Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aquilaria crassna Wood (ACW) Powder Preparation
2.3. Preparation of Aquilaria crassna Wood Dispersion
2.4. Air-Dried Sheet Preparation
2.5. Preparation of Aquilaria crassna Wood-Filled Rubber Sheet
2.6. Preparation of NR Thin Films
2.7. Characterization
2.7.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.7.2. Analysis of ACW
2.7.3. Characterization of Air-Dried Sheet (ADS)
2.7.4. Gel Content
2.7.5. Measurement of Initial Plasticity
2.7.6. Plasticity Retention Index (PRI) Determination
2.7.7. Mooney Viscosity
2.7.8. Tensile Properties
2.7.9. Antifungal Performance
3. Results and Discussion
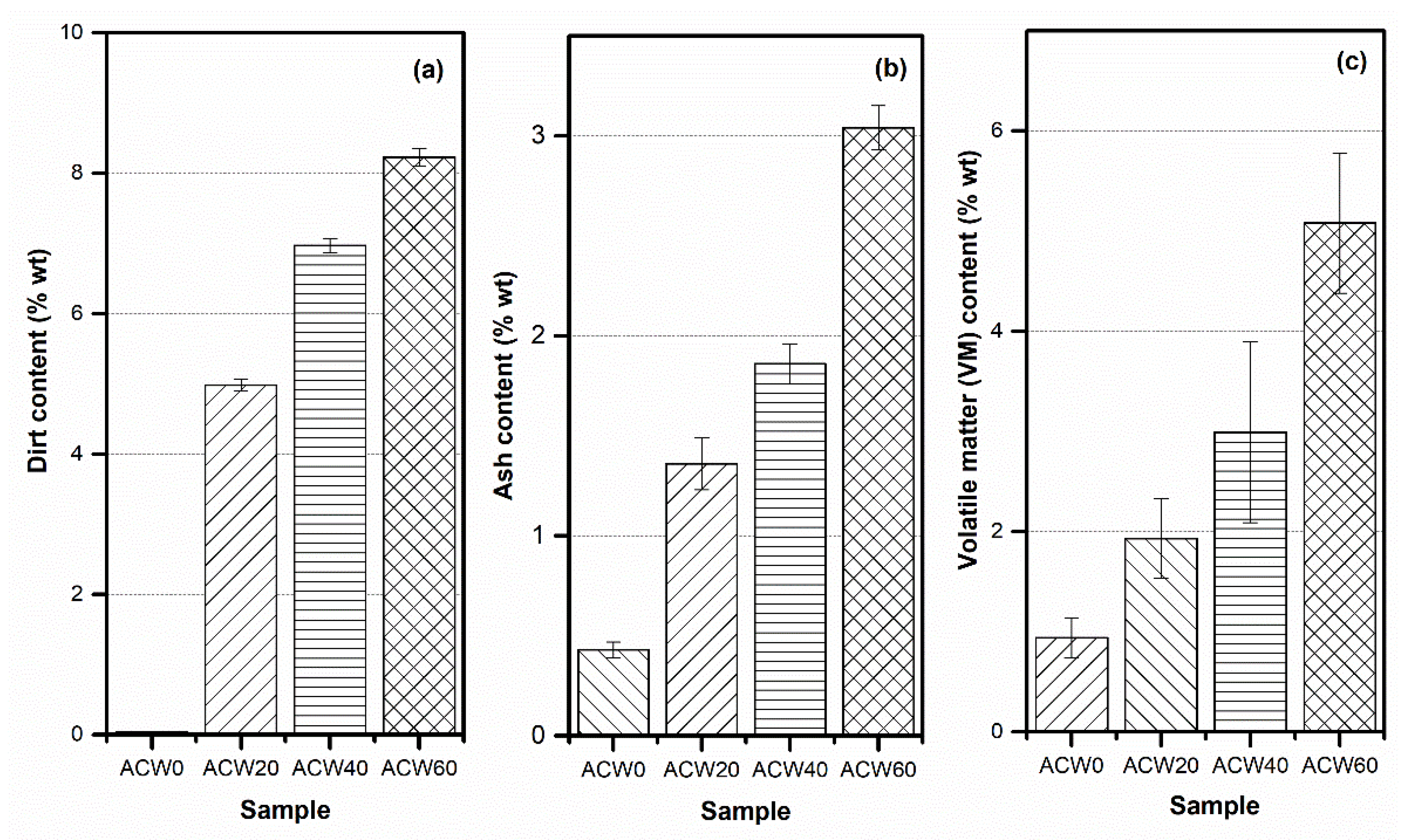
3.1. Characterization of the ACW Powder
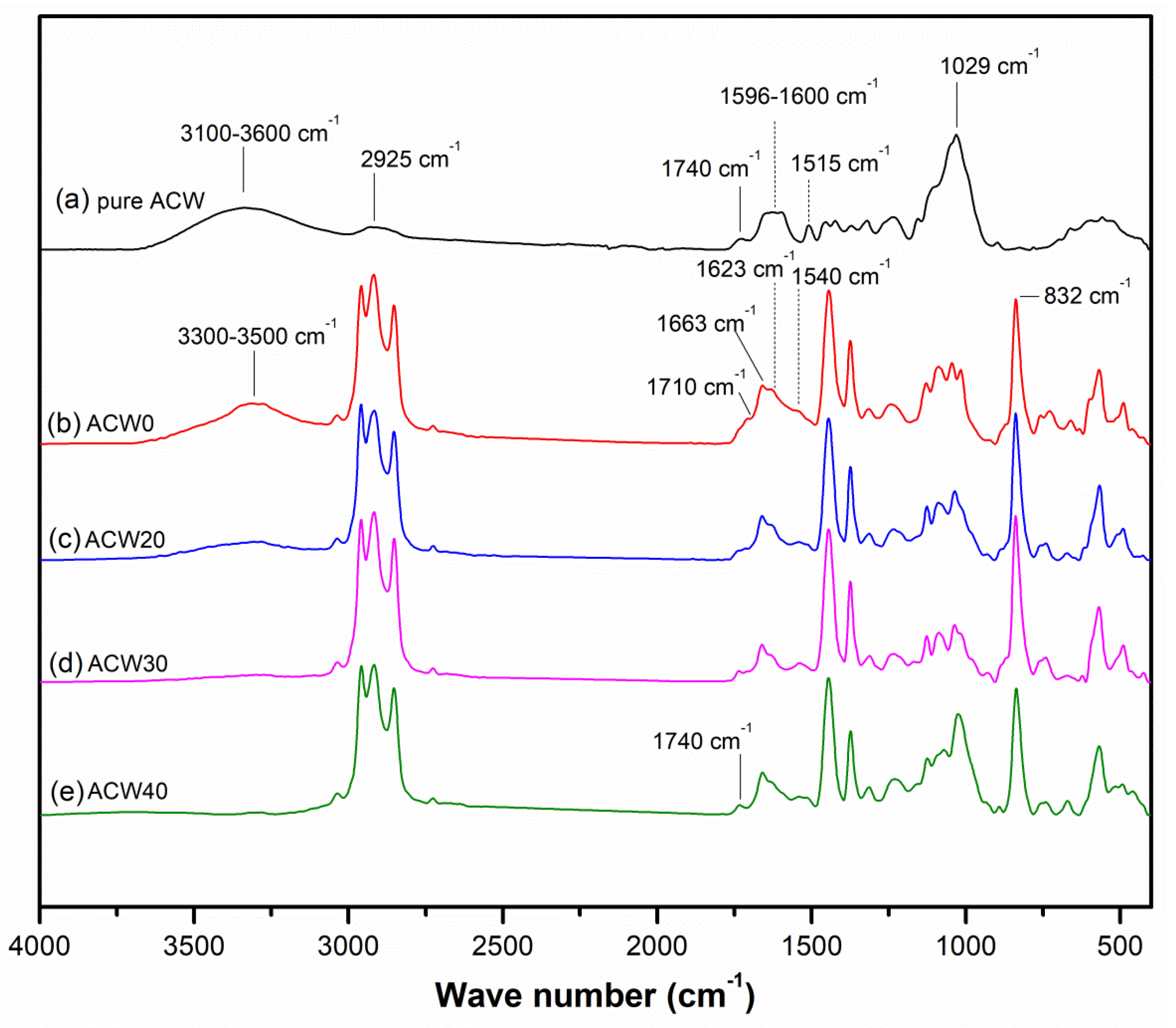
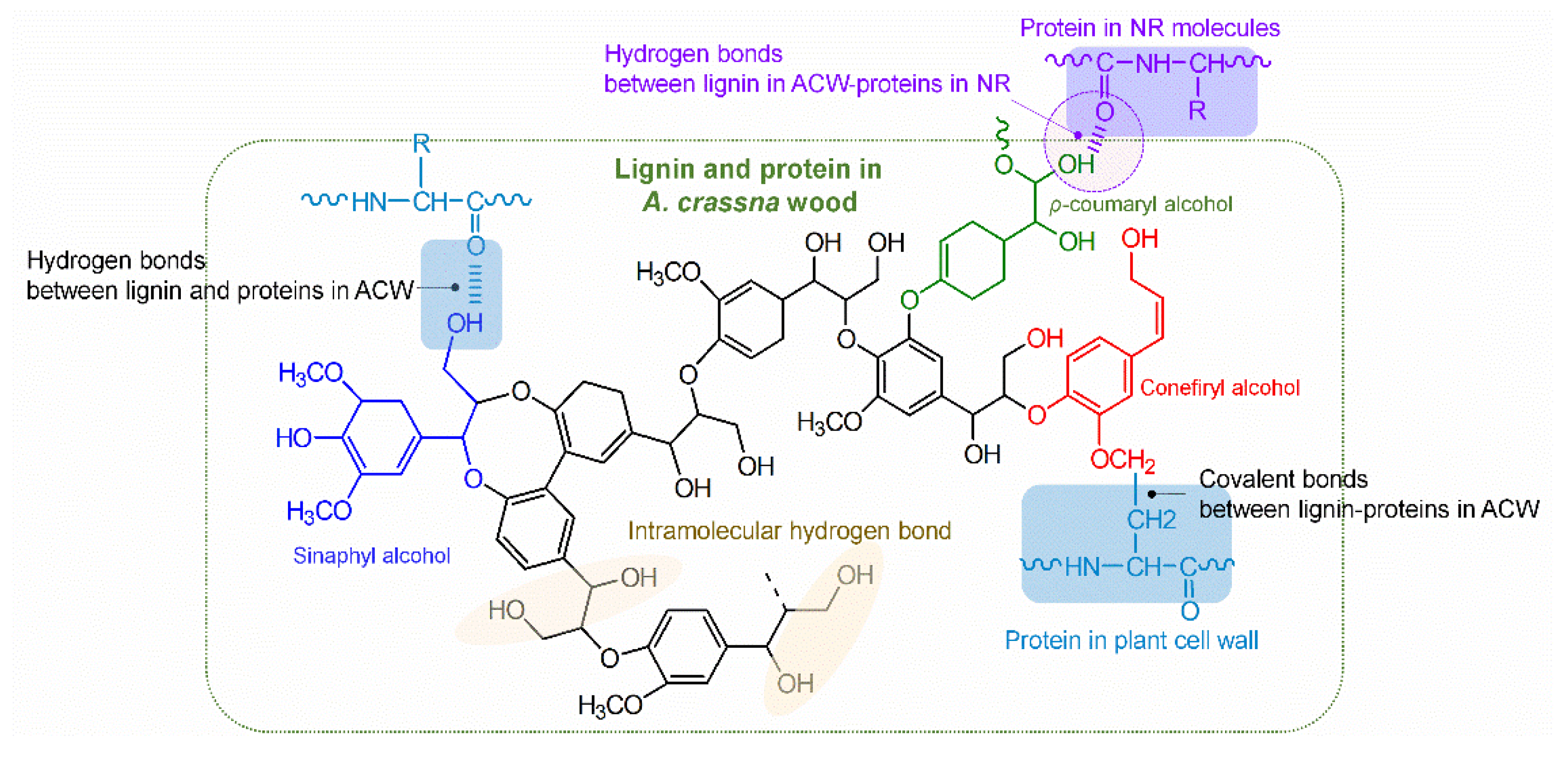
3.2. FT–IR Spectra
3.3. Physical Properties of Raw NR
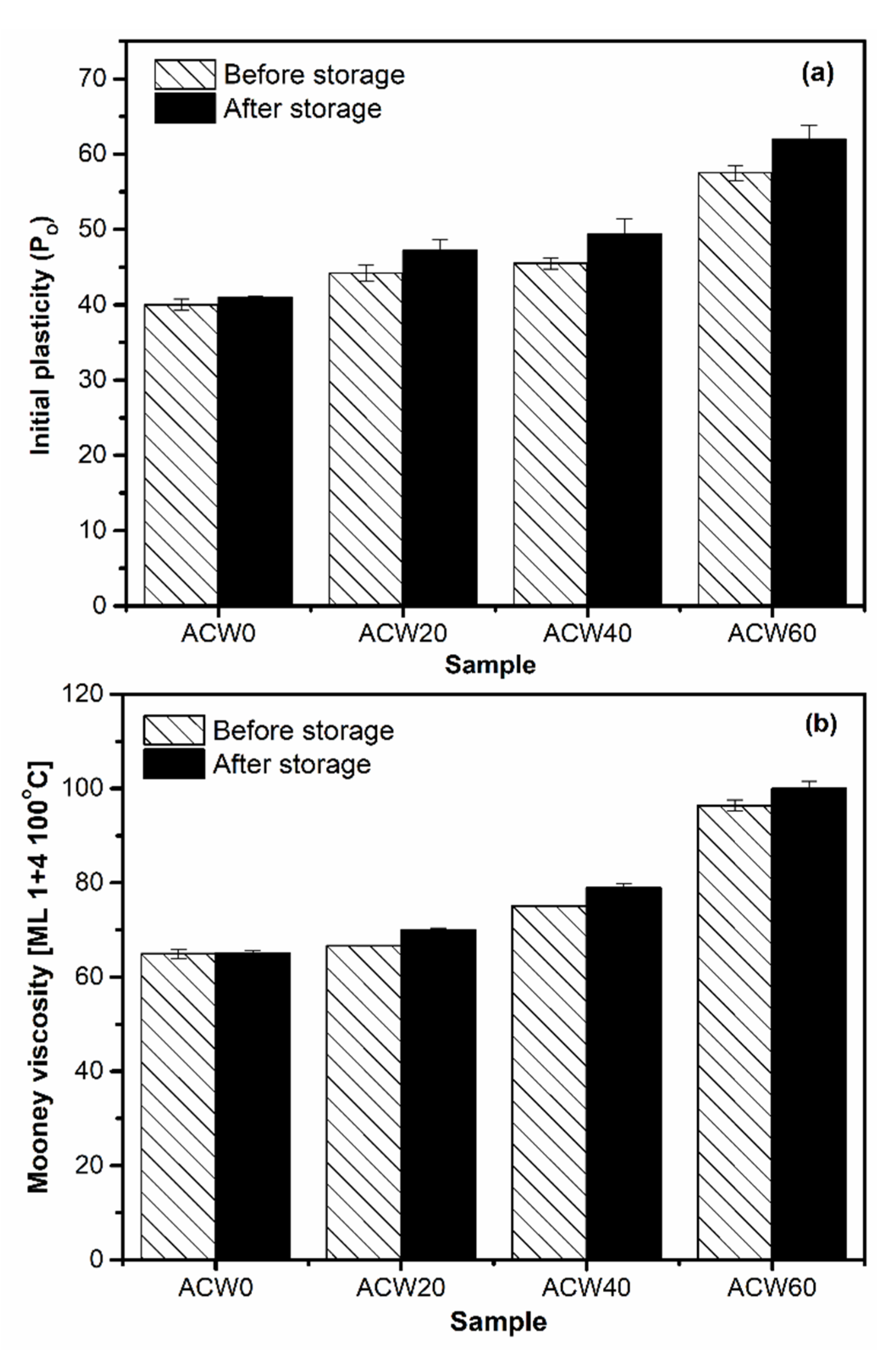
3.4. Plasticity of Rubber
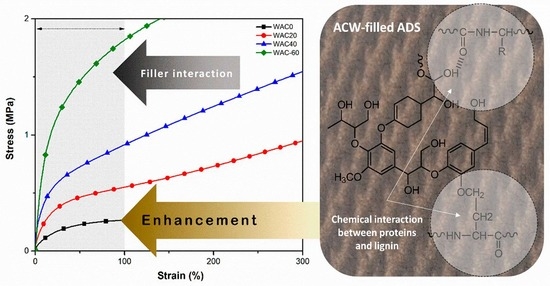
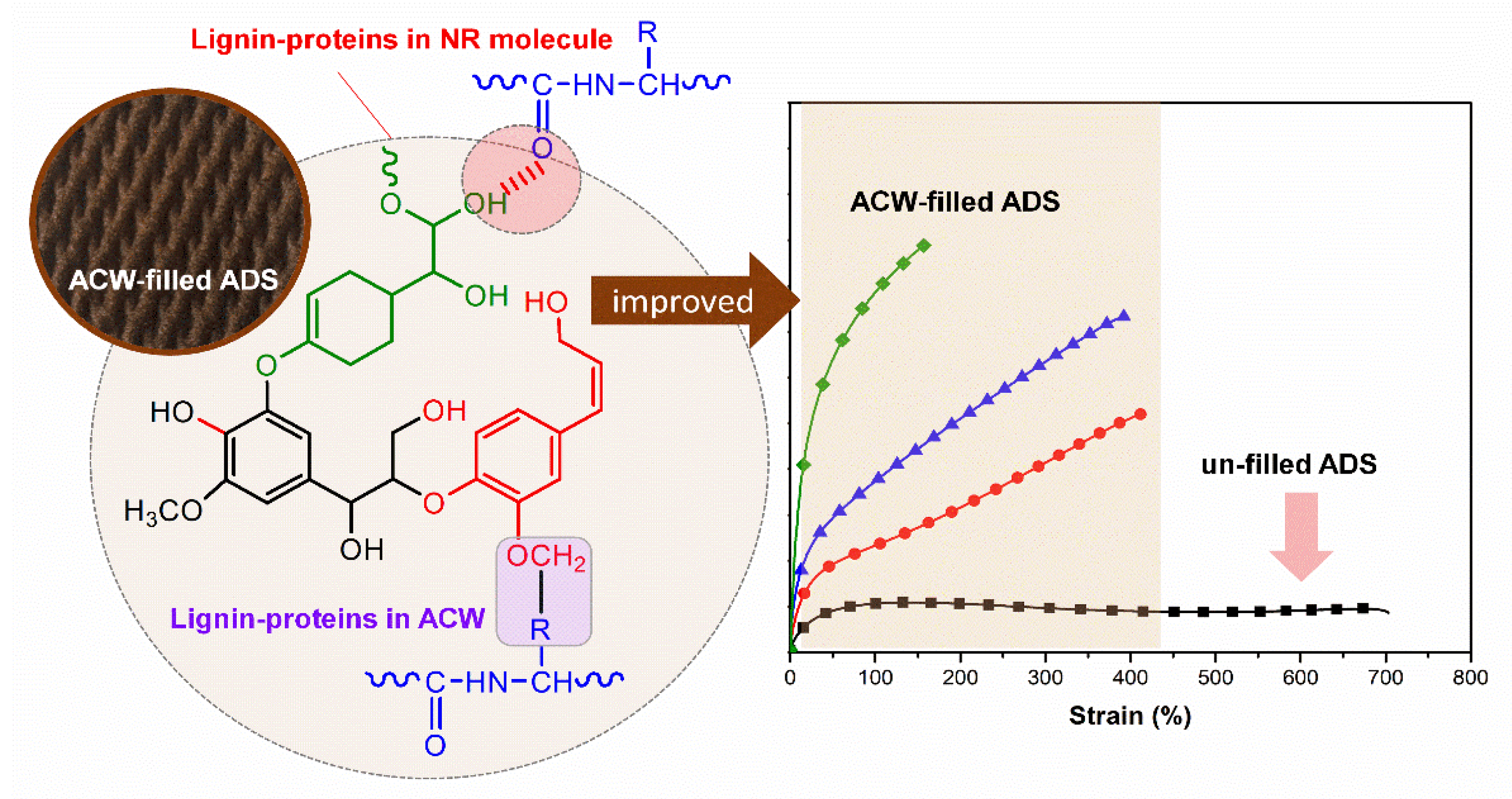
3.5. Tensile Properties
3.6. Antifungal Performance of the Rubber Sheet
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amnuaypornsri, S.; Sakdapipanich, J.; Toki, S.; Hsiao, B.S.; Ichikawa, N.; Tanaka, Y. Strain-induced crystallization of natural rubber: Effect of proteins and phospholipids. Rubber Chem. Technol. 2008, 81, 753–766. [Google Scholar] [CrossRef]
- Limhengha, S.; Limnararat, S.; Sriseubsai, W. Effect of ENR50/STR5L Blends on Properties of Foodstuff Conveyor Belts Compound. Key Eng. Mater. 2016, 701, 243–249. [Google Scholar] [CrossRef]
