095. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Noumbissi, S.; Scarano, A.; Gupta, S. A literature review study on atomic ions dissolution of titanium and its alloys in implant dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.J.; Chou, H.H.; Ou, K.L.; Sugiatno, E.; Ruslin, M.; Waris, R.A.; Huang, C.F.; Liu, C.M.; Peng, P.W. Evaluation of surface characteristics and hemocompatibility on the oxygen plasma-modified biomedical titanium. Metals 2018, 8, 513. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive Effects of Low-Temperature Argon-Oxygen Plasma on a Titanium Implant Surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Ao, X.; Xie, P.; Wu, J.; Dong, Y.; Yu, D.; Wang, J.; Zhu, Z.; Xu, H.H.K.; Chen, W. Effects of novel non-thermal atmospheric plasma treatment of titanium on physical and biological improvements and in vivo osseointegration in rats. Sci. Rep. 2020, 10, 10637. [Google Scholar] [CrossRef]
- Andrade-Del Olmo, J.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Xu, J.; Chirume, W.M.; Zhang, J.; Zhang, H. Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review. Coatings 2021, 11, 1401. [Google Scholar] [CrossRef]
- Barik, A.; Chakravorty, N. Targeted Drug Delivery from Titanium Implants: A Review of Challenges and Approaches. In Trends in Biomedical Research; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1251, pp. 1–17. [Google Scholar]
- Duan, Y.; Wu, Y.; Yan, R.; Lin, M.; Sun, S.; Ma, H. Self-healing and self-strengthening dual-function polyelectrolytes coating for corrosion protection of titanium sheet. Prog. Org. Coat. 2021, 155, 106232. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef] [Green Version]
- Nathanael, A.J.; Oh, T.H. Biopolymer coatings for biomedical applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Ou, K.; Shih, Y.; Huang, C.; Chen, C.; Liu, C.-M. Preparation of bioactive amorphous-like titanium oxide layer on titanium by plasma oxidation treatment. Appl. Surf. Sci. 2008, 255, 2046–2051. [Google Scholar] [CrossRef]
- Fan, Z.; Zhi, C.; Wu, L.; Zhang, P.; Feng, C.; Deng, L.; Yu, B.; Qian, L. UV/Ozone-Assisted Rapid Formation of High-Quality Tribological Self-Assembled Monolayer. Coatings 2019, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Jalali, N.; Moztarzadeh, F.; Asgari, A.; Zamanian, A.; Verma, K.D.; Mozafari, M. Improving Cellular Response of Titanium Surface through Electrochemical Anodization for Biomedical Applications: A Critical Review. Trends Biomater. Artif. Organs 2015, 29, 86–91. [Google Scholar]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Cools, P.; De Geyter, N.; Vanderleyden, E.; Dubruel, P.; Morent, R. Surface analysis of titanium cleaning and activation processes: Non-thermal plasma versus other techniques. Plasma Chem. Plasma Process. 2014, 34, 917–932. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; John, H. Titanium dioxide based self-cleaning smart surfaces: A short review. J. Environ. Chem. Eng. 2020, 8, 104211. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Zhou, L.; Guo, Z.; Rong, M.; Liu, X.; Lai, C.; Ding, X. The Effects of Different Wavelength UV Photofunctionalization on Micro-Arc Oxidized Titanium. PLoS ONE 2013, 8, e68086. [Google Scholar] [CrossRef]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res.-Part A 2014, 102, 3618–3630. [Google Scholar] [CrossRef]
- Lv, L.; Xie, Y.; Li, K.; Hu, T.; Lu, X.; Cao, Y.; Zheng, X. Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization. Adv. Healthc. Mater. 2018, 7, 1800675. [Google Scholar] [CrossRef] [PubMed]
- Pop-Georgievski, O.; Kubies, D.; Zemek, J.; Neykova, N.; Demianchuk, R.; Chánová, E.M.; Šlouf, M.; Houska, M.; Rypácek, F. Self-assembled anchor layers/polysaccharide coatings on titanium surfaces: A study of functionalization and stability. Beilstein J. Nanotechnol. 2015, 6, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Sotgiu, G.; Orsini, M.; Porcelli, F.; de Santis, S.; Petrucci, E. Wettability of micro and nanostructured surface of titanium based electrodes: Influence of chemical and electrochemical etching. Chem. Eng. Trans. 2021, 86, 1417–1422. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Khadiri, M.; Elyaagoubi, M.; Idouhli, R.; Koumya, Y.; Zakir, O.; Benzakour, J.; Benyaich, A.; Abouelfida, A.; Outzourhit, A. Electrochemical Study of Anodized Titanium in Phosphoric Acid. Adv. Mater. Sci. Eng. 2020, 2020, 5769071. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Brewis, D.M. Review of surface pretreatments for titanium alloys. Int. J. Adhes. Adhes. 1995, 15, 161–172. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P.; Cromer, T.F. Surface Properties of Hydroxyl Groups in the Air-Formed Oxide Film on Titanium. J. Electrochem. Soc. 1999, 146, 2849–2852. [Google Scholar] [CrossRef]
