Performance of Citric Acid-Bonded Oriented Board from Modified Fibrovascular Bundle of Salacca (Salacca zalacca (Gaertn.) Voss) Frond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
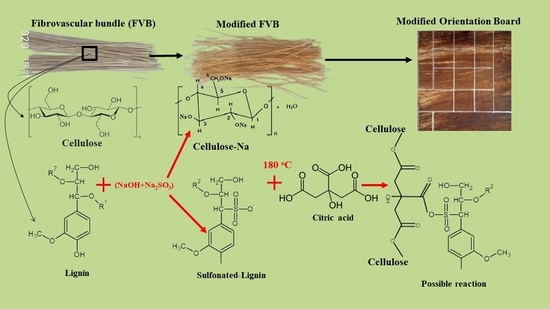
2.2. Chemical Treatments and Chemical Content Changes
2.3. Contact Angle and Mechanical Properties of Modified Fibrovascular Bundles
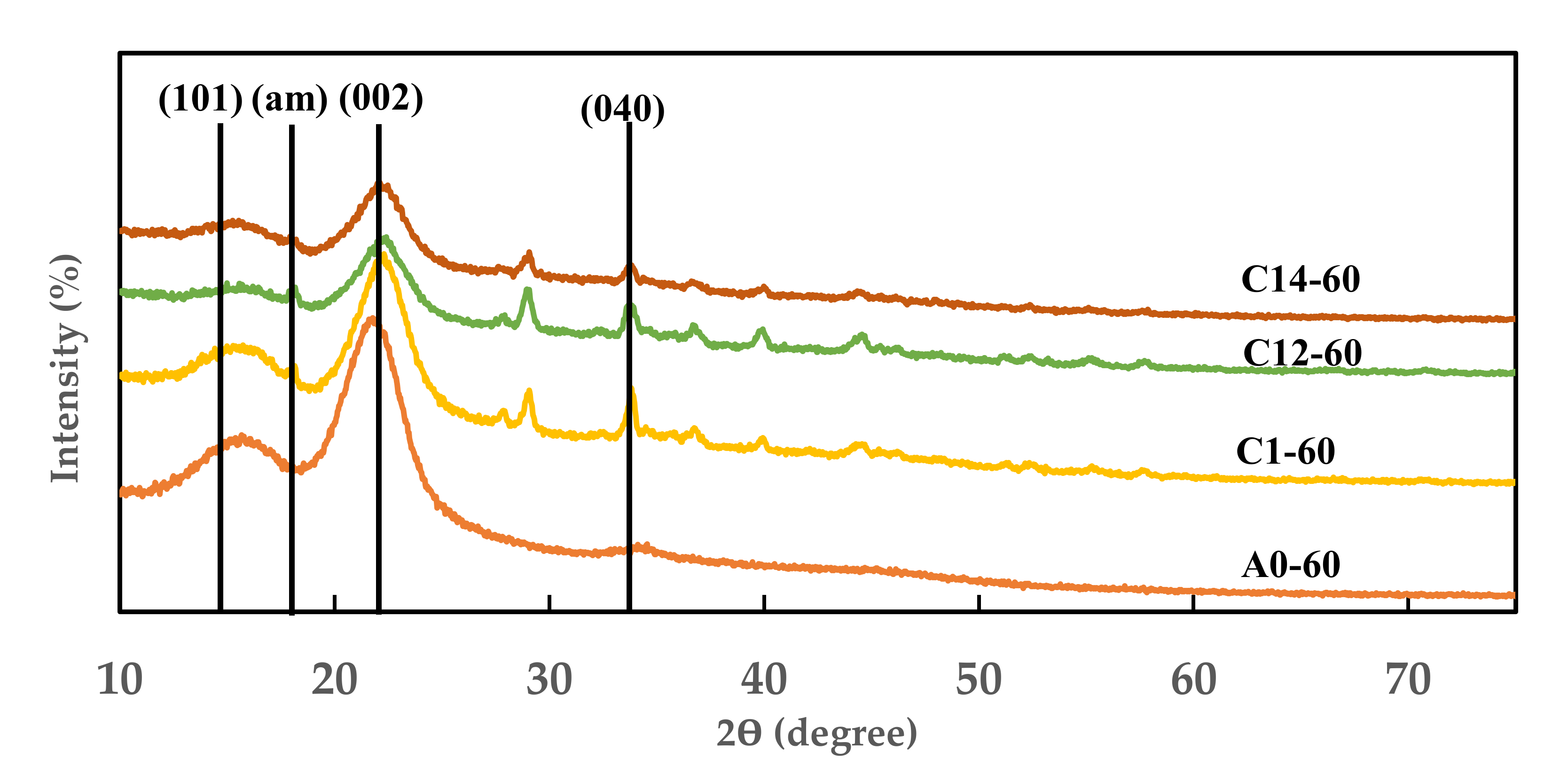
2.4. Index Crystallinity of Modified FVB
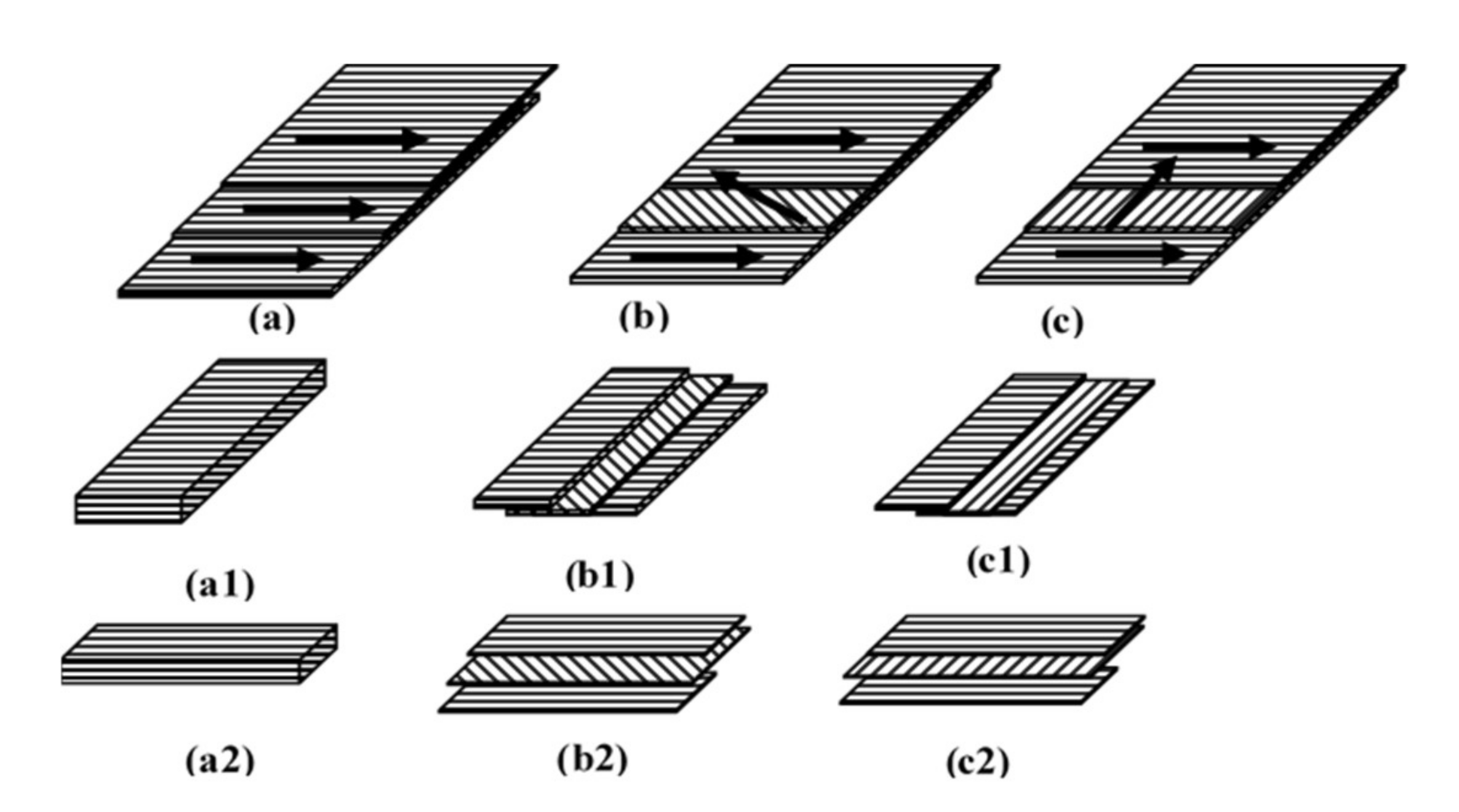
2.5. The Manufacture of Oriented Board
2.6. Evaluation of Oriented Board Properties
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
3. Results and Discussion
3.1. Properties Change of Modified FVB
3.2. Modulus of Rupture (MOR) and Modulus of Elasticity (MOE) of a Modified Orientation Board
3.3. Strength of Internal Bond (IB)
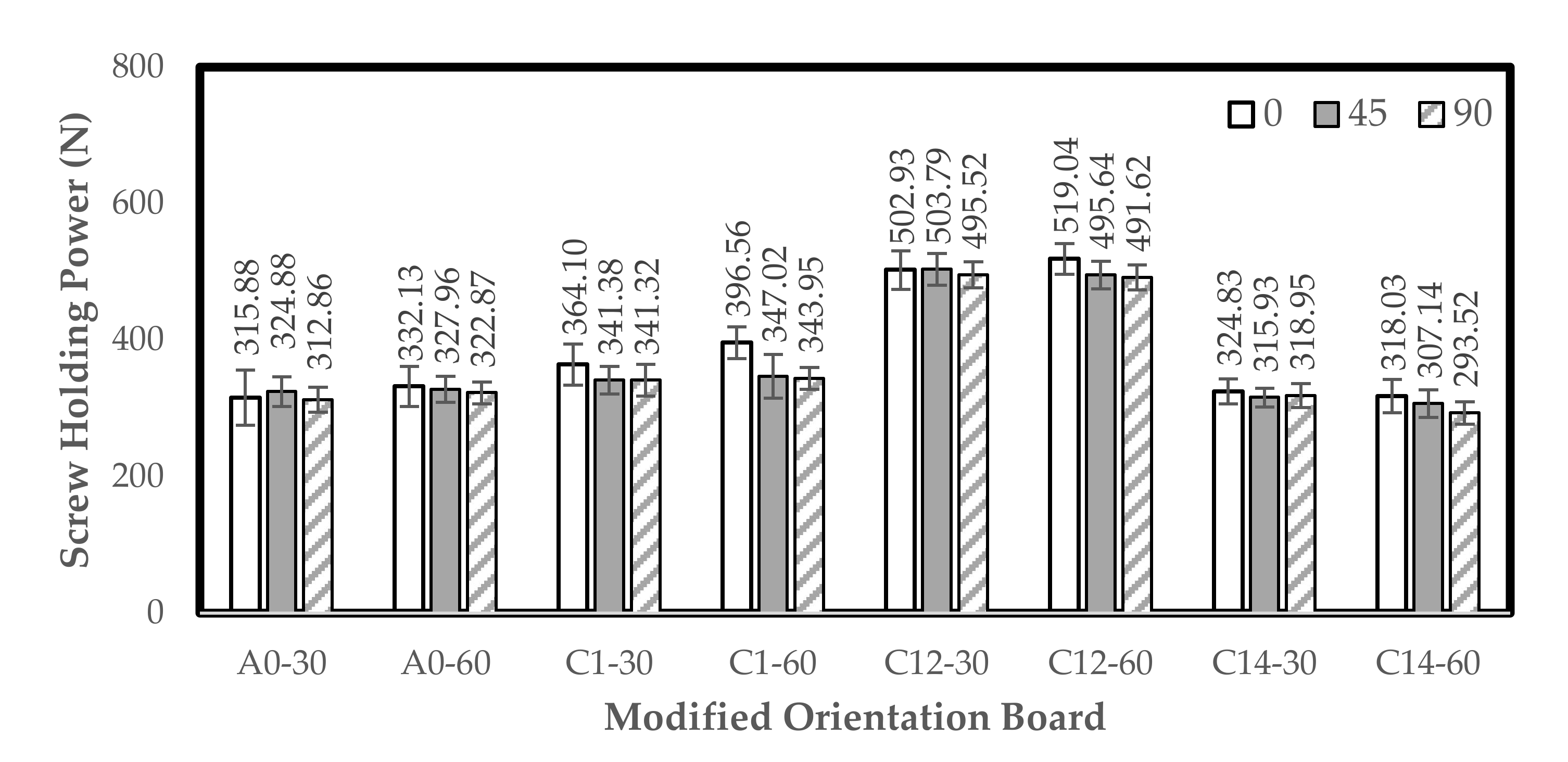
3.4. Screw Holding Power (SHP)
3.5. Thickness Swelling (TS) and Water Absorption (WA)
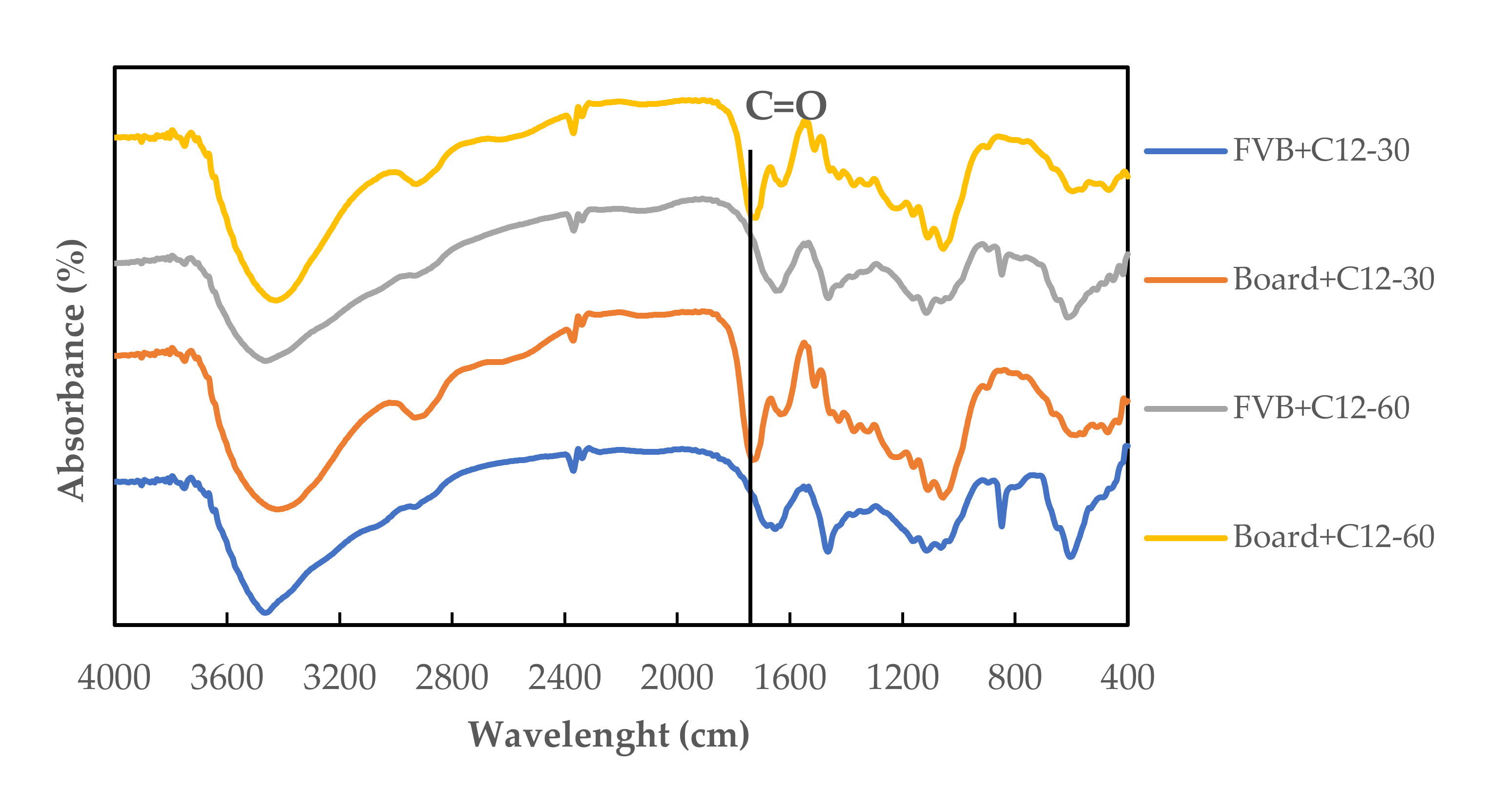
3.6. Fourier Transform Infrared (FTIR) Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hakim, L.; Widyorini, R.; Nugroho, W.D.; Prayitno, T.A. Anatomical, chemical, and mechanical properties of fibrovascular bundles of salacca (snake fruit) frond. BioResources 2019, 14, 7943–7957. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Tamanna, T.A.; Belal, S.A.; Shibly, M.A.H.; Khan, A.N. Characterization of a new natural fiber extracted from Corypha taliera fruit. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Sinebe, J.; Chukwuneke, J.L.; Omenyi, S.N. Surface energetics effects on mechanical strength of fibre reinforced polymer matrix. J. Phys. Conf. Ser. 2019, 1378, 042016. [Google Scholar] [CrossRef]
- Ariawan, D.; Rivai, T.S.; Surojo, E.; Hidayatulloh, S.; Akbar, H.I.; Prabowo, A.R. Effect of alkali treatment of Salacca Zalacca fiber (SZF) on mechanical properties of HDPE composite reinforced with SZF. Alex. Eng. J. 2020, 59, 3981–3989. [Google Scholar] [CrossRef]