- Prasertsit, K.; Rattanawan, N.; Ratanapisit, J. Effects of wood vinegar as an additive for natural rubber products. Songklanakarin J. Sci. Technol. 2011, 33, 425–430. [Google Scholar]
- Chungsiriporn, J.; Pongyeela, P.; Iewkittayakorn, J. Use of wood vinegar as fungus and malodor retarding agent for natural rubber products. Songklanakarin J. Sci. Technol. 2018, 40, 87–92. [Google Scholar]
- Kalasee, W.; Dangwilailux, P. Effect of Wood Vinegar Substitutes on Acetic Acid for Coagulating Natural Para Rubber Sheets during the Drying Process. Appl. Sci. 2021, 11, 7891. [Google Scholar] [CrossRef]
- Cademartori, P.H.G.; dos Santos, P.S.; Serrano, L.; Labidi, J.; Gatto, D.A. Effect of thermal treatment on physicochemical properties of Gympie messmate wood. Ind. Crops Prod. 2013, 45, 360–366. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.-H.; Guo, Z.-K.; Wang, H.; Zuo, W.-J.; Dong, W.-H.; Mei, W.-L.; Dai, H.-F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Templeton, M.D.; Lamb, C.J. Specific localization of a plant cell wall glycine-rich protein in protoxylem cells of the vascular system. Proc. Natl. Acad. Sci. USA 1989, 86, 1529–1533. [Google Scholar] [CrossRef] [Green Version]
- Martius, C. Density, humidity, and nitrogen content of dominant wood species of floodplain forests (várzea) in Amazonia. Holz Als Roh-Und Werkst. 1992, 50, 300–303. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Paoli, G.D.; Peart, D.R.; Leighton, M.; Samsoedin, I. An ecological and economic assessment of the nontimber forest product gaharu wood in Gunung Palung National Park, West Kalimantan, Indonesia. Conserv. Biol. 2001, 15, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Parcheta, P.; Surówka, J. Softwood-lignin/natural rubber composites containing novel plasticizing agent: Preparation and characterization. Ind. Crops Prod. 2017, 95, 675–685. [Google Scholar] [CrossRef]
