Hybrid Nanoparticles for Haloperidol Encapsulation: Quid Est Optimum?
Abstract
:1. Introduction
2. Materials
2.1. Preparation of the ZIF-8 Nanoparticles
2.2. Synthesis of Mesoporous Silica Nanoparticles
2.3. Preparation of Eudragit/Brij98 Nanoparticles with Loaded Haloperidol
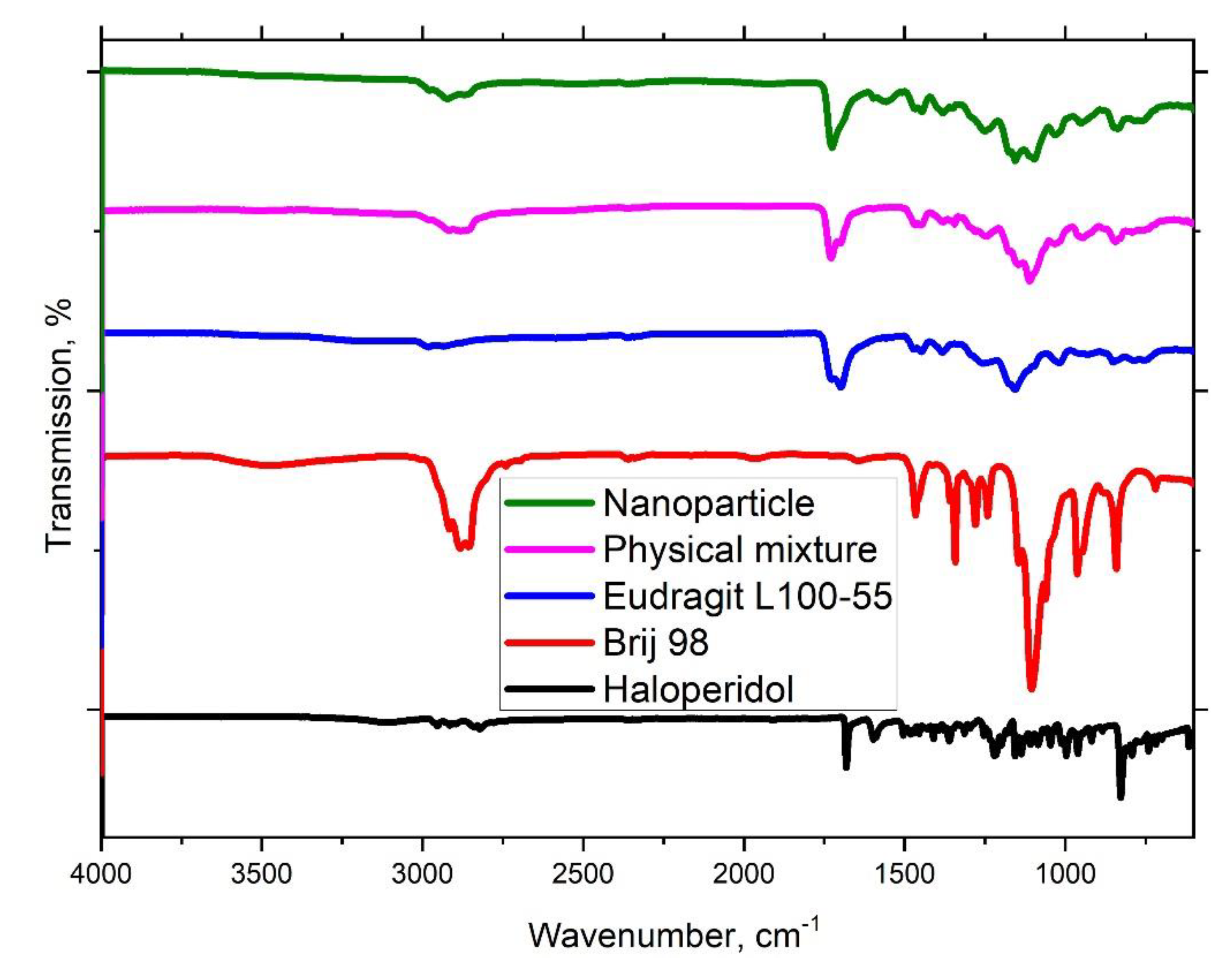
2.4. Physicochemical Characterisation
2.5. In Vivo Catalepsy Recording Test
2.5.1. Animals
2.5.2. Behavioural Techniques
3. Results and Discussions
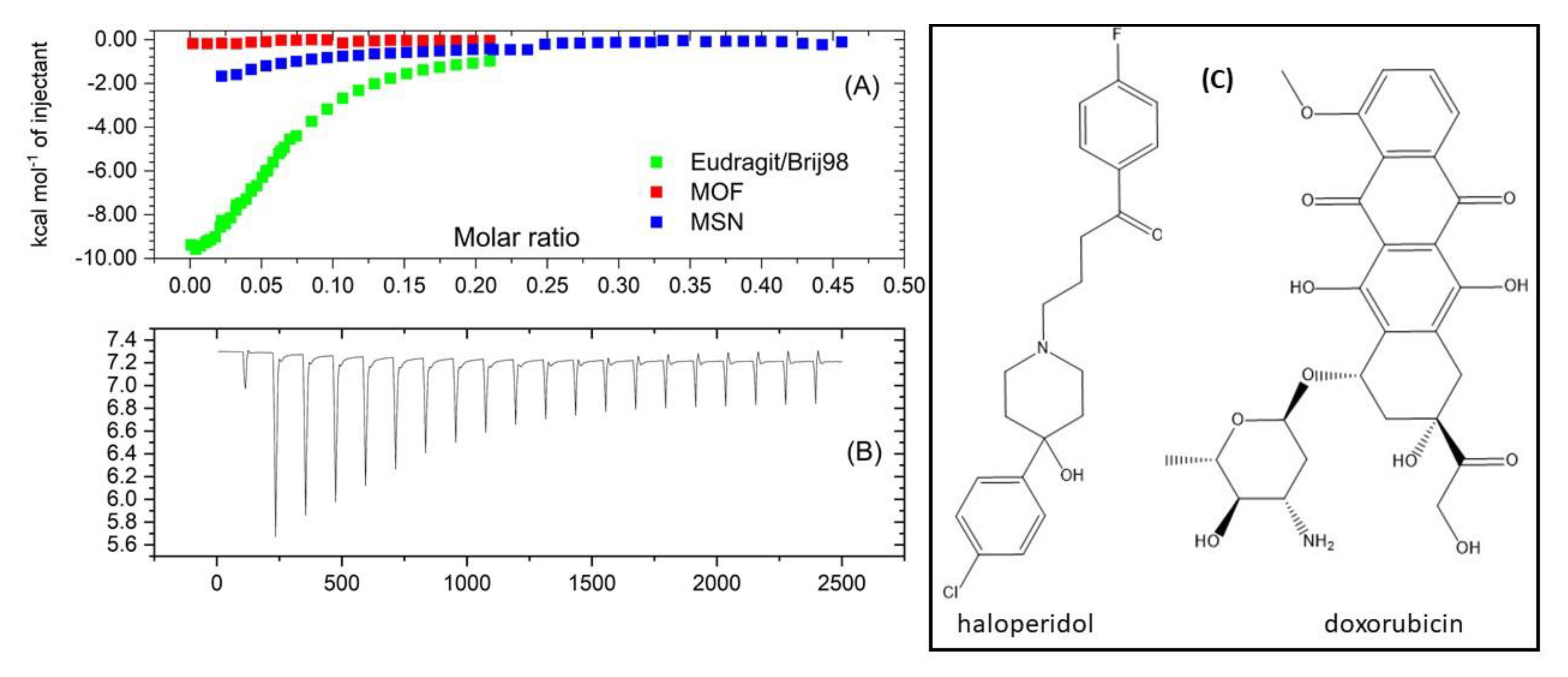
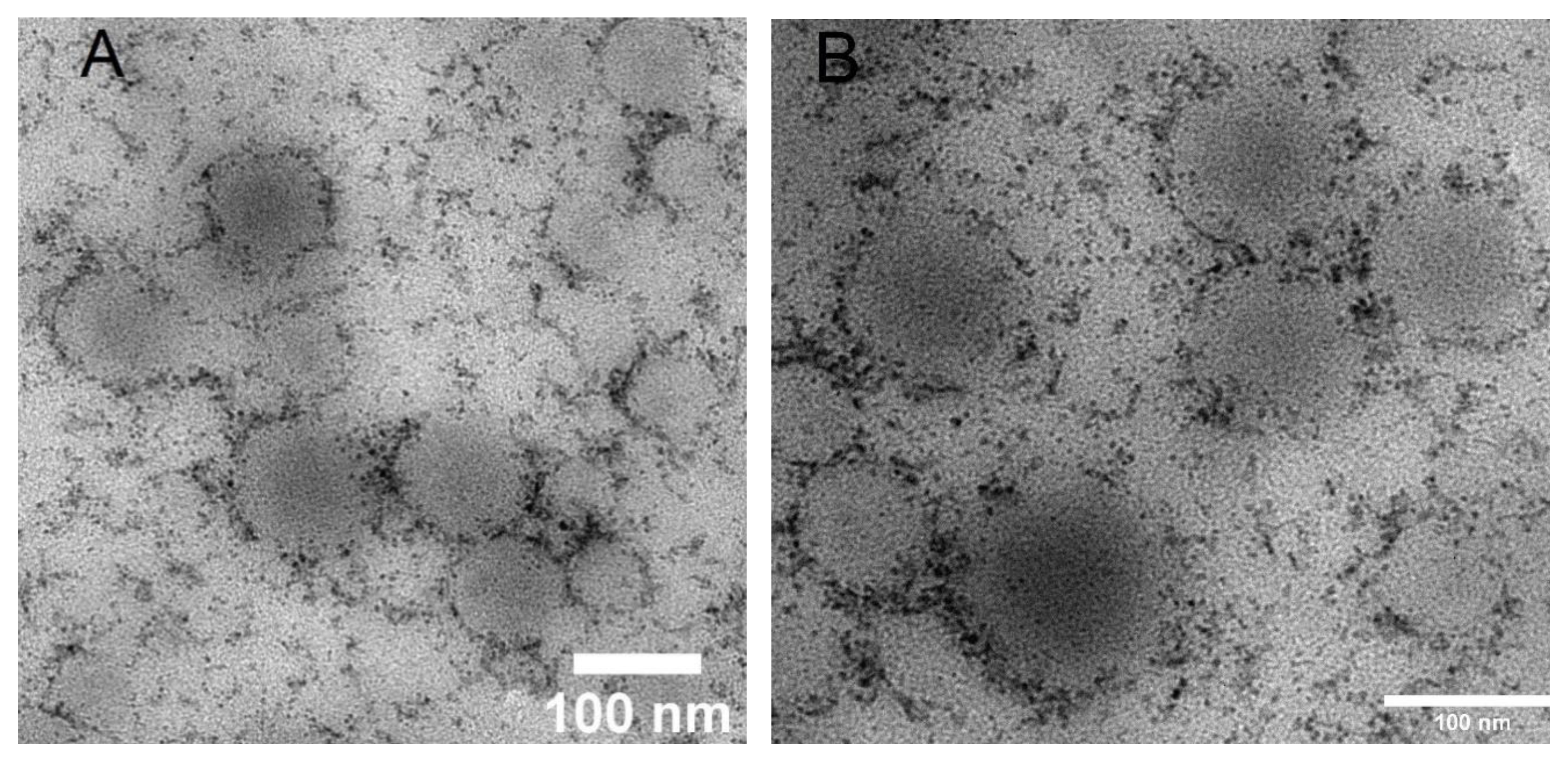
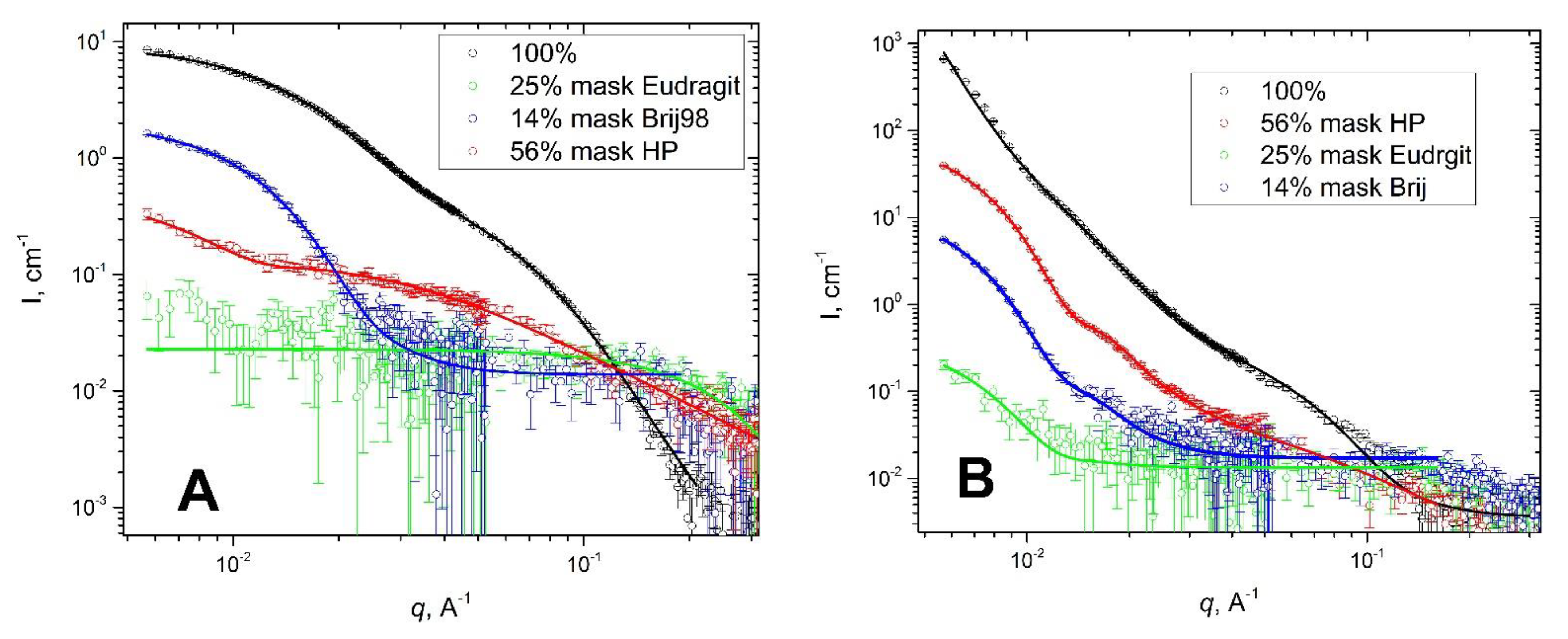
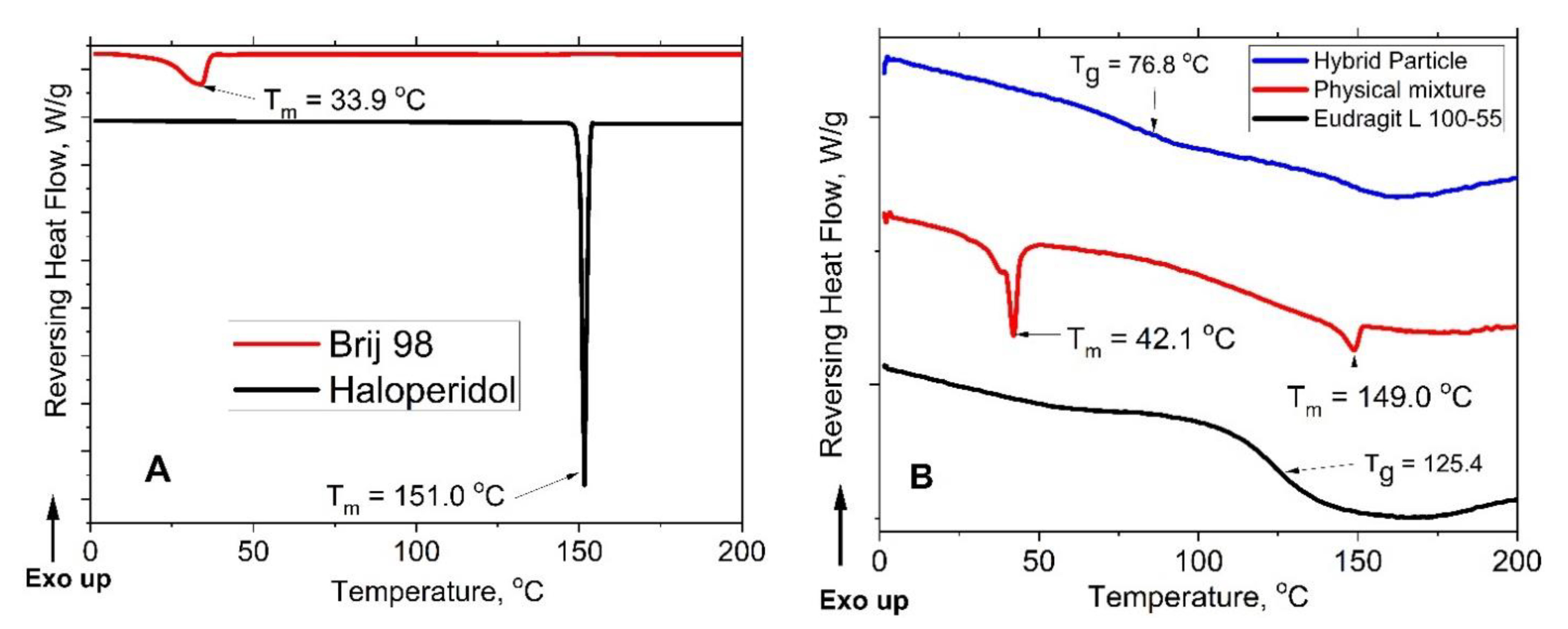
3.1. Haloperidol Loading
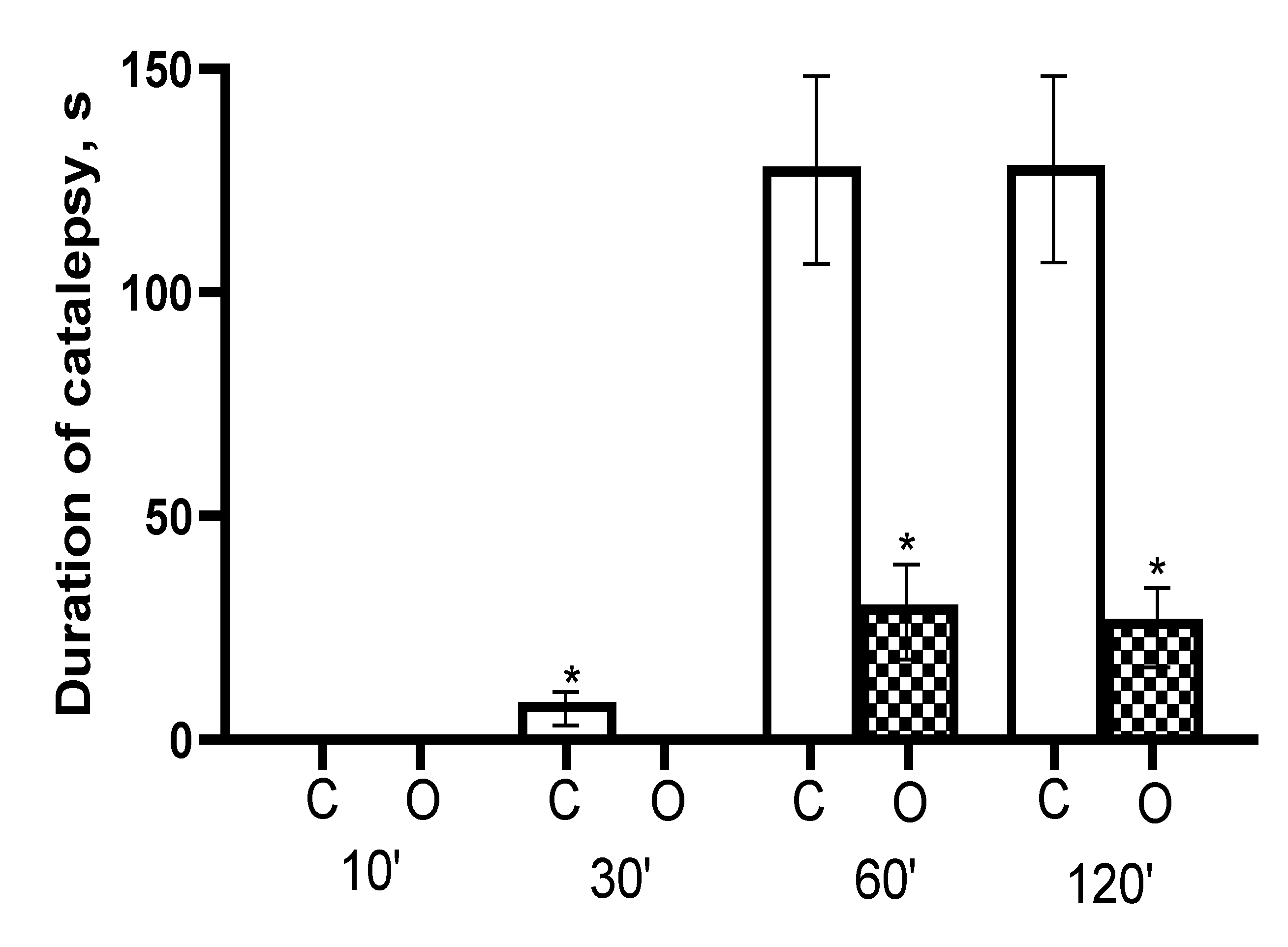
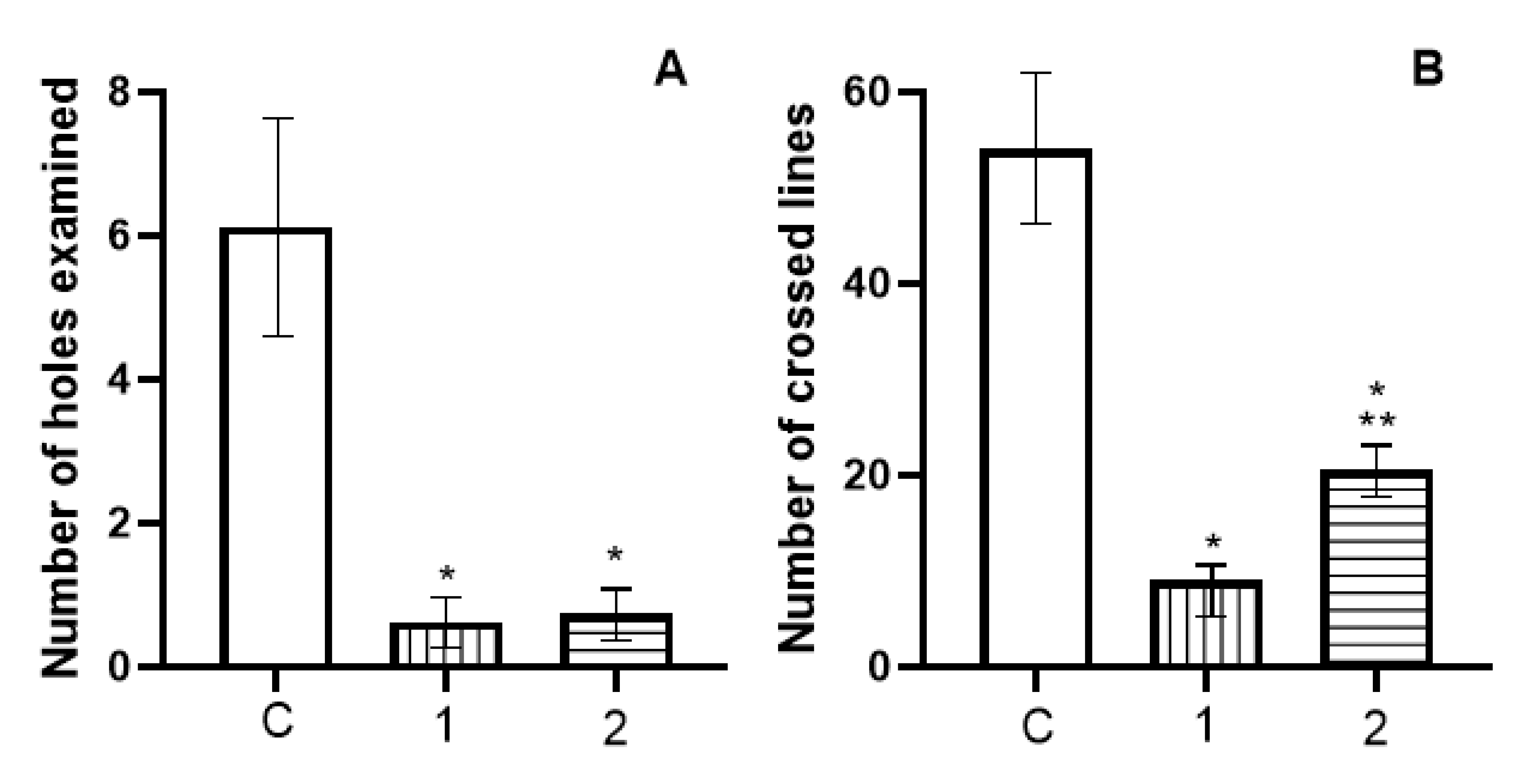
3.2. In Vivo Catalepsy Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, M.; Filippov, S.; Stepanek, P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? Eur. Polym. J. 2015, 65, 82–97. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, I.A.; Lázaro, S.A.; Forgan, R.S. Enhancing anticancer cytotoxicity through bimodal drug delivery from ultrasmall Zr MOF nanoparticles. Chem. Commun. 2018, 54, 2792–2795. [Google Scholar] [CrossRef] [Green Version]
- Wuttke, S.; Dietl, C.; Hinterholzinger, F.M.; Hintz, H.; Langhals, H.; Bein, T. Turn-on fluorescence triggered by selective internal dye replacement in MOFs. Chem. Commun. 2013, 50, 3599–3601. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Cho, M.S.; Lee, Y. Ferrocene-Encapsulated Zn Zeolitic Imidazole Framework (ZIF-8) for Optical and Electrochemical Sensing of Amyloid-β Oligomers and for the Early Diagnosis of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2019, 11, 11743–11748. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.A.; Wong-Foy, A.G.; Matzger, A.J. The Metal–Organic Framework Collapse Continuum: Insights from Two-Dimensional Powder X-ray Diffraction. Chem. Mater. 2018, 30, 6559–6565. [Google Scholar] [CrossRef]