- Deesoruth, A.; Ramasawmy, H.; Chummun, J. Investigation into the use of alkali treated screwpine (Pandanus Utilis) fibres as reinforcement in epoxy matrix. Int. J. Plast. Technol. 2014, 18, 263–279. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2019, 55, 829–892. [Google Scholar] [CrossRef]
- Boumediri, H.; Bezazi, A.; Del Pino, G.G.; Haddad, A.; Scarpa, F.; Dufresne, A. Extraction and characterization of vascular bundle and fiber strand from date palm rachis as potential bio-reinforcement in composite. Carbohydr. Polym. 2019, 222, 114997. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Ntalos, G.A.; Kakaras, I. Mechanical and physical properties of cement-bonded OSB. Holz Roh Werkst. 2006, 64, 517–518. [Google Scholar] [CrossRef]
- Hassani, V.; Papadopoulos, A.N.; Schmidt, O.; Maleki, S.; Papadopoulos, A.N. Mechanical and physical properties of oriented strand lumber (OSL): The effect of fortification level of nanowollastonite on UF resin. Polymers 2019, 11, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciannamea, E.M.; Martucci, J.F.; Stefani, P.M.; Ruseckaite, R.A. Bonding quality of chemically-modified soybean protein concentrate-based adhesives in particleboards from rice husks. J. Am. Oil Chem. Soc. 2012, 4302, 1733–1741. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Olivato, J.B.; Müller, C.M.O.; Carvalho, G.M.; Yamashita, F.; Grossmann, M.V.E. Physical and structural characterisation of starch/polyester blends with tartaric acid. Mater. Sci. Eng. C 2014, 39, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cao, Y.; Fang, Q.; Liu, Z. Effects of treatment temperature on properties of starch-based adhesives. BioResources 2015, 10, 3520–3530. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Kawai, S. Characterization of wood-based molding bonded with citric acid. J. Wood Sci. 2011, 58, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Liao, R.; Xu, J.; Umemura, K. Low density sugarcane bagasse particleboard bonded with citric acid and sucrose: Effect of board density and additive content. BioResources 2015, 11, 2174–2185. [Google Scholar] [CrossRef]