- Ehabe, E.; Ngolemasango, F.; Bonfils, F.; Sainte-Beuve, J. Precision associated with determination of dirt content of natural rubber. J. Appl. Polym. Sci. 2001, 81, 957–962. [Google Scholar] [CrossRef]
- Giraldo-Vásquez, D.H.; Velásquez-Restrepo, S.M. Variation of technological properties of field natural rubber lattices from Hevea brasiliensis clones and natural rubber-based compounds. Dyna 2017, 84, 80–87. [Google Scholar] [CrossRef]
- Vimalasiri, P.; Tillekeratne, L.; Weeraman, S.; Dekumpitiya, A. A rapid and accurate method for determining the volatile matter content of raw natural rubber. Polym. Test. 1987, 7, 317–323. [Google Scholar] [CrossRef]
- Nun-anan, P.; Wisunthorn, S.; Pichaiyut, S.; Vennemann, N.; Kummerlöwe, C.; Nakason, C. Influence of alkaline treatment and acetone extraction of natural rubber matrix on properties of carbon black filled natural rubber vulcanizates. Polym. Test. 2020, 89, 106623. [Google Scholar] [CrossRef]
- Baimark, Y.; Niamsa, N. Study on wood vinegars for use as coagulating and antifungal agents on the production of natural rubber sheets. Biomass Bioenergy 2009, 33, 994–998. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Ahmed Hassan, L.E.; Khadeer Ahamed, M.B.; Abdul Majid, A.S.; Abdul Majid, A.M.S. In vitro antimetastatic activity of Agarwood (Aquilaria crassna) essential oils against pancreatic cancer cells. Alex. J. Med. 2016, 52, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Esteves, B.; Velez Marques, A.; Domingos, I.; Pereira, H. Chemical changes of heat treated pine and eucalypt wood monitored by FTIR. Maderas Cienc. Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.; Faix, O.; Pereira, H. Determination of lignin content of Eucalyptus globulus wood using FTIR spectroscopy. Holzforschung 1998, 52, 46–50. [Google Scholar] [CrossRef]
- Nallasamy, P.; Mohan, S. Vibrational spectra of cis-1, 4-polyisoprene. Arab. J. Sci. Eng. 2004, 29, 17–26. [Google Scholar]
- Thuong, N.T.; Yamamoto, O.; Nghia, P.T.; Cornish, K.; Kawahara, S. Effect of naturally occurring crosslinking junctions on green strength of natural rubber. Polym. Adv. Technol. 2017, 28, 303–311. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Kraft lignin/poly (ethylene oxide) blends: Effect of lignin structure on miscibility and hydrogen bonding. J. Appl. Polym. Sci. 2005, 98, 1437–1444. [Google Scholar] [CrossRef]
- Aik-Hwee, E.; Tanaka, Y.; Seng-Neon, G. FTIR studies on amino groups in purified Hevea rubber (short communication). J. Nat. Rubber Res. 1992, 7, 152–155. [Google Scholar]
- Chaikumpollert, O.; Yamamoto, Y.; Suchiva, K.; Kawahara, S. Protein-free natural rubber. Colloid Polym. Sci. 2012, 290, 331–338. [Google Scholar] [CrossRef]
- Tengroth, C.; Gasslander, U.; Andersson, F.O.; Jacobsson, S.P. Cross-linking of gelatin capsules with formaldehyde and other aldehydes: An FTIR spectroscopy study. Pharm. Dev. Technol. 2005, 10, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 2nd ed.; Academic Press, Inc.: New York, NY, USA; San Francisco, CA, USA; London, UK, 1975. [Google Scholar]
