Development of a Novel X-ray Compatible 3D-Printed Bone Model to Characterize Different K-Wire Fixation Methods in Support of the Treatment of Pediatric Radius Fractures
Abstract
1. Introduction
2. Materials and Methods
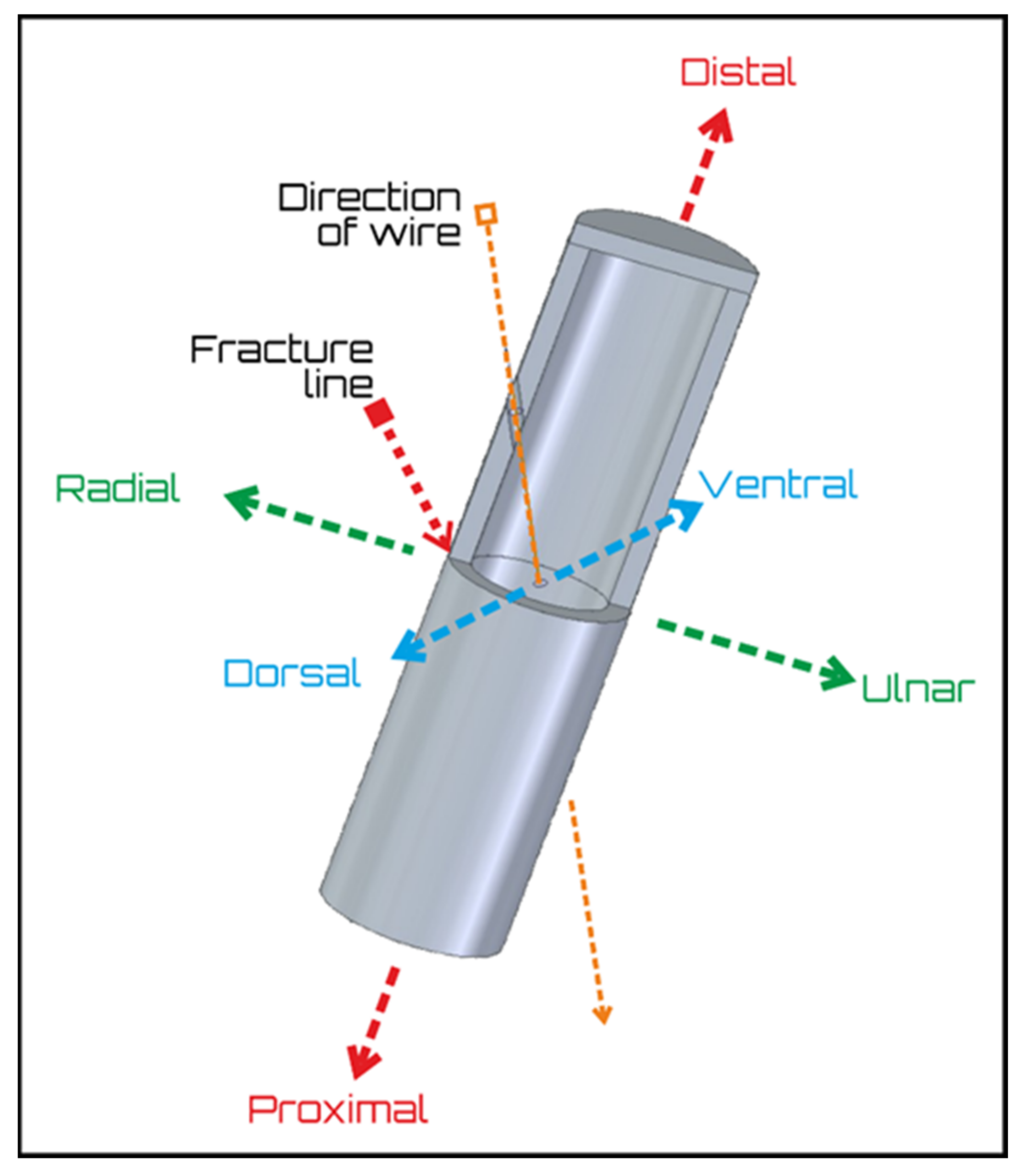
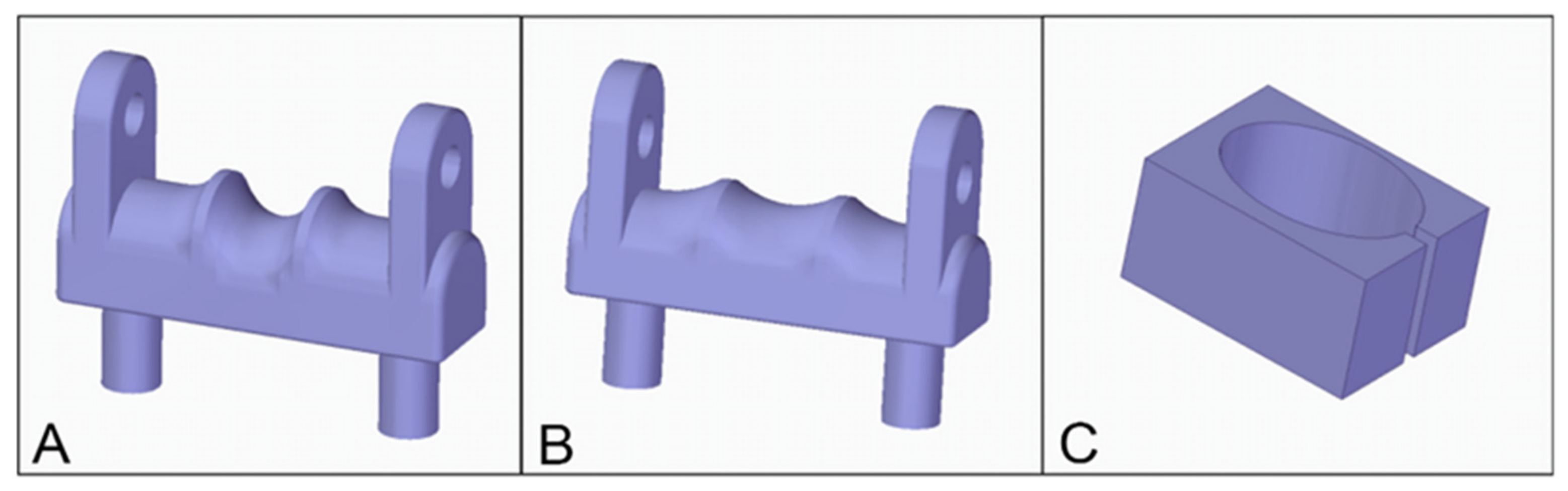
2.1. Basic Model Description
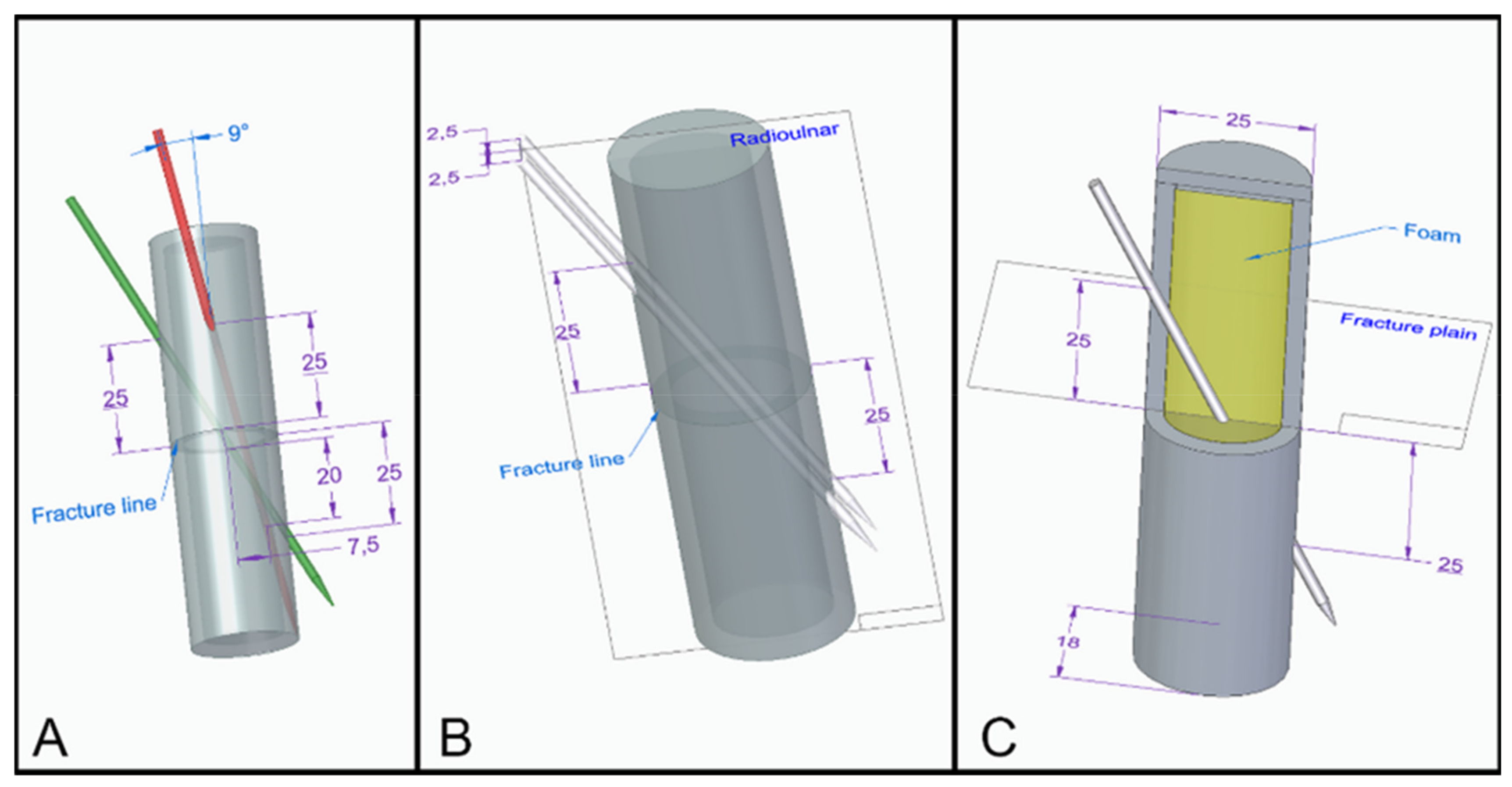
2.2. Groups
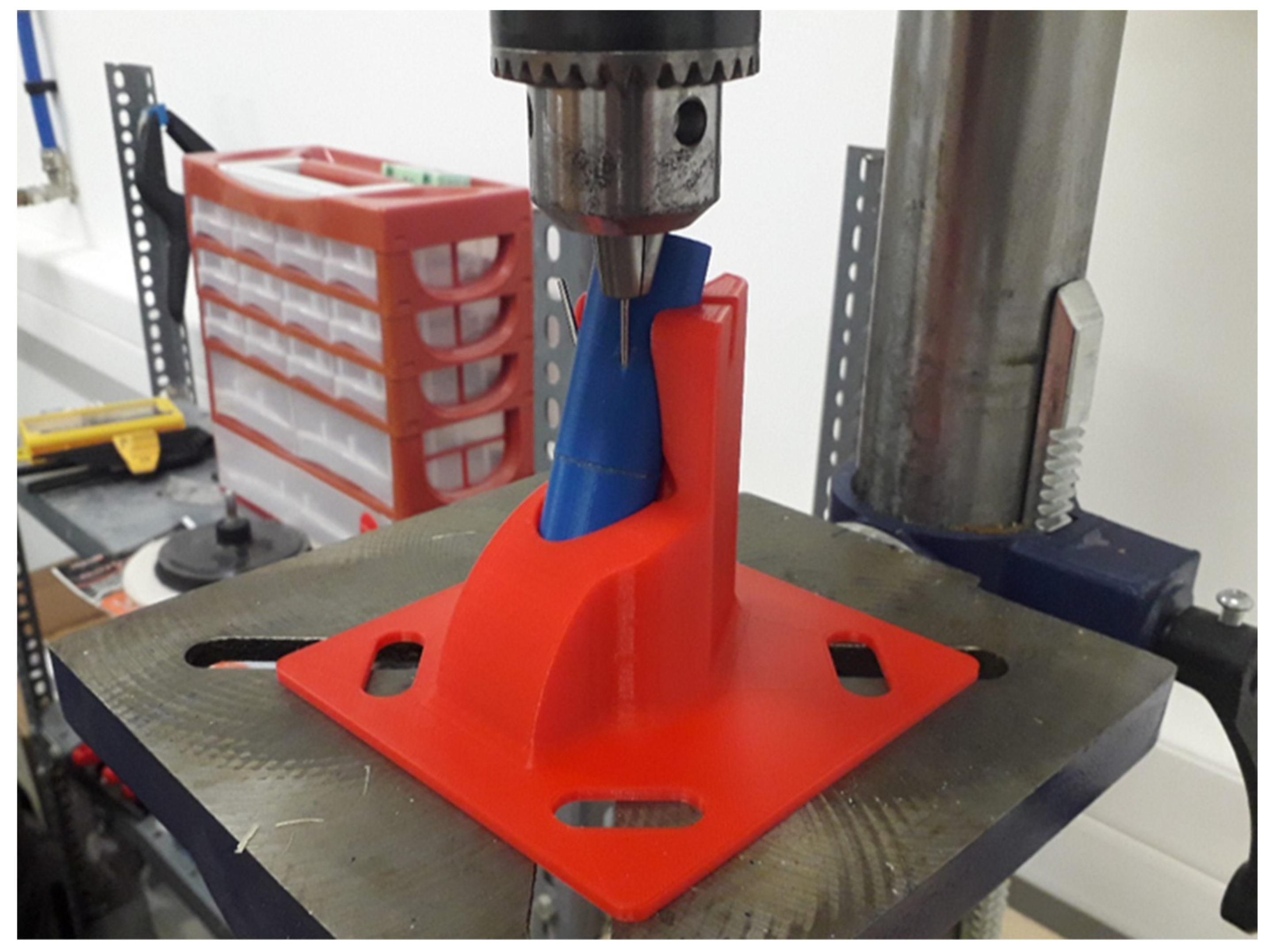
2.3. Mechanical and Structural Tests, Imaging