- Son, Y.; Lee, M.K.; Park, Y.C. Contact Angle Relaxation on Amorphous, Mixed-Phase (Anatase + Rutile), and Anatase TiO2Films and Its Mechanism. Langmuir 2021, 37, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Gadois, C.; Światowska, J.; Zanna, S.; Marcus, P. Influence of titanium surface treatment on adsorption of primary amines. J. Phys. Chem. C 2013, 117, 1297–1307. [Google Scholar] [CrossRef]
- So, S.; Riboni, F.; Hwang, I.; Paul, D.; Hammond, J.; Tomanec, O.; Zboril, R.; Sadoway, D.R.; Schmuki, P. The double-walled nature of TiO2 nanotubes and formation of tube-in-tube structures—A characterization of different tube morphologies. Electrochim. Acta 2017, 231, 721–731. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Li, J.; Fu, X.; Li, C.; Wang, H.; Liu, S.; Guo, L.; Xin, S.; Liang, C.; et al. Improvement of biological properties of titanium by anodic oxidation and ultraviolet irradiation. Appl. Surf. Sci. 2014, 307, 202–208. [Google Scholar] [CrossRef]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.P. Collagen-functionalised titanium surfaces for biological sealing of dental implants: Effect of immobilisation process on fibroblasts response. Colloids Surf. B Biointerfaces 2014, 122, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Paredes, V.; Salvagni, E.; Rodríguez-Castellón, E.; Manero, J.M. Comparative Study of Surface Chemical Composition and Oxide Layer Modification upon Oxygen Plasma Cleaning and Piranha Etching on a Novel Low Elastic Modulus Ti25Nb21Hf Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 3770–3776. [Google Scholar] [CrossRef]
- Paredes, V.; Salvagni, E.; Rodriguez, E.; Gil, F.J.; Manero, J.M. Assessment and comparison of surface chemical composition and oxide layer modification upon two different activation methods on a cocrmo alloy. J. Mater. Sci. Mater. Med. 2014, 25, 311–320. [Google Scholar] [CrossRef]
- Freitas, S.C.; Correa-Uribe, A.; Martins, M.C.L.; Pelaez-Vargas, A. Self-Assembled Monolayers for Dental Implants. Int. J. Dent. 2018, 2018, 4395460. [Google Scholar] [CrossRef]
- Somasundaram, S. Silane coatings of metallic biomaterials for biomedical implants: A preliminary review. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 2901–2918. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, C.; Huskens, J. Reactive self-assembled monolayers: From surface functionalization to gradient formation. Mater. Horiz. 2014, 1, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, N.; Schweiss, R.; Lützow, K.; Werner, C.; Groth, T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef]
- Kim, S.; Yoo, H. Self-assembled monolayers: Versatile uses in electronic devices from gate dielectrics, dopants, and biosensing linkers. Micromachines 2021, 12, 565. [Google Scholar] [CrossRef]
- Ahn, J.K.; Oh, S.J.; Park, H.; Song, Y.; Kwon, S.J.; Shin, H.B. Vapor-phase deposition-based self-assembled monolayer for an electrochemical sensing platform. AIP Adv. 2020, 10, 045213. [Google Scholar] [CrossRef]
- Sun, C.; Liao, X.; Huang, P.; Shan, G.; Ma, X.; Fu, L.; Zhou, L.; Kong, W. A self-assembled electrochemical immunosensor for ultra-sensitive detection of ochratoxin A in medicinal and edible malt. Food Chem. 2020, 315, 126289. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Munro, K.; Ebralize, I.I.; Narouz, M.R.; Padmos, J.D.; Hao, H.; Crudden, C.M.; Horton, J.H. N-Heterocyclic Carbene Self-Assembled Monolayers on Gold as Surface Plasmon Resonance Biosensors. Langmuir 2017, 33, 13936–13944. [Google Scholar] [CrossRef] [PubMed]
- Suni, I.I. Substrate materials for biomolecular immobilization within electrochemical biosensors. Biosensors 2021, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Quimada Mondarte, E.A.; Palai, D.; Sekine, T.; Kashiwazaki, A.; Murakami, D.; Tanaka, M.; Hayashi, T. Protein- and Cell-Resistance of Zwitterionic Peptide-Based Self-Assembled Monolayers: Anti-Biofouling Tests and Surface Force Analysis. Front. Chem. 2021, 9, 748017. [Google Scholar] [CrossRef] [PubMed]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef] [PubMed]
- Schmaltz, T.; Sforazzini, G.; Reichert, T.; Frauenrath, H. Self-Assembled Monolayers as Patterning Tool for Organic Electronic Devices. Adv. Mater. 2017, 29, 1605286. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chang, C.Y. Enhanced output performance and stability of triboelectric nanogenerators by employing silane-based self-assembled monolayers. J. Mater. Chem. C 2020, 8, 4542–4548. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chemie Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef]