- Eng, A.; Tanaka, Y.; Gan, S. Some properties of epoxidised deproteinised natural rubber. J. Nat. Rubber Res. 1997, 12, 82–89. [Google Scholar]
- Tuampoemsab, S.; Sakdapipanich, J.; Tanaka, Y. Influence of some non-rubber components on aging behavior of purified natural rubber. Rubber Chem. Technol. 2007, 80, 159–168. [Google Scholar] [CrossRef]
- Hill, C.A. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Manteghi, A.; Ahmadi, S.; Arabi, H. Enhanced thermo-oxidative stability through covalent attachment of hindered phenolic antioxidant on surface functionalized polypropylene. Polymer 2018, 138, 41–48. [Google Scholar] [CrossRef]
- Cong, F.; Diehl, B.G.; Hill, J.L.; Brown, N.R.; Tien, M. Covalent bond formation between amino acids and lignin: Cross-coupling between proteins and lignin. Phytochemistry 2013, 96, 449–456. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A. Sulfur-free lignin as reinforcing component of styrene–butadiene rubber. J. Appl. Polym. Sci. 2005, 97, 924–929. [Google Scholar] [CrossRef]
- Pillai, K.V.; Renneckar, S. Cation−π interactions as a mechanism in technical lignin adsorption to cationic surfaces. Biomacromolecules 2009, 10, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Nimpaiboon, A.; Amnuaypornsri, S.; Sakdapipanich, J. Role of gel content on the structural changes of masticated natural rubber. Adv. Mater. Res. 2014, 844, 101–104. [Google Scholar] [CrossRef]
- Yangthong, H.; Nun-anan, P.; Faibunchan, P.; Karrila, S.; Limhengha, S. The enhancement of cure and mechanical properties of natural rubber vulcanizates with waste Aquilaria crassna wood. Ind. Crops Prod. 2021, 171, 113922. [Google Scholar] [CrossRef]