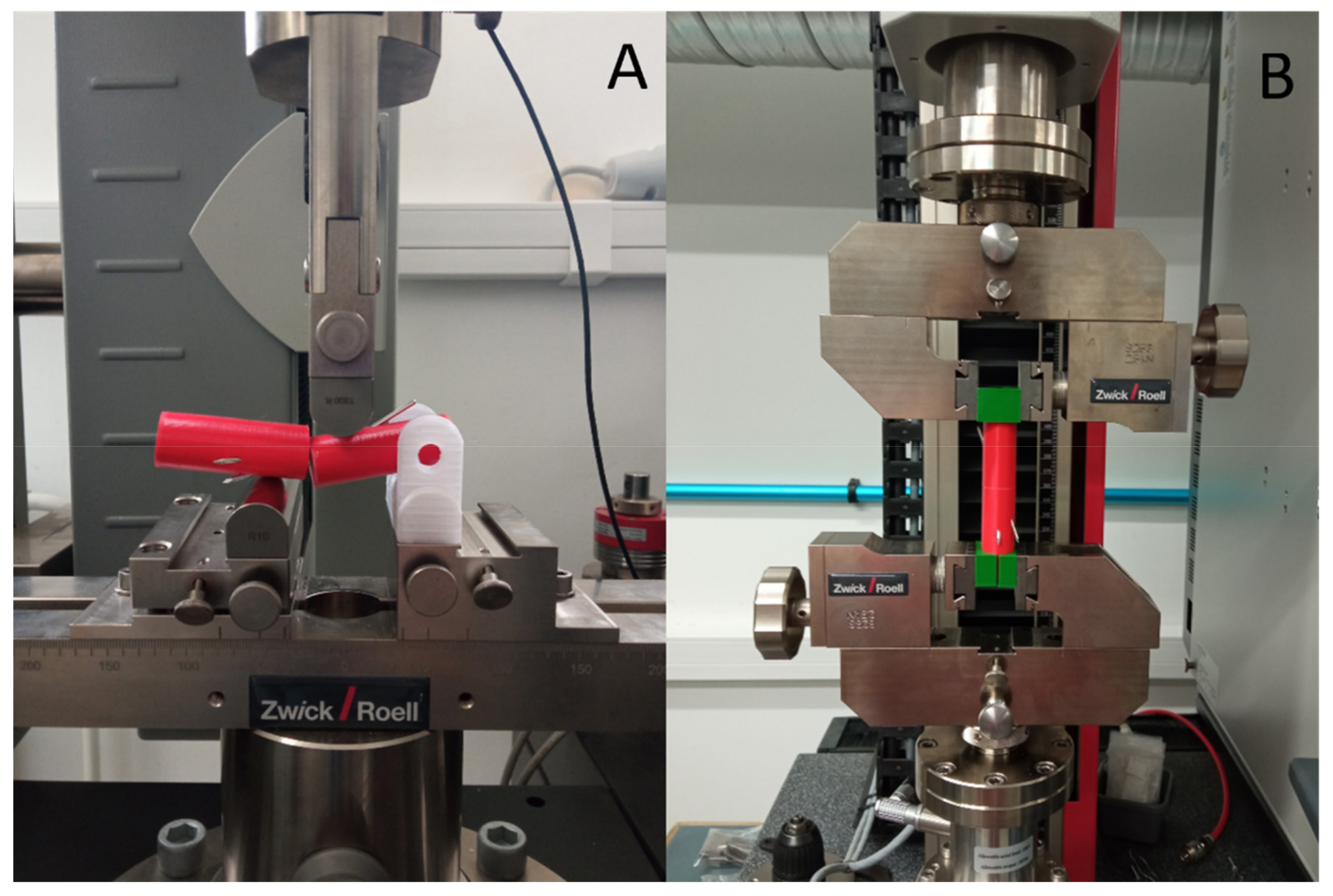
2.3.1. Mechanical Testing of the Intact and Fixed Models
Three-Point Bending Test
Torsion Test
Imaging with Digital Microscopy and X-ray
2.3.2. Statistical Analysis
3. Results
3.1. Mechanical Characterization of the Intact Bone Model
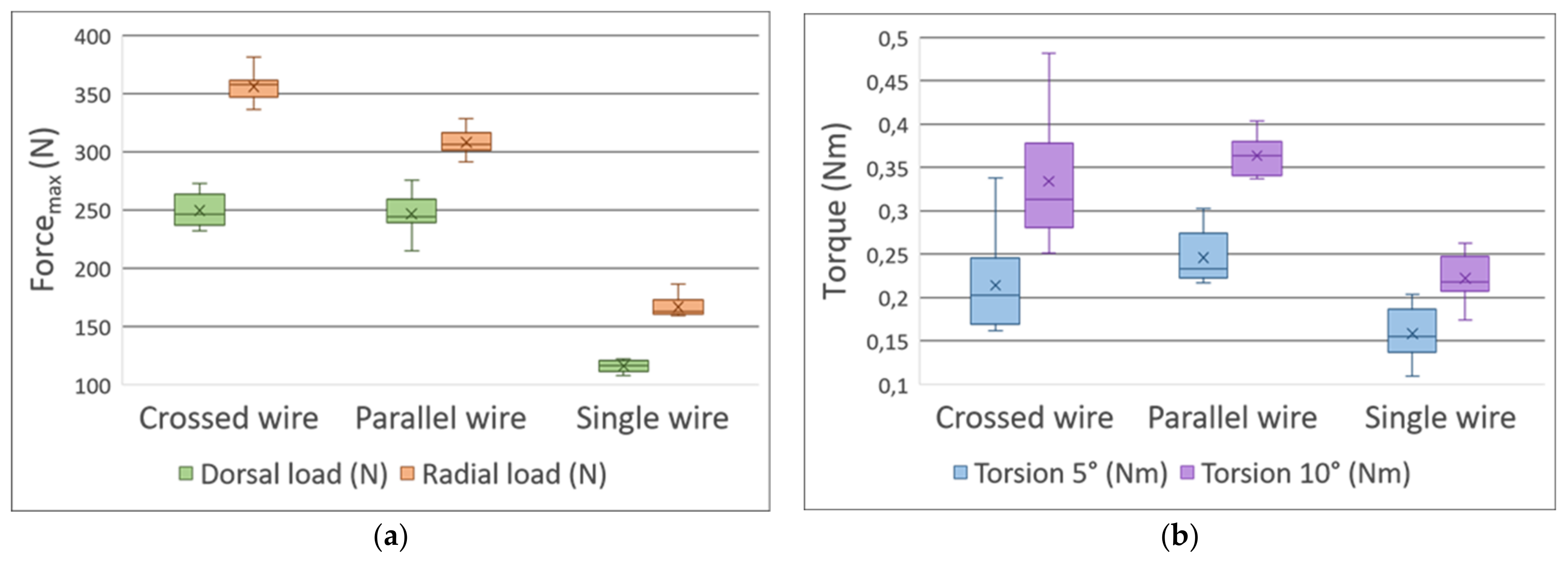
3.2. Comparison of Fixation Methods
3.2.1. Three-Point Bending Test—Dorsal Load
3.2.2. Three-Point Bending Test—Radial Load
3.2.3. Torsion Test
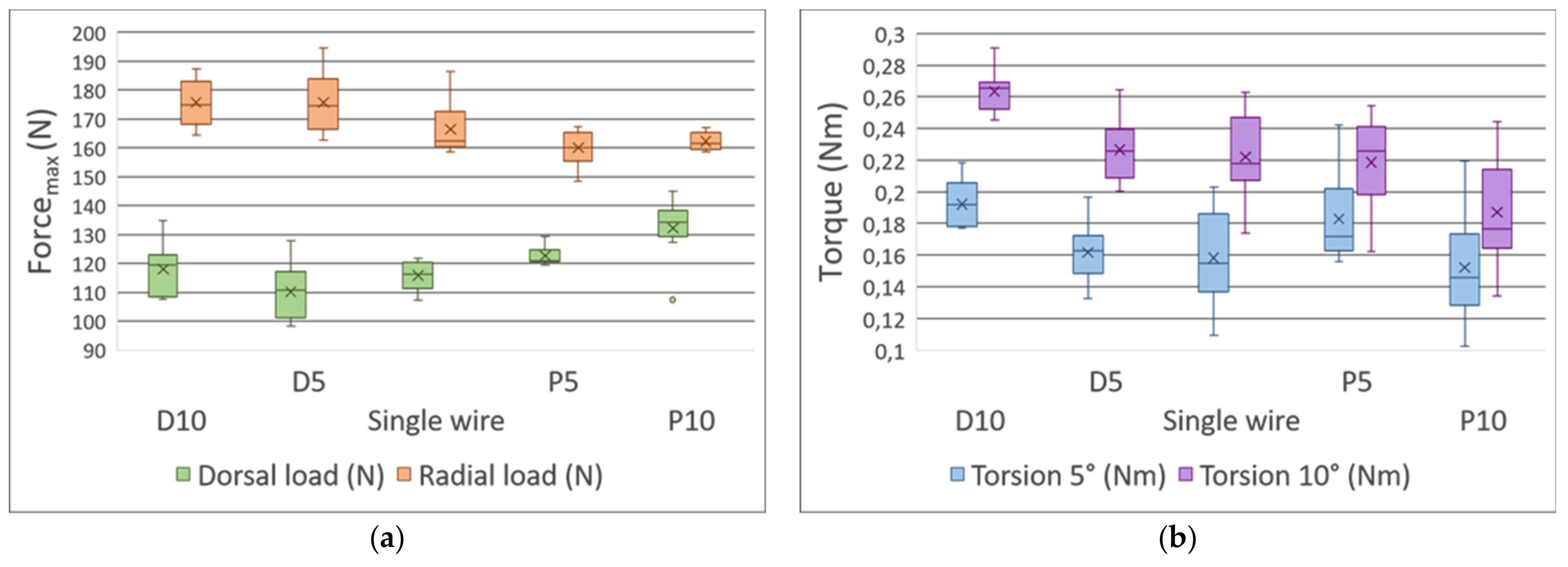