- Hasan, A.; Pandey, L.M. Self-Assembled Monolayers in Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081007167. [Google Scholar]
- Wang, L.; Schubert, U.S.; Hoeppener, S. Surface chemical reactions on self-assembled silane based monolayers. Chem. Soc. Rev. 2021, 50, 6507–6540. [Google Scholar] [CrossRef]
- Singh, V.; Mondal, P.C.; Singh, A.K.; Zharnikov, M. Molecular sensors confined on SiOx substrates. Coord. Chem. Rev. 2017, 330, 144–163. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C 2016, 59, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial Properties of Triethoxysilylpropyl Succinic Anhydride Silane (TESPSA) on Titanium Dental Implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Cano, A.; Cintas, P.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.Á.; Crespo, L.; Saldaña, L.; Vilaboa, N.; González-Martín, M.L.; Babiano, R. Controlled silanization-amination reactions on the Ti6Al4V surface for biomedical applications. Colloids Surf. B Biointerfaces 2013, 106, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Gonella, G.; Pinto, G.; Grachev, V.; Canepa, M.; Cavalleri, O. Anchoring of Aminophosphonates on Titanium Oxide for Biomolecular Coupling. J. Phys. Chem. C 2019, 123, 16843–16850. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.C.; Huang, T.S.; Cho, Y.C.; Huang, Y.T.; Walinski, C.J.; Chiang, P.C.; Rusilin, M.; Pai, F.T.; Huang, C.C.; Huang, M.S. The potential of a nanostructured titanium oxide layer with self-assembled monolayers for biomedical applications: Surface properties and biomechanical behaviors. Appl. Sci. 2020, 10, 590. [Google Scholar] [CrossRef] [Green Version]
- Metoki, N.; Liu, L.; Beilis, E.; Eliaz, N.; Mandler, D. Preparation and characterization of alkylphosphonic acid self-assembled monolayers on titanium alloy by chemisorption and electrochemical deposition. Langmuir 2014, 30, 6791–6799. [Google Scholar] [CrossRef]
- Calliess, T.; Sluszniak, M.; Winkel, A.; Pfaffenroth, C.; Dempwolf, W.; Heuer, W.; Menzel, H.; Windhagen, H.; Stiesch, M. Antimicrobial surface coatings for a permanent percutaneous passage in the concept of osseointegrated extremity prosthesis. Biomed. Tech. 2012, 57, 467–471. [Google Scholar] [CrossRef]
- Pfaffenroth, C.; Winkel, A.; Dempwolf, W.; Gamble, L.J.; Castner, D.G.; Stiesch, M.; Menzel, H. Self-Assembled Antimicrobial and Biocompatible Copolymer Films on Titanium. Macromol. Biosci. 2011, 11, 1515–1525. [Google Scholar] [CrossRef]
- Viornery, C.; Chevolot, Y.; Léonard, D.; Aronsson, B.O.; Péchy, P.; Mathieu, H.J.; Descouts, P.; Grätzel, M. Surface modification of titanium with phosphonic acid to improve bone bonding: Characterization by XPS and ToF-SIMS. Langmuir 2002, 18, 2582–2589. [Google Scholar] [CrossRef]
- Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Petković, M. A new insight into coating’s formation mechanism between TiO2 and alendronate on titanium dental implant. Materials 2020, 13, 3220. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chemie-Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Carey, F.A. Organic chemistry. J. Chem. Soc. 1877, 32, 725–791. [Google Scholar]
- Karty, J.; Melzer, M. Organic Chemistry: Principles and Mechanisms; WW Norton: New York, NY, USA, 2018; ISBN 9780393630756. [Google Scholar]
- Holmberg, K.V.; Abdolhosseini, M.; Li, Y.; Chen, X.; Gorr, S.U.; Aparicio, C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013, 9, 8224–8231. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Vyas, V.; Kaur, T.; Kar, S.; Thirugnanam, A. Biofunctionalization of commercially pure titanium with chitosan/hydroxyapatite biocomposite via silanization: Evaluation of biological performances. J. Adhes. Sci. Technol. 2017, 31, 1768–1781. [Google Scholar] [CrossRef]
- Kucharíková, S.; Gerits, E.; De Brucker, K.; Braem, A.; Ceh, K.; Majdič, G.; Španič, T.; Pogorevc, E.; Verstraeten, N.; Tournu, H.; et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016, 71, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Auernheimer, J.; Zukowski, D.; Dahmen, C.; Kantlehner, M.; Enderle, A.; Goodman, S.L.; Kessler, H. Titanium implant materials with improved biocompatibility through coating with phosphonate-anchored cyclic RGD peptides. ChemBioChem 2005, 6, 2034–2040. [Google Scholar] [CrossRef]
- Amalric, J.; Mutin, P.H.; Guerrero, G.; Ponche, A.; Sotto, A.; Lavigne, J.P. Phosphonate monolayers functionalized by silver thiolate species as antibacterial nanocoatings on titanium and stainless steel. J. Mater. Chem. 2009, 19, 141–149. [Google Scholar] [CrossRef]