- McMahan, C.; Lhamo, D. Study of Amino Acid Modifiers in Guayule Natural Rubber. Rubber Chem. Technol. 2015, 88, 310–323. [Google Scholar] [CrossRef]
- Ratanapisit, J.; Apiraksakul, S.; Rerngnarong, A.; Chungsiriporn, J.; Bunyakarn, C. Preliminary evaluation of production and characterization of wood vinegar from rubberwood. Songklanakarin J. Sci. Technol. 2009, 31, 343–349. [Google Scholar]
- Jong, L. Modulus enhancement of natural rubber through the dispersion size reduction of protein/fiber aggregates. Ind. Crops Prod. 2014, 55, 25–32. [Google Scholar] [CrossRef]
- Novriyanti, E.; Santosa, E. The role of phenolics in agarwood formation of Aquilaria crassna Pierre ex Lecomte and Aquilaria microcarpa Baill Trees. Indones. J. For. Res. 2011, 8, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.-H.; Matsushita, Y.-I.; Sugamoto, K.; Matsui, T. Antimicrobial effect of the wood vinegar from Cryptomeria japonica sapwood on plant pathogenic microorganisms. J. Microbiol. Biotechnol. 2005, 15, 1106–1109. [Google Scholar]
- Kamarulzaman, N.; Idris, N.; Nor, Z.M. Characteristics of odour concentration from rubber processing factories via olfactometry technique. Chem. Eng. Trans. 2012, 30, 121–126. [Google Scholar]





| Ingredient | Parts per Hundred of Rubber (phr) |
|---|---|
| Aquilaria crassna wood powder | 20 |
| bentonite | 1 |
| vultamol | 1 |
| water | 78 |
| Wavelength (cm−1) | Assignment |
|---|---|
| 1240 | Amide III band (in-phase combination of C–N stretch and N–H bend modes) [21] |
| 1308 | CH2 wagging [19] |
| 1375 | CH3 asymmetric deformation [19] |