- Kusumah, S.S.; Umemura, K.; Yoshioka, K.; Miyafuji, H.; Kanayama, K. Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard I: Effects of pre-drying treatment and citric acid content on the board properties. Ind. Crop. Prod. 2016, 84, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Widyorini, R.; Umemura, K.; Soraya, D.K.; Dewi, G.K.; Nugroho, W.D. Effect of citric acid content and extractives treatment on the manufacturing process and properties of citric acid-bonded salacca Frond. BioResources 2019, 14, 4171–4180. [Google Scholar]
- Hakim, L.; Widyorini, R.; Nugroho, W.D.; Prayitno3, T.A. Radial variability of fibrovascular bundle properties of salacca (Salacca zalacca) fronds cultivated on Turi Agrotourism in Yogyakarta, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 861. [Google Scholar] [CrossRef]
- Schellbach, S.L.; Monteiro, S.N.; Drelich, J.W. A novel method for contact angle measurements on natural fibers. Mater. Lett. 2016, 164, 599–604. [Google Scholar] [CrossRef]
- Munawar, S.S.; Umemura, K.; Kawai, S. Characterization of the morphological, physical, and mechanical properties of seven nonwood plant fiber bundles. J. Wood Sci. 2007, 53, 108–113. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Darmanto, S.; Rochardjo, H.S.B.; Widyorini, R. Effects of alkali and steaming on mechanical properties of snake fruit (Salacca) fiber. In Proceedings of the International Conference on Engineering Science and Nanotechnology 2016 (ICESNANO), Solo, Indonesia, 3–5 August 2017; Volume 1. [Google Scholar] [CrossRef] [Green Version]
- Shanmugasundaram, N.; Rajendran, I.; Ramkumar, T. Characterization of untreated and alkali treated new cellulosic fiber from an Areca palm leaf stalk as potential reinforcement in polymer composites. Carbohydr. Polym. 2018, 195, 566–575. [Google Scholar] [CrossRef]
- Ganapathy, T.; Sathiskumar, R.; Senthamaraikannan, P.; Saravanakumar, S.; Khan, A. Characterization of raw and alkali treated new natural cellulosic fibres extracted from the aerial roots of banyan tree. Int. J. Biol. Macromol. 2019, 138, 573–581. [Google Scholar] [CrossRef]