- Hu, X.; Neoh, K.G.; Shi, Z.; Kang, E.T.; Poh, C.; Wang, W. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials 2010, 31, 8854–8863. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Pérez-Álvarez, L.; Pacha-Olivenza, M.Á.; Ruiz-Rubio, L.; Gartziandia, O.; Vilas-Vilela, J.L.; Alonso, J.M. Antibacterial catechol-based hyaluronic acid, chitosan and poly (N-vinyl pyrrolidone) coatings onto Ti6Al4V surfaces for application as biomedical implant. Int. J. Biol. Macromol. 2021, 183, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J. Minireviews 1, 3-Dipolar Cycloadditions of Azides and Alkynes: A Universal Ligation Tool in Polymer and Materials Science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Lahann, J. Click Chemistry for Biotechnology and Materials Science; John Wiley & Sons: Chichester, UK, 2009; ISBN 9780470699706. [Google Scholar]
- Chen, Y.; Tonfg, Z.-R. (Eds.) Click Chemistry: Approaches, Applications, and Challenges; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; ISBN 978-1-53611-903-9. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- He, R.; Hu, X.; Tan, H.C.; Feng, J.; Steffi, C.; Wang, K.; Wang, W. Surface modification of titanium with curcumin: A promising strategy to combat fibrous encapsulation. J. Mater. Chem. B 2015, 3, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Xiong, M.; Hu, G.; Zhan, J.; Li, T.; Wang, L.; Wang, Y. Antimicrobial Titanium Surface via Click-Immobilization of Peptide and Its in Vitro/Vivo Activity. ACS Biomater. Sci. Eng. 2019, 5, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Heijink, A.; Schwartz, J.; Zobitz, M.E.; Nicole Crowder, K.; Lutz, G.E.; Sibonga, J.D. Self-assembled monolayer films of phosphonates for bonding RGD to titanium. Clin. Orthop. Relat. Res. 2008, 466, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Rechenmacher, F.; Neubauer, S.; Mas-Moruno, C.; Dorfner, P.M.; Polleux, J.; Guasch, J.; Conings, B.; Boyen, H.G.; Bochen, A.; Sobahi, T.R.; et al. A molecular toolkit for the functionalization of titanium-based biomaterials that selectively control integrin-mediated cell adhesion. Chem.-A Eur. J. 2013, 19, 9218–9223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouirfa, H.; Evans, M.D.M.; Castner, D.G.; Bean, P.; Mercier, D.; Galtayries, A.; Falentin-Daudré, C.; Migonney, V. Grafting of architecture controlled poly(styrene sodium sulfonate) onto titanium surfaces using bio-adhesive molecules: Surface characterization and biological properties. Biointerphases 2017, 12, 02C418. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.A.; Lyskawa, J.; Zobrist, C.; Fournier, D.; Jimenez, M.; Traisnel, M.; Gengembre, L.; Woisel, P. A “clickable” titanium surface platform. Langmuir 2010, 26, 15920–15924. [Google Scholar] [CrossRef]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized therapeutic surface coatings for dental implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Mijangos, C.; Hernández, R. Polyelectrolyte multilayer films based on natural polymers: From fundamentals to Bio-Applications. Polymers 2021, 13, 2254. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-Based Delivery of Nucleic Acid Therapeutics for Enhanced Bone and Cartilage Repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Junter, G.A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, R.; Fu, Y.; Kao, W.J. Release Mechanisms and Applications of Drug Delivery Systems for Extended-Release. Expert Opin. Drug Deliv. 2020, 17, 1289–1304. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–84. [Google Scholar] [CrossRef]
- Del Olmo, J.A.; Alonso, J.M.; Martínez, V.S.; Ruiz-Rubio, L.; González, R.P.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible hyaluronic acid-divinyl sulfone injectable hydrogels for sustained drug release with enhanced antibacterial properties against Staphylococcus aureus. Mater. Sci. Eng. C 2021, 125, 112102. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef] [PubMed]
- Pavlukhina, S.; Sukhishvili, S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv. Drug Deliv. Rev. 2011, 63, 822–836. [Google Scholar] [CrossRef]
- Wang, X.; Venkatraman, S.S.; Boey, F.Y.C.; Loo, J.S.C.; Tan, L.P. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials 2006, 27, 5588–5595. [Google Scholar] [CrossRef]
- Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: A Review. Regen. Eng. Transl. Med. 2021, 7, 91–114. [Google Scholar] [CrossRef]
- Alhaique, F.; Casadei, M.A.; Cencetti, C.; Coviello, T.; Di Meo, C.; Matricardi, P.; Montanari, E.; Pacelli, S.; Paolicelli, P. From macro to nano polysaccharide hydrogels: An opportunity for the delivery of drugs. J. Drug Deliv. Sci. Technol. 2016, 32, 88–99. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Sáez, V.; Hernáez, E.; López, L. Liberación controlada de fármacos. Aplicaciones biomédicas. Rev. Iberoam. Polímeros 2003, 4, 111–122. [Google Scholar]