| 1448 | CH2 deformation [19] |
| 1509 | C–H stretching vibration of wood [13] |
| 1540 | Amide II: N–H and C–N stretching vibration of proteins in NR molecules [19] |
| 1620 | Aromatic ring stretching vibration [13] |
| 1630 | Amide I: R1–(C=O)–NH–R2 stretching vibration of proteins in NR molecules [19] |
| 1660 | C=C stretching vibration of cis-1,4-isoprene units [19] |
| 1710 | –(C=O)–OH stretching vibration of lipids in natural rubber [19] |
| 1740–1720 | C=O stretching vibration of aldehydes [13] |
| 3280 | N–H stretching vibration of proteins [19] |
| Element | Concentration (%) |
|---|---|
| C | 27.359 |
| O | 36.444 |
| N | 31.905 |
| H | 2.296 |
| Na | 0.034 |
| Mg | 0.076 |
| Al | 0.104 |
| Si | 0.195 |
| P | 0.043 |
| S | 0.145 |
| Cl | 0.136 |
| K | 0.243 |
| Ca | 0.874 |
| Ti | 0.011 |
| Mn | 0.028 |
| Fe | 0.090 |
| Cu | 0.011 |
| Zn | 0.006 |
| Sr | 0.002 |
| Property\Sample | ACW0 | ACW20 | ACW40 | ACW60 |
|---|---|---|---|---|
| dirt content (% wt) | 0.03 ± 0.06 | 6.88 ± 0.06 | 6.97 ± 0.06 | 8.23 ± 0.06 |
| ash content (% wt) | 0.43 ± 0.04 | 1.36 ± 20.04 | 1.86 ± 40.04 | 3.04 ± 60.04 |
| volatile matter (VM) content (% wt) | 0.94 ± 0.2 | 01.93 ± 0.40 | 2.99 ± 0.90 | 5.08 ± 1.00 |
| nitrogen content (% wt) | 0.410 ± 0.020 | 0.447 ± 0.122 | 0.487 ± 0.070 | 0.513 ± 0.115 |
| Sample | Initial Plasticity (PO) | Plasticity Retention Index (PRI) | Mooney Viscosity [ML 1+4 (100 °C)] | Gel Content (%wt) |
|---|---|---|---|---|
| ACW0 | 38.2 ± 0.7 | 100.6 ± 2.0 | 64.9 ± 1.0 | 8.78 ± 0.14 |
| ACW20 | 40.1 ± 1.1 | 91.2 ± 3.0 | 66.6 ± 0.1 | 20.53 ± 0.75 |
| ACW40 | 50.1 ± 0.8 | 64.0 ± 1.0 | 75.1 ± 0.1 | 31.57 ± 1.85 |