- Shen, J.; Ma, M.; Zhang, H.; Yu, H.; Xue, F.; Hao, N.; Chen, H. Microfluidics-Assisted Surface Trifunctionalization of a Zeolitic Imidazolate Framework Nanocarrier for Targeted and Controllable Multitherapies of Tumors. ACS Appl. Mater. Interfaces 2020, 12, 45838–45849. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Javanbakht, S.; Baradaran, B.; Shahbazi, M.-A.; Hashemzaei, M.; Mokhtarzadeh, A.; Santos, H.A. Synthesis and therapeutic potential of stimuli-responsive metal-organic frameworks. Chem. Eng. J. 2020, 408, 127233. [Google Scholar] [CrossRef]
- Maleki, A.; Shahbazi, M.; Alinezhad, V.; Santos, H.A. The Progress and Prospect of Zeolitic Imidazolate Frameworks in Cancer Therapy, Antibacterial Activity, and Biomineralization. Adv. Health Mater. 2020, 9, e2000248. [Google Scholar] [CrossRef]
- Yan, J.; Liu, C.; Wu, Q.; Zhou, J.; Xu, X.; Zhang, L.; Wang, D.; Yang, F.; Zhang, H. Mineralization of pH-Sensitive Doxorubicin Prodrug in ZIF-8 to Enable Targeted Delivery to Solid Tumors. Anal. Chem. 2020, 92, 11453–11461. [Google Scholar] [CrossRef]
- Zhang, J.; Niemelä, M.; Westermarck, J.; Rosenholm, J.M. Mesoporous silica nanoparticles with redox-responsive surface linkers for charge-reversible loading and release of short oligonucleotides. Dalton Trans. 2014, 43, 4115–4126. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2018, 109, 1100–1111. [Google Scholar] [CrossRef]
- Pada, A.-K.; Desai, D.; Sun, K.; Govardhanam, N.P.; Törnquist, K.; Zhang, J.; Rosenholm, J.M. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. Int. J. Mol. Sci. 2019, 20, 3408. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Xu, X.; Zhou, J.; Liu, C.; Zhang, L.; Wang, D.; Yang, F.; Zhang, H. Fabrication of a pH/Redox-Triggered Mesoporous Silica-Based Nanoparticle with Microfluidics for Anticancer Drugs Doxorubicin and Paclitaxel Codelivery. ACS Appl. Bio Mater. 2020, 3, 1216–1225. [Google Scholar] [CrossRef]