- Liu, J.; Lin, S.; Liu, X.; Qin, Z.; Yang, Y.; Zang, J.; Zhao, X. Fatigue-resistant adhesion of hydrogels. Nat. Commun. 2020, 11, 1071. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yin, Y.A.; Liu, Y.X.; Lin, L.; Liu, M.J. Design and fabrication of functional hydrogels through interfacial engineering. Chinese J. Polym. Sci. 2017, 35, 1181–1193. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Deng, Z.; Chen, Y.; Yu, Q.; Tang, W.; Sun, T.L.; Zhang, Y.S.; Yue, K. Tough Bonding, On-Demand Debonding, and Facile Rebonding between Hydrogels and Diverse Metal Surfaces. Adv. Mater. 2019, 31, 1904732. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, S.; Chen, K.; Wang, F.; Feng, C.; Xu, L.; Zhang, D. 3D printed chitosan-gelatine hydrogel coating on titanium alloy surface as biological fixation interface of artificial joint prosthesis. Int. J. Biol. Macromol. 2021, 182, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Soylu, H.M.; Chevallier, P.; Copes, F.; Ponti, F.; Candiani, G.; Yurt, F.; Mantovani, D. A Novel Strategy to Coat Dopamine-Functionalized Titanium Surfaces With Agarose-Based Hydrogels for the Controlled Release of Gentamicin. Front. Cell. Infect. Microbiol. 2021, 11, 678081. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Portillo Lara, R.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, F.S.; Volpe Bavuso, A.; Cusimano, M.G.; Pitarresi, G.; Giammona, G.; Schillaci, D. A polycarboxylic/amino functionalized hyaluronic acid derivative for the production of pH sensible hydrogels in the prevention of bacterial adhesion on biomedical surfaces. Int. J. Pharm. 2015, 478, 70–77. [Google Scholar] [CrossRef]
- Boot, W.; Gawlitta, D.; Nikkels, P.G.J.; Pouran, B.; van Rijen, M.H.P.; Dhert, W.J.A.; Vogely, H.C. Hyaluronic Acid-Based Hydrogel Coating Does Not Affect Bone Apposition at the Implant Surface in a Rabbit Model. Clin. Orthop. Relat. Res. 2017, 475, 1911–1919. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Han, J.J.; Park, Y.D.; Cho, T.H.; Hwang, S.J. Effect of sustained release of rhBMP-2 from dried and wet hyaluronic acid hydrogel carriers compared with direct dip coating of rhBMP-2 on peri-implant osteogenesis of dental implants in canine mandibles. J. Cranio-Maxillofac. Surg. 2016, 44, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does Implant Coating With Antibacterial-Loaded Hydrogel Reduce Bacterial Colonization and Biofilm Formation in Vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitarresi, G.; Palumbo, F.S.; Calascibetta, F.; Fiorica, C.; Di Stefano, M.; Giammona, G. Medicated hydrogels of hyaluronic acid derivatives for use in orthopedic field. Int. J. Pharm. 2013, 449, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Qin, X.; Barbeck, M.; Hou, Y.; Xu, H.; Liu, L.; Liu, C. Mussel-Inspired Carboxymethyl Chitosan Hydrogel Coating of Titanium Alloy with Antibacterial and Bioactive Properties. Materials 2021, 14, 6901. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, M.; Yang, C.; Yin, X.; Duan, K.; Wang, J.; Feng, B. Macrophage phenotype switch by sequential action of immunomodulatory cytokines from hydrogel layers on titania nanotubes. Colloids Surf. B Biointerfaces 2018, 163, 336–345. [Google Scholar] [CrossRef]
- Ordikhani, F.; Dehghani, M.; Simchi, A. Antibiotic-loaded chitosan–Laponite films for local drug delivery by titanium implants: Cell proliferation and drug release studies. J. Mater. Sci. Mater. Med. 2015, 26, 269. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, K.; Jiang, G.; Xiong, Y.; Du, Y.; Shi, X. Electrical signal guided ibuprofen release from electrodeposited chitosan hydrogel. Int. J. Polym. Sci. 2014, 2014, 736898. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- Yu, W.P.; Gong, Y.; Wang, Z.; Lu, C.; Ding, J.L.; Liu, X.L.; Zhu, G.D.; Lin, F.; Xu, J.J.; Zhou, J.L. The biofunctionalization of titanium nanotube with chitosan/genipin heparin hydrogel and the controlled release of IL-4 for anti-coagulation and anti-thrombus through accelerating endothelialization. RSC Adv. 2021, 11, 16510–16521. [Google Scholar] [CrossRef]