3.3. The Distance between the Fracture Gap and the Exit Point Regarding the K-Wires Affected the Stability of the Fixation
3.3.1. Three-Point Bending Test—Dorsal Load
3.3.2. Three-Point Bending Test—Radial Load
3.3.3. Torsion Tests
3.4. Results of Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Descriptive Statistics | ||||
|---|---|---|---|---|
| Dorsal Load (N) | Radial Load (N) | Torsion 5° (Nm) | Torsion 10° (Nm) | |
| Mean | 1335 | 1798 | 4.33 | 6.52 |
| Median | 1328 | 1789 | 4.29 | 6.53 |
| Standard deviation | 41.7 | 72.3 | 0.23 | 0.0739 |
| Variance | 1736 | 5228 | 0.0529 | 0.00547 |
| Shapiro–Wilk W | 0.975 | 0.961 | 0.957 | 0.927 |
| Shapiro–Wilk p | 0.908 | 0.818 | 0.762 | 0.574 |
Appendix B
| Descriptive Statistics | |||||
|---|---|---|---|---|---|
| Fixation Method | Dorsal Load (N) | Radial Load (N) | Torsion 5° (Nm) | Torsion 10° (Nm) | |
| Mean | Crossed wire | 249 | 356 | 0.214 | 0.334 |
| Parallel wire | 246 | 308 | 0.246 | 0.364 | |