| ACW60 | 61.2 ± 1.0 | 45.1 ± 1.5 | 96.4 ± 1.2 | 50.22 ± 1.19 |
| Property\Sample | ACW0 | ACW20 | ACW40 | ACW60 |
|---|---|---|---|---|
| 100% modulus (MPa) | 0.26 ± 0.01 | 0.56 ± 0.02 | 0.95 ± 0.01 | 1.94 ± 0.03 |
| 300% modulus (MPa) | 0.24 ± 0.02 | 0.99 ± 0.04 | 1.61 ± 0.03 | - |
| Green strength (MPa) | 0.27 ± 0.01 | 1.29 ± 0.02 | 1.84 ± 0.01 | 2.19 ± 0.03 |
| Elongation at break (%) | 669.52 ± 29.17 | 434.22 ± 17.08 | 386.92 ± 11.16 | 158.36 ± 15.77 |
| Aquilaria crassna Wood Content (phr) | % Area of Fungal Growth |
|---|---|
| 0 | 97.62 ± 2.45 |
| 20 | 73.70 ± 1.65 |
| 40 | 69.11 ± 0.56 |
| 60 | 65.23 ± 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nun-Anan, P.; Suchat, S.; Mahathaninwong, N.; Chueangchayaphan, N.; Karrila, S.; Limhengha, S. Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets. Polymers 2021, 13, 4178. https://doi.org/10.3390/polym13234178
Nun-Anan P, Suchat S, Mahathaninwong N, Chueangchayaphan N, Karrila S, Limhengha S. Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets. Polymers. 2021; 13(23):4178. https://doi.org/10.3390/polym13234178
Chicago/Turabian StyleNun-Anan, Phattarawadee, Sunisa Suchat, Narissara Mahathaninwong, Narong Chueangchayaphan, Seppo Karrila, and Suphatchakorn Limhengha. 2021. "Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets" Polymers 13, no. 23: 4178. https://doi.org/10.3390/polym13234178
APA StyleNun-Anan, P., Suchat, S., Mahathaninwong, N., Chueangchayaphan, N., Karrila, S., & Limhengha, S. (2021). Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets. Polymers, 13(23), 4178. https://doi.org/10.3390/polym13234178