- Mishra, S.K.; Teotia, A.K.; Kumar, A.; Kannan, S. Mechanically tuned nanocomposite coating on titanium metal with integrated properties of biofilm inhibition, cell proliferation, and sustained drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 23–35. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, E.J.; Kim, H.E.; Jang, J.H.; Koh, Y.H. Silica-chitosan hybrid coating on Ti for controlled release of growth factors. J. Mater. Sci. Mater. Med. 2011, 22, 2757–2764. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Lee, E.J.; Han, C.M.; Won, J.E.; Knowles, J.C.; Kim, H.W. Tailoring solubility and drug release from electrophoretic deposited chitosan-gelatin films on titanium. Surf. Coatings Technol. 2014, 242, 232–236. [Google Scholar] [CrossRef]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.K.; Ke, C.J.; Lin, Y.W.; Lin, F.H.; Tsai, T.H.; Sun, J.S. Transglutaminase cross-linked gelatin-alginate-antibacterial hydrogel as the drug delivery-coatings for implant-related infections. Polymers 2021, 13, 414. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Zhang, Y.; Lin, Y. Controlled release of dopamine coatings on titanium bidirectionally regulate osteoclastic and osteogenic response behaviors. Mater. Sci. Eng. C 2021, 129, 112376. [Google Scholar] [CrossRef]
- Choi, J.; Konno, T.; Takai, M.; Ishihara, K. Smart controlled preparation of multilayered hydrogel for releasing bioactive molecules. Curr. Appl. Phys. 2009, 9, e259–e262. [Google Scholar] [CrossRef]
- Li, D.; Lv, P.; Fan, L.; Huang, Y.; Yang, F.; Mei, X.; Wu, D. The immobilization of antibiotic-loaded polymeric coatings on osteoarticular Ti implants for the prevention of bone infections. Biomater. Sci. 2017, 5, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Konno, T.; Takai, M.; Ishihara, K. Regulation of cell proliferation by multi-layered phospholipid polymer hydrogel coatings through controlled release of paclitaxel. Biomaterials 2012, 33, 954–961. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Ricci, M.A.; Cafagna, D.; Savino, A.M.; Sabbatini, L.; Orciani, M.; Ceci, E.; Novello, L.; Tantillo, G.M.; et al. Ciprofloxacin-modified electrosynthesized hydrogel coatings to prevent titanium-implant-associated infections. Acta Biomater. 2011, 7, 882–891. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Del Olmo, J.; Maiz-Fernández, S.; Valverde-De Mingo, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Recubrimientos poliméricos avanzados en base a técnicas de ensamblaje capa a capa. Rev. Plásticos Mod. 2018, 116, 5–13. [Google Scholar]
- Pahal, S.; Gakhar, R.; Raichur, A.M.; Varma, M.M. Polyelectrolyte multilayers for bio-applications: Recent advancements. IET Nanobiotechnol. 2017, 11, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, F.; Belbekhouche, S.; Oniszczuk, J.; Carbonnier, B. Smart Polyelectrolyte Multilayer Coatings for Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128167700. [Google Scholar]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; McShane, M.; Lvov, Y.M.; Ji, Q.; Hill, J.P. Layer-by-layer assembly for drug delivery and related applications. Expert Opin. Drug Deliv. 2011, 8, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Mann, J.K.; Kundu, S.C. Silk fibroin/gelatin multilayered films as a model system for controlled drug release. Eur. J. Pharm. Sci. 2009, 37, 160–171. [Google Scholar] [CrossRef]
- Loo, S.C.J.; Tan, Z.Y.S.; Chow, Y.J.; Lin, S.L.I. Drug Release From Irradiated PLGA and PLLA Multi-Layered Films. J. Pharm. Sci. 2010, 99, 3060–3071. [Google Scholar] [CrossRef]
- Valverde, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Pacha Olivenza, M.A.; García Blanco, M.B.; Díaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef]
- Yin, X.; Yang, C.; Wang, Z.; Zhang, Y.; Li, Y.; Weng, J.; Feng, B. Alginate/chitosan modified immunomodulatory titanium implants for promoting osteogenesis in vitro and in vivo. Mater. Sci. Eng. C 2021, 124, 112087. [Google Scholar] [CrossRef]
- Wu, C.; Shao, X.; Lin, X.; Gao, W.; Fang, Y.; Wang, J. Surface modification of titanium with collagen/hyaluronic acid and bone morphogenetic protein 2/7 heterodimer promotes osteoblastic differentiation. Dent. Mater. J. 2020, 39, 1072–1079. [Google Scholar] [CrossRef]
- Li, H.; Nie, B.; Zhang, S.; Long, T.; Yue, B. Immobilization of type I collagen/hyaluronic acid multilayer coating on enoxacin loaded titania nanotubes for improved osteogenesis and osseointegration in ovariectomized rats. Colloids Surf. B Biointerfaces 2019, 175, 409–420. [Google Scholar] [CrossRef]
- Song, Y.; Ma, A.; Ning, J.; Zhong, X.; Zhang, Q.; Zhang, X.; Hong, G.; Li, Y.; Sasaki, K.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int. J. Nanomed. 2018, 13, 6751–6767. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium surface priming with phase-transited lysozyme to establish a silver nanoparticle-loaded chitosan/hyaluronic acid antibacterial multilayer via layer-by-layer self-assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Wang, Y.; Zhang, J.; Zhao, S.; Yang, G. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci. Rep. 2015, 5, 16336. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Liu, M.; Li, N.; Zhang, L.; Meng, F.; Zhao, L.; Liu, M.; Zhang, Y. Chitosan-miRNA functionalized microporous titanium oxide surfaces via a layer-by-layer approach with a sustained release profile for enhanced osteogenic activity. J. Nanobiotechnol. 2020, 18, 127. [Google Scholar] [CrossRef]