- Xu, X.; Koivisto, O.; Liu, C.; Zhou, J.; Miihkinen, M.; Jacquemet, G.; Wang, D.; Rosenholm, J.M.; Shu, Y.; Zhang, H. Effective Delivery of the CRISPR/Cas9 System Enabled by Functionalized Mesoporous Silica Nanoparticles for GFP-Tagged Paxillin Knock-In. Adv. Ther. 2020. [Google Scholar] [CrossRef]
- Douroumis, D.; Onyesom, I.; Maniruzzaman, M.; Mitchell, J. Mesoporous silica nanoparticles in nanotechnology. Crit. Rev. Biotechnol. 2012, 33, 229–245. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Filippov, S.K.; Hruby, M.; Mackova, H.; Spirkova, M.; Stepanek, P. Novel pH-Responsive Nanoparticles. Langmuir 2008, 24, 9295–9301. [Google Scholar] [CrossRef]
- Filippov, S.K.; Starovoytova, L.; Koňák, C.; Hruby, M.; Macková, H.; Karlsson, G.; Stepanek, P. pH Sensitive Polymer Nanoparticles: Effect of Hydrophobicity on Self-Assembly. Langmuir 2010, 26, 14450–14457. [Google Scholar] [CrossRef]
- Bogomolova, A.; Filippov, S.K.; Starovoytova, L.; Angelov, B.; Konarev, P.; Sedlacek, O.; Hruby, M.; Stepanek, P. Study of Complex Thermosensitive Amphiphilic Polyoxazolines and Their Interaction with Ionic Surfactants. Are Hydrophobic, Thermosensitive, and Hydrophilic Moieties Equally Important? J. Phys. Chem. B 2014, 118, 4940–4950. [Google Scholar] [CrossRef]
- Filippov, S.K.; Bogomolova, A.Y.; Kaberov, L.; Velychkivska, N.; Starovoytova, L.; Cernochova, Z.; Rogers, S.E.; Lau, W.M.; Khutoryanskiy, V.V.; Cook, M.T. Internal Nanoparticle Structure of Temperature-Responsive Self-Assembled PNIPAM-b-PEG-b-PNIPAM Triblock Copolymers in Aqueous Solutions: NMR, SANS, and Light Scattering Studies. Langmuir 2016, 32, 5314–5323. [Google Scholar] [CrossRef] [Green Version]