- Sghaier, A.E.O.B.; Chaabouni, Y.; Msahli, S.; Sakli, F. Morphological and crystalline characterization of NaOH and NaOCl treated Agave americana L. fiber. Ind. Crop. Prod. 2012, 36, 257–266. [Google Scholar] [CrossRef]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Katoh, M.; Ueki, T.; Waterhouse, G.I.; Li, Y. Influence of alkali treatment on internal microstructure and tensile properties of abaca fibers. Ind. Crop. Prod. 2014, 65, 27–35. [Google Scholar] [CrossRef]
- Vijay, R.; Singaravelu, D.L.; Vinod, A.; Sanjay, M.; Siengchin, S.; Jawaid, M.; Khan, A.; Parameswaranpillai, J. Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens. Int. J. Biol. Macromol. 2018, 125, 99–108. [Google Scholar] [CrossRef]
- Arnata, I.W.; Suprihatin, S.; Fahma, F.; Richana, N.; Candra Sunarti, T. Cellulose production from sago frond with alkaline delignification and bleaching on various types of bleach agents. Orient. J. Chem. 2019, 35, 8–19. [Google Scholar] [CrossRef]
- Darmanto, S.; Rochardjo, H.S.B.; Jamasri; Widyorini, R. Effect of sonication treatment on fibrilating snake fruit (Sallaca) frond fiber. AIP Conf. Proc. 2018, 1931, 030064. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Z.; Qin, D.; Yu, Y.; Tian, G.; Lu, F.; Fei, B.; Wang, G. Contact angles of single bamboo fibers measured in different environments and compared with other plant fibers and bamboo strips. BioResources 2013, 8, 2827–2838. [Google Scholar] [CrossRef] [Green Version]
- Yorseng, K.; Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S. Influence of accelerated weathering on the mechanical, fracture morphology, thermal stability, contact angle, and water absorption properties of natural fiber fabric-based epoxy hybrid composites. Polymers 2020, 12, 2254. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.; Filho, Z.A.; Noseda, M.D.; Kaur, S.B.; Soccol, R.C. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment—A biorefinery approach. Bioresour. Technol. 2015, 194, 172–178. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Huang, J.; Ma, K. The structure–mechanical relationship of palm vascular tissue. J. Mech. Behav. Biomed. Mater. 2014, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Yunus, W.M.Z.W.; Chieng, B.W. Static mechanical, interfacial, and water absorption behaviors of Alkali Treated Oil palm mesocarp fiber reinforced poly(butylene succinate) biocomposites. BioResources 2015, 10, 123–136. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Ratnam, C.T.; Manickam, S. Enhancements in crystallinity, thermal stability, tensile modulus and strength of sisal fibres and their PP composites induced by the synergistic effects of alkali and high intensity ultrasound (HIU) treatments. Ultrason. Sonochem. 2017, 34, 729–742. [Google Scholar] [CrossRef]
- Moradbak, A.; Tahir, P.M.; Mohamed, A.Z.; Halis, R.B. Alkaline sulfite anthraquinone and methanol pulping of bamboo (Gigantochloa scortechinii). BioResources 2015, 11, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Munawar, S.S.; Umemura, K.; Kawai, S. Manufacture of oriented board using mild steam treatment of plant fiber bundles. J. Wood Sci. 2008, 54, 369–376. [Google Scholar] [CrossRef]
- Baharin, A.; Fattah, N.A.; Abu Bakar, A.; Ariff, Z. Production of laminated natural fibre board from banana tree wastes. Procedia Chem. 2016, 19, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Widyorini, R.; Umemura, K.; Septiano, A.; Soraya, D.K.; Dewi, G.K.; Nugroho, W.D. Manufacture and properties of citric acid-bonded composite board made from salacca frond: Effects of Maltodextrin Addition, Pressing Temperature, and Pressing Method. BioResources 2018, 13, 8662–8676. [Google Scholar] [CrossRef]