- Tang, J.; Yan, D.; Chen, L.; Shen, Z.; Wang, B.; Weng, S.; Wu, Z.; Xie, Z.; Fang, K.; Hong, C.; et al. Enhancement of local bone formation on titanium implants in osteoporotic rats by biomimetic multilayered structures containing parathyroid hormone (PTH)-related protein. Biomed. Mater. 2020, 15, 045011. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Zustiak, S.P.; Simchi, A. Surface Modifications of Titanium Implants by Multilayer Bioactive Coatings with Drug Delivery Potential: Antimicrobial, Biological, and Drug Release Studies. Jom 2016, 68, 1100–1108. [Google Scholar] [CrossRef]
- Pérez-Anes, A.; Gargouri, M.; Laure, W.; Van Den Berghe, H.; Courcot, E.; Sobocinski, J.; Tabary, N.; Chai, F.; Blach, J.F.; Addad, A.; et al. Bioinspired Titanium Drug Eluting Platforms Based on a Poly-β-cyclodextrin-Chitosan Layer-by-Layer Self-Assembly Targeting Infections. ACS Appl. Mater. Interfaces 2015, 7, 12882–12893. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, L.; Shen, X.; Li, M.; Luo, Z.; Cai, K.; Hu, Y. Construction of multilayered molecular reservoirs on a titanium alloy implant for combinational drug delivery to promote osseointegration in osteoporotic conditions. Acta Biomater. 2020, 105, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Liu, C.; Feng, X.; Wei, L.; Shao, L. Self-assembly chitosan/gelatin composite coating on icariin-modified TiO2 nanotubes for the regulation of osteoblast bioactivity. Mater. Des. 2016, 92, 471–479. [Google Scholar] [CrossRef]
- Li, W.; Xu, D.; Hu, Y.; Cai, K.; Lin, Y. Surface modification of titanium substrates with silver nanoparticles embedded sulfhydrylated chitosan/gelatin polyelectrolyte multilayer films for antibacterial application. J. Mater. Sci. Mater. Med. 2014, 25, 1435–1448. [Google Scholar] [CrossRef]
- Lv, H.; Chen, Z.; Yang, X.; Cen, L.; Zhang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, Y.; Zhao, Y.; Yuan, Z.; Ding, Y.; Cai, K. Surface modification of titanium substrates for enhanced osteogenetic and antibacterial properties. Colloids Surf. B Biointerfaces 2017, 160, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, Y.; Yang, C.; Weng, J.; Wang, J.; Zhou, J.; Feng, B. Alginate/chitosan multilayer films coated on IL-4-loaded TiO2 nanotubes for modulation of macrophage phenotype. Int. J. Biol. Macromol. 2019, 133, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, L.; Han, L.; Wang, K.; Lu, X.; Fang, L.; Qu, S.; Chan, C.W. Self-assembled Biodegradable Nanoparticles and Polysaccharides as Biomimetic ECM Nanostructures for the Synergistic effect of RGD and BMP-2 on Bone Formation. Sci. Rep. 2016, 6, 25090. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Hu, Y.; Hou, Y.; Sun, Y.; Chen, M.; Li, M.; Tan, L.; Luo, Z.; Cai, K. Construction of a reactive oxygen species-responsive biomimetic multilayered titanium implant for in situ delivery of α-melanocyte-stimulating hormone to improve bone remolding in osteoporotic rats. Appl. Mater. Today 2021, 23, 101059. [Google Scholar] [CrossRef]
- de Avila, E.D.; Castro, A.G.B.; Tagit, O.; Krom, B.P.; Löwik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J.J.J.P. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Verza, B.S.; van den Beucken, J.J.J.P.; Brandt, J.V.; Jafelicci Junior, M.; Barão, V.A.R.; Piazza, R.D.; Tagit, O.; Spolidorio, D.M.P.; Vergani, C.E.; de Avila, E.D. A long-term controlled drug-delivery with anionic beta cyclodextrin complex in layer-by-layer coating for percutaneous implants devices. Carbohydr. Polym. 2021, 257, 117604. [Google Scholar] [CrossRef]
- Peterson, A.M.; Pilz-Allen, C.; Kolesnikova, T.; Möhwald, H.; Shchukin, D. Growth factor release from polyelectrolyte-coated titanium for implant applications. ACS Appl. Mater. Interfaces 2014, 6, 1866–1871. [Google Scholar] [CrossRef] [PubMed]

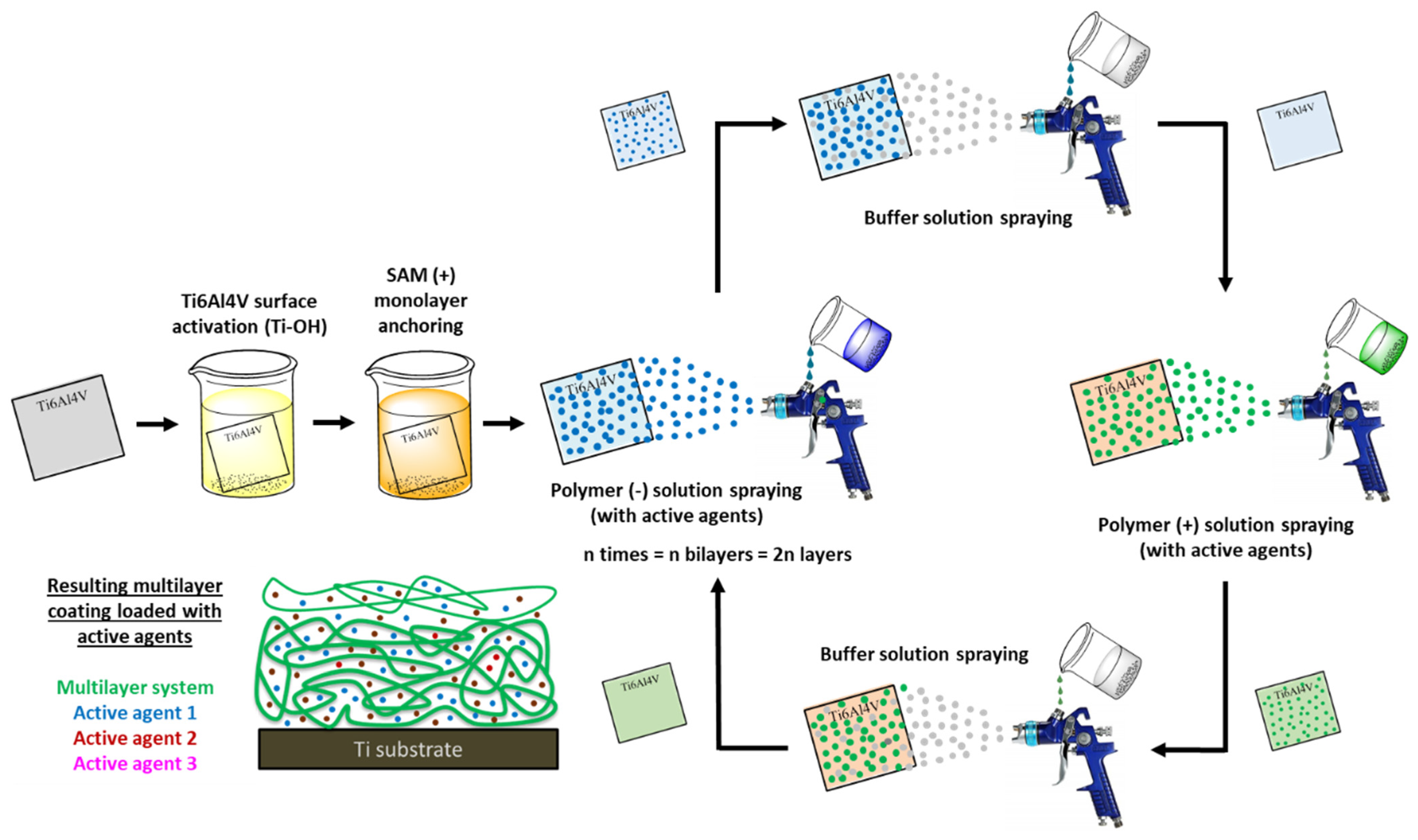
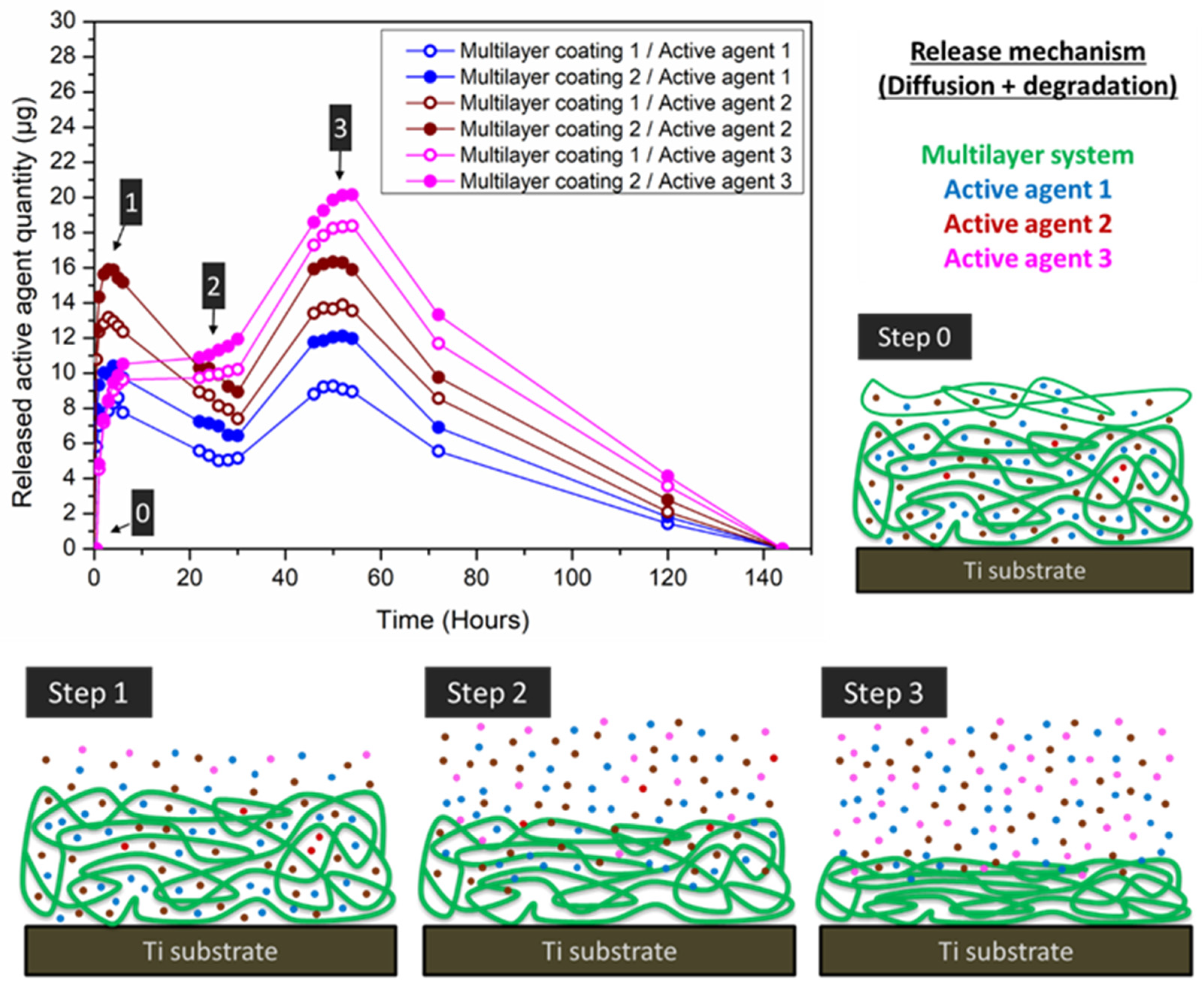
| Hydrogel Coating | Released Active Agent (s) | Biomedical Application | Reference |
|---|---|---|---|
| Polycarboxylic/amino functionalized hyaluronic acid | Vancomycin | Prevention of bacterial adhesion | [154] |
| Hyaluronic acid | Vancomycin | Enhancement of osseointegration | [155] |
| Recombinant human bone morphogenetic protein (rhBMG)-2 | Enhancement of peri-implant osteogenesis | [156] | |
| Hyaluronic acid and polylactic acid | Vancomycin Gentamicin Amikacin Tobramycin N-acetylcysteine Sodium salicylate | Enhancement of antibacterial properties | [157] |