- Sedlacek, O.; Filippov, S.; Svec, P.; Hruby, M. SET-LRP Synthesis of Well-Defined Light-Responsible Block Copolymer Micelles. Macromol. Chem. Phys. 2019, 220. [Google Scholar] [CrossRef]
- Duncan, R. Polymer therapeutics at a crossroads? Finding the path for improved translation in the twenty-first century. J. Drug Target. 2017, 25, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Vicent, M.J. Polymer therapeutics-prospects for 21st century: The end of the beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Sitenkov, A.Y.; Cascone, S.; Lamberti, G.; Barba, A.A.; Moustafine, R. Hydrophilic drug encapsulation in shell-core microcarriers by two stage polyelectrolyte complexation method. Int. J. Pharm. 2017, 518, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Porfiryeva, N.; Nasibullin, S.F.; Abdullina, S.G.; Tukhbatullina, I.K.; Moustafine, R.I.; Khutoryanskiy, V.V. Acrylated Eudragit® E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. Int. J. Pharm. 2019, 562, 241–248. [Google Scholar] [CrossRef]
- Timergalieva, V.R.; Gennari, C.G.M.; Cilurzo, F.; Moustafine, R.I. Interpolyelectrolyte complexes based on Carbopol and oppositely charged polymer as new carriers for oral controlled diclofenac delivery. Polym. Adv. Technol. 2021, 32, 2744–2752. [Google Scholar] [CrossRef]
- Porfiryeva, N.N.; Semina, I.I.; Salakhov, I.A.; Moustafine, R.I.; Khutoryanskiy, V.V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102432. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Song, J.G.; Lee, S.H.; Han, H.-K. Development of an M cell targeted nanocomposite system for effective oral protein delivery: Preparation, in vitro and in vivo characterization. J. Nanobiotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Cai, X.; Wang, X.; He, M.; Wang, Y.; Lan, M.; Zhao, Y.; Gao, F. Colon-targeted delivery of tacrolimus using pH-responsive polymeric nanoparticles for murine colitis therapy. Int. J. Pharm. 2021, 606, 120836. [Google Scholar] [CrossRef]