- Khakpour, H.; Ayatollahi, M.R.; Akhavan-Safar, A.; da Silva, L.F.M. Mechanical properties of structural adhesives enhanced with natural date palm tree fibers: Effects of length, density and fiber type. Compos. Struct. 2020, 237l, 111950. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Calado, V.M.A.; Gonçalves, L.R.B.; Coelho, M.A.Z. Effect of chemical treatments on properties of green coconut fiber. Carbohydr. Polym. 2010, 79, 832–838. [Google Scholar] [CrossRef]
- Elanchezhian, C.; Ramnath, B.V.; Ramakrishnan, G.; Rajendrakumar, M.; Naveenkumar, V.; Saravanakumar, M.K. Review on mechanical properties of natural fiber composites. Mater. Today Proc. 2018, 5, 1785–1790. [Google Scholar] [CrossRef]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; Oudrhiri Hassani, F.; Laachachi, A.; El Bouari, A. Effect of surface modification on morphological, mechanical and thermal conductivity of hemp fiber: Characterization of the interface of hemp -Polyurethane composite. Case Stud. Therm. Eng. 2017, 10, 550–559. [Google Scholar] [CrossRef]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis Sativa L.) for composite applications. Ind. Crop. Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Santoso, M.; Widyorini, R.; Prayitno, T.A.; Sulistyo, J. Bonding performance of maltodextrin and citric acid for particleboard made from nipa fronds. J. Korean Wood Sci. Technol. 2017, 45, 432–443. [Google Scholar] [CrossRef]
- Widyorini, R.; Umemura, K.; Isnan, R.; Putra, D.R.; Awaludin, A.; Prayitno, T.A. Manufacture and properties of citric acid-bonded particleboard made from bamboo materials. Eur. J. Wood Prod. 2016, 74, 7–65. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature. J. Wood Sci. 2014, 61, 40–44. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K.; Kanayama, K. Effects of the addition of citric acid on tannin-sucrose adhesive and physical properties of the particleboard. BioResources 2015, 11, 1333. [Google Scholar] [CrossRef]
- Walther, T.; Kartal, S.N.; Hwang, W.J.; Umemura, K.; Kawai, S. Strength, decay and termite resistance of oriented kenaf fiberboards. J. Wood Sci. 2007, 53, 481–486. [Google Scholar] [CrossRef]