| Vancomycin Tobramycin | Enhancement of antibacterial properties | [158] | |
| Carboxymethyl chitosan | Silver nanoparticles | Improve antibacterial and bioactive properties | [159] |
| Carboxymethyl chitosan and chitosan | Interleukin-4 (IL-4) and interferon-γ (IFN-γ) cytokines | Immunomodulation and anti-inflammatory properties | [160] |
| Chitosan | Vancomycin | Bone regeneration | [161] |
| Ibuprofen | Drug elution on conductive implants | [162] | |
| Ibuprofen | Controlled drug delivery system | [163] | |
| Interleukin-4 (IL-4) and heparin | Anti-inflammatory, anti-coagulation and anti-thrombus | [164] | |
| Silver nanoparticles and naproxen | Enhancement of antibacterial and anti-inflammatory properties | [165] | |
| Chitosan and silica xerogel | Fibroblast growth factor | Bioactivity enhancement | [166] |
| Chitosan and gelatin | Ampicillin | Tissue engineering | [167] |
| Gelatin | Antimicrobial peptide (AMP) and silicate nanoparticles | Prevention of infections and promotion of bone formation | [168] |
| Gelatin and alginate | Vancomycin Gentamicin | Reduction of implant-related infection | [169] |
| Alginate | Dopamine | Regulation of osteoclastic and osteogenic responses | [170] |
| Alginate and 4-vynilphenylboronic acid | Vascular endothelial growth factor (VEGF) | Local drug delivery system | [171] |
| Starch | Vancomycin | Prevention of bone infections | [172] |
| Polyvinyl alcohol (PVA) and phospholipid polymer (PMBV) | Paclitaxel | Anticancer therapy | [173] |
| poly(2-hydroxyethyl methacrylate) | Ciprofloxacin | Prevent implant associated infections | [174] |
| poly(ethylene–glycol diacrylate) and acrylic acid | Silver nanoparticles | Enhancement of antibacterial properties | [175] |
| Multilayer Coating | Released Active Agent (s) | Biomedical Application | Reference |
|---|---|---|---|
| Hyaluronic acid and collagen | Enoxacin | Improvement of osteogenesis and osseointegration | [186] |
| Hyaluronic acid and chitosan | Icariin | Improvement of osteogenesis | [187] |
| Silver nanoparticles | Prevention of implant associated infections | [188] | |
| Antimicrobial peptide-collagen | Long-term sustained antimicrobial activity | [189] | |
| microRNAs | Enhancement of osteogenic activity | [190] | |
| Hyaluronic acid and polylysine | Parathyroid hormone-related protein (PTHrP) | Enhancement of local bone formation | [191] |
| Chitosan and bioactive glass | Vancomycin | Prevent implant associated infections | [192] |
| Chitosan and β-cyclodextrin | Gentamicin | Enhancement of antibacterial properties | [193] |
| Calcitriol (VD3) | Promotion of osseointegration | [194] | |
| Chitosan and gelatin | Icariin | Regulation of osteoblast bioactivity | [195] |
| Silver nanoparticles | Enhancement of antibacterial properties | [196] | |
| Chitosan and alginate | Minocycline | Enhancement of antibacterial properties | [197] |
| Gentamycin | Improvement of bone osseointegration and reduction of bacterial infections | [198] | |
| Interleukin-4 (IL-4) cytokine | Modulation of macrophage phenotype for tissue repair | [199] | |
| Chitosan, alginate and bovine serum albumin (BSA) | Bone morphogenetic protein-2 (BMP-2) | Tissue engineering | [200] |
| Dextran and gelatin | A-melanocyte-stimulating hormone (α-MSH) | Improvement of bone remolding | [201] |
| Polyacrylic acid and poly-L-lysine | Tetracycline | Enhancement of antibacterial properties | [202] |
| Polyacrylic acid, poly-L-lysine and β-cyclodextrin | Tetracycline | Enhancement of antibacterial properties | [203] |
| Poly (methacrylic acid) and poly-L-histidine | Bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor (FGF) | Increase of bone growth | [204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bodón, J.; Andrade del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. https://doi.org/10.3390/polym14010165
Sánchez-Bodón J, Andrade del Olmo J, Alonso JM, Moreno-Benítez I, Vilas-Vilela JL, Pérez-Álvarez L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers. 2022; 14(1):165. https://doi.org/10.3390/polym14010165
Chicago/Turabian StyleSánchez-Bodón, Julia, Jon Andrade del Olmo, Jose María Alonso, Isabel Moreno-Benítez, José Luis Vilas-Vilela, and Leyre Pérez-Álvarez. 2022. "Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies" Polymers 14, no. 1: 165. https://doi.org/10.3390/polym14010165
APA StyleSánchez-Bodón, J., Andrade del Olmo, J., Alonso, J. M., Moreno-Benítez, I., Vilas-Vilela, J. L., & Pérez-Álvarez, L. (2022). Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers, 14(1), 165. https://doi.org/10.3390/polym14010165