- Bukhovets, A.V.; Fotaki, N.; Khutoryanskiy, V.V.; Moustafine, R.I. Interpolymer Complexes of Eudragit® Copolymers as Novel Carriers for Colon-Specific Drug Delivery. Polymers 2020, 12, 1459. [Google Scholar] [CrossRef]
- Riabtseva, A.; Kaberov, L.I.; Kučka, J.; Bogomolova, A.; Stepanek, P.; Filippov, S.K.; Hruby, M. Polyelectrolyte pH-Responsive Protein-Containing Nanoparticles: The Physicochemical Supramolecular Approach. Langmuir 2017, 33, 764–772. [Google Scholar] [CrossRef]
- Harenberg, J.; Malsch, R. Eigenschaften und Analytik von Heparinoiden. Hämostaseologie 1996, 16, 1–5. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Suresh, S. Solubility behaviour of haloperidol in individual solvents determination of partial solubility parameters. Eur. J. Pharm. Biopharm. 1999, 47, 289–294. [Google Scholar] [CrossRef]
- De la Cruz-Moreno, M.P.; Montejo, C.; Aguilar-Ros, A.; Dewe, W.; Beck, B.; Stappaerts, J.; Tack, J.; Augustijns, P. Exploring drug solubility in fasted human intestinal fluid aspirates: Impact of inter-individual variability, sampling site and dilution. Int. J. Pharm. 2017, 528, 471–484. [Google Scholar] [CrossRef]
- Patel, N.G.; Serajuddin, A.T. Development of FDM 3D-printed tablets with rapid drug release, high drug-polymer miscibility and reduced printing temperature by applying the acid-base supersolubilization (ABS) principle. Int. J. Pharm. 2021, 600, 120524. [Google Scholar] [CrossRef]
- Katare, Y.K.; Daya, R.P.; Gray, C.S.; Luckham, R.E.; Bhandari, J.; Chauhan, A.S.; Mishra, R.K. Brain Targeting of a Water Insoluble Antipsychotic Drug Haloperidol via the Intranasal Route Using PAMAM Dendrimer. Mol. Pharm. 2015, 12, 3380–3388. [Google Scholar] [CrossRef] [Green Version]