- Hung, K.; Wu, T.; Chen, Y.; Wu, J. Assessing the effect of wood acetylation on mechanical properties and extended creep behavior of wood/recycled-polypropylene composites. Constr. Build. Mater. 2016, 108, 139–145. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, J.-H. Mechanical and interfacial properties of plastic composite panels made from esterified bamboo particles. J. Wood Sci. 2010, 56, 216–221. [Google Scholar] [CrossRef]
- Kemalasari, D.; Widyorini, R. Karakteristik Papan Partikel dari Pelepah Salak Pondoh (Saliacca sp.) dengan Penambahan Asam Sitrat. In Proceedings of the Prosiding Seminar Nasional MAPEKI XVIII, Bandung, Indonesia, 4–5 November 2015; pp. 542–548. [Google Scholar]
- Kusumah, S.S.; Astari, L.; Subyakto, W.; Zhao, Z. The utilization of citric acid as an environmentally friendly of chemical modification agent of the lignocellulosic materials: A review. J. Lignocellul. Technol. 2017, 2, 1–7. [Google Scholar]
- Menezzi, C.; Del Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with wood carbohydrates and lignin of citric acid as a bond promoter of wood veneer panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef] [Green Version]






| Treatment | NaOH+Na2SO3 Combination | Immersion Time (Minutes) |
|---|---|---|
| A0-30 | Untreated, water immersion | 30 |
| A0-60 | Untreated, water immersion | 60 |
| C1-30 | 1% NaOH | 30 |
| C1-60 | 1% NaOH | 60 |
| C12-30 | 1% NaOH + 0.2% Na2SO3 | 30 |
| C12-60 | 1% NaOH + 0.2% Na2SO3 | 60 |
| C14-30 | 1% NaOH + 0.4% Na2SO3 | 30 |
| C14-60 | 1% NaOH + 0.4% Na2SO3 | 60 |
| Treatment | Contact Angle (°) | Density (g/cm3) | CrI (%) | α-Cellulose (% wt) | Hemicellulose (% wt) | Lignin (% wt) |
|---|---|---|---|---|---|---|
| A0-30 | 92.30 ± 7.66 | 0.40 ± 0.09 | – | 43.11 ± 0.71 | 32.24 ± 0.87 | 28.18 ± 1.13 |
| A0-60 | 84.47 ± 3.25 | 0.40 ± 0.08 | 53.04 | 43.13 ± 0.90 | 32.17 ± 0.79 | 28.09 ± 0.96 |
| C1-30 | 55.83 ± 3.41 | 0.39 ± 0.12 | – | 46.45 ± 0.52 | 30.73 ± 0.94 | 27.11 ± 1.03 |
| C1-60 | 46.20 ± 5.04 | 0.39 ± 0.17 | 56.25 | 48.15 ± 0.77 | 31.00 ± 1.57 | 26.33 ± 2.31 |