- Piazza, J.; Hoare, T.; Molinaro, L.; Terpstra, K.; Bhandari, J.; Selvaganapathy, P.R.; Gupta, B.; Mishra, R.K. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)–block-poly(d,l)-lactic-co-glycolic acid (PEG–PLGA) nanoparticles for the treatment of schizophrenia. Eur. J. Pharm. Biopharm. 2014, 87, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Natfji, A.A.; Nikitin, D.O.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V.; Lin, H.; Stephens, G.J.; Watson, K.A.; Osborn, H.M.; Greco, F. Conjugation of haloperidol to PEG allows peripheral localisation of haloperidol and eliminates CNS extrapyramidal effects. J. Control. Release 2020, 322, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Vargas, C.; Zhao, H.; Piszczek, G.; Brautigam, C.A.; Schuck, P. High-Precision Isothermal Titration Calorimetry with Automated Peak-Shape Analysis. Anal. Chem. 2012, 84, 5066–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheuermann, T.H.; Brautigam, C.A. High-precision, automated integration of multiple isothermal titration calorimetric thermograms: New features of NITPIC. Methods 2014, 76, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.; Mata, J.; Garvey, C.J.; Wu, C.-M.; Hamilton, W.A.; Abbeywick, P.; Bartlett, D.; Bartsch, F.; Baxter, P.; Booth, N.; et al. QUOKKA, the pinhole small-angle neutron scattering instrument at the OPAL Research Reactor, Australia: Design, performance, operation and scientific highlights. J. Appl. Crystallogr. 2018, 51, 294–314. [Google Scholar] [CrossRef]
- Kline, S.R. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- Phattanarudee, S.; T.J., M.; Towiwat, P. Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, ISBN 9780128003756. [Google Scholar]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Feng, Y.; Bai, J.; Liu, Z.; Zhao, X. Optimized mesoporous silica nanoparticle-based drug delivery system with removable manganese oxide gatekeeper for controlled delivery of doxorubicin. J. Colloid Interface Sci. 2021, 592, 227–236. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, M.; Yuan, P.; Liu, T.; Tian, R.; Bai, Y.; Zhang, Y.; Chen, X. The facile formation of hierarchical mesoporous silica nanocarriers for tumor-selective multimodal theranostics. Biomater. Sci. 2021, 9, 5237–5246. [Google Scholar] [CrossRef]
- Frickenstein, A.; Hagood, J.; Britten, C.; Abbott, B.; McNally, M.; Vopat, C.; Patterson, E.; MacCuaig, W.; Jain, A.; Walters, K.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef]
- Ahmed, A.; Karami, A.; Sabouni, R.; Husseini, G.A.; Paul, V. pH and Ultrasound Dual-responsive Drug Delivery System Based On PEG–folate-functionalized Iron-based Metal–Organic Framework for Targeted Doxorubicin Delivery. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127062. [Google Scholar] [CrossRef]
- Li, X.; Porcino, M.; Qiu, J.; Constantin, D.; Martineau-Corcos, C.; Gref, R. Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy. Nanomaterials 2021, 11, 945. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Haris, M.H.; Ghadiri, A.M.; Moghaddam, F.M.; Fatahi, Y.; Dinarvand, R.; Jarahiyan, A.; Ahmadi, S.; Shokouhimehr, M. Polymer-Coated NH2-UiO-66 for the Codelivery of DOX/pCRISPR. ACS Appl. Mater. Interfaces 2021, 13, 10796–10811. [Google Scholar] [CrossRef]
- Sedlak, M.; Falus, P.; Steinhart, M.; Gummel, J.; Stepanek, P.; Filippov, S.K. Temperature-Induced Formation of Polymeric Nanoparticles: In Situ SAXS and QENS Experiments. Macromol. Chem. Phys. 2013, 214, 2841–2847. [Google Scholar] [CrossRef]
- Shan, X.; Williams, A.C.; Khutoryanskiy, V.V. Polymer structure and property effects on solid dispersions with haloperidol: Poly(N-vinyl pyrrolidone) and poly(2-oxazolines) studies. Int. J. Pharm. 2020, 590, 119884. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Sitenkov, A.Y.; Bukhovets, A.V.; Nasibullin, S.F.; Appeltans, B.; Kabanova, T.V.; Khutoryanskiy, V.; Mooter, G.V.D. Indomethacin-containing interpolyelectrolyte complexes based on Eudragit® E PO/S 100 copolymers as a novel drug delivery system. Int. J. Pharm. 2017, 524, 121–133. [Google Scholar] [CrossRef]
- Hauptstein, S.; Bonengel, S.; Rohrer, J.; Bernkop-Schnürch, A. Preactivated thiolated poly (methacrylic acid-co-ethyl acrylate): Synthesis and evaluation of mucoadhesive potential. Eur. J. Pharm. Sci. 2014, 63, 132–139. [Google Scholar] [CrossRef]
- England, R.J.A.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. 1999, 24, 67–68. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippov, S.K.; Khusnutdinov, R.R.; Inham, W.; Liu, C.; Nikitin, D.O.; Semina, I.I.; Garvey, C.J.; Nasibullin, S.F.; Khutoryanskiy, V.V.; Zhang, H.; et al. Hybrid Nanoparticles for Haloperidol Encapsulation: Quid Est Optimum? Polymers 2021, 13, 4189. https://doi.org/10.3390/polym13234189
Filippov SK, Khusnutdinov RR, Inham W, Liu C, Nikitin DO, Semina II, Garvey CJ, Nasibullin SF, Khutoryanskiy VV, Zhang H, et al. Hybrid Nanoparticles for Haloperidol Encapsulation: Quid Est Optimum? Polymers. 2021; 13(23):4189. https://doi.org/10.3390/polym13234189
Chicago/Turabian StyleFilippov, Sergey K., Ramil R. Khusnutdinov, Wali Inham, Chang Liu, Dmitry O. Nikitin, Irina I. Semina, Christopher J. Garvey, Shamil F. Nasibullin, Vitaliy V. Khutoryanskiy, Hongbo Zhang, and et al. 2021. "Hybrid Nanoparticles for Haloperidol Encapsulation: Quid Est Optimum?" Polymers 13, no. 23: 4189. https://doi.org/10.3390/polym13234189
APA StyleFilippov, S. K., Khusnutdinov, R. R., Inham, W., Liu, C., Nikitin, D. O., Semina, I. I., Garvey, C. J., Nasibullin, S. F., Khutoryanskiy, V. V., Zhang, H., & Moustafine, R. I. (2021). Hybrid Nanoparticles for Haloperidol Encapsulation: Quid Est Optimum? Polymers, 13(23), 4189. https://doi.org/10.3390/polym13234189