| C12-30 | 44.38 ± 7.65 | 0.38 ± 0.06 | – | 53.38 ± 1.67 | 27.92 ± 0.76 | 22.05 ± 0.89 |
| C12-60 | 34.88 ± 3.88 | 0.37 ± 0.18 | 58.33 | 50.71 ± 1.46 | 24.72 ± 0.66 | 21.33 ± 0.85 |
| C14-30 | 31.98 ± 4.60 | 0.37 ± 0.09 | – | 39.62 ± 1.55 | 20.85 ± 0.69 | 21.22 ± 0.92 |
| C14-60 | 24.02 ± 2.80 | 0.35 ± 0.12 | 47.43 | 35.15 ± 0.72 | 21.91 ± 0.98 | 18.78 ± 1.21 |
| Treatment | Maximum Load (N) | Tensile Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| A0-30 | 121.01 ± 13.53 | 217.19 ± 28.11 | 2.23 ± 0.44 |
| A0-60 | 117.28 ± 8.82 | 222.10 ± 18.63 | 2.23 ± 0.42 |
| C1-30 | 117.68 ± 8.77 | 253.32 ± 25.81 | 2.52 ± 0.40 |
| C1-60 | 122.57 ± 9.94 | 275.41 ± 21.28 | 2.29 ± 0.41 |
| C12-30 | 119.92 ± 9.33 | 313.93 ± 41.43 | 2.55 ± 0.24 |
| C12-60 | 110.30 ± 6.67 | 331.66 ± 33.38 | 2.28 ± 0.36 |
| C14-30 | 77.83 ± 6.06 | 267.72 ± 39.52 | 2.18 ± 0.43 |
| C14-60 | 57.15 ± 6.86 | 237.99 ± 43.45 | 1.91 ± 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, L.; Widyorini, R.; Nugroho, W.D.; Prayitno, T.A. Performance of Citric Acid-Bonded Oriented Board from Modified Fibrovascular Bundle of Salacca (Salacca zalacca (Gaertn.) Voss) Frond. Polymers 2021, 13, 4090. https://doi.org/10.3390/polym13234090
Hakim L, Widyorini R, Nugroho WD, Prayitno TA. Performance of Citric Acid-Bonded Oriented Board from Modified Fibrovascular Bundle of Salacca (Salacca zalacca (Gaertn.) Voss) Frond. Polymers. 2021; 13(23):4090. https://doi.org/10.3390/polym13234090
Chicago/Turabian StyleHakim, Luthfi, Ragil Widyorini, Widyanto Dwi Nugroho, and Tibertius Agus Prayitno. 2021. "Performance of Citric Acid-Bonded Oriented Board from Modified Fibrovascular Bundle of Salacca (Salacca zalacca (Gaertn.) Voss) Frond" Polymers 13, no. 23: 4090. https://doi.org/10.3390/polym13234090
APA StyleHakim, L., Widyorini, R., Nugroho, W. D., & Prayitno, T. A. (2021). Performance of Citric Acid-Bonded Oriented Board from Modified Fibrovascular Bundle of Salacca (Salacca zalacca (Gaertn.) Voss) Frond. Polymers, 13(23), 4090. https://doi.org/10.3390/polym13234090