| Single wire | 116 | 166 | 0.158 | 0.222 | |
| Median | Crossed wire | 246 | 357 | 0.202 | 0.313 |
| Parallel wire | 244 | 306 | 0.233 | 0.364 | |
| Single wire | 116 | 162 | 0.155 | 0.218 | |
| Standard deviation | Crossed wire | 14.1 | 12.6 | 0.0576 | 0.0721 |
| Parallel wire | 16.9 | 10.8 | 0.032 | 0.0225 | |
| Single wire | 5.15 | 9.45 | 0.0303 | 0.0272 | |
| Variance | Crossed wire | 198 | 160 | 0.00332 | 0.0052 |
| Parallel wire | 285 | 117 | 0.00103 | 5.07 × 10−4 | |
| Single wire | 26.5 | 89.2 | 9.21 × 10−4 | 7.37 × 10−4 | |
| Shapiro–Wilk W | Crossed wire | 0.935 | 0.959 | 0.834 | 0.909 |
| Parallel wire | 0.944 | 0.974 | 0.812 | 0.947 | |
| Single wire | 0.933 | 0.781 | 0.974 | 0.96 | |
| Shapiro–Wilk p | Crossed wire | 0.531 | 0.792 | 0.049 | 0.307 |
| Parallel wire | 0.627 | 0.927 | 0.028 | 0.656 | |
| Single wire | 0.509 | 0.012 | 0.927 | 0.796 | |
Appendix C
| ANOVA—Test Results | |||||||
|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | p | η2 | ||
| Fixation method | Dorsal Load (N) | 104,690 | 2 | 52345 | 309 | < 0.001 | 0.963 |
| Radial Load (N) | 174,647 | 2 | 87324 | 715 | <0.001 | 0.983 | |
| Subgroups of single wire fixation | Dorsal Load (N) | 2475 | 4 | 618.8 | 9.56 | <0.001 | 0.489 |
| Radial Load (N) | 1951 | 4 | 487.8 | 7.87 | <0.001 | 0.411 | |
| Torsion 5° (Nm) | 0.0107 | 4 | 0.00268 | 3.8 | 0.01 | 0.275 | |
| Torsion 10° (Nm) | 0.0265 | 4 | 0.00662 | 9.94 | <0.001 | 0.498 | |
Appendix D
| Post Hoc Comparisons—Test Results | |||||||
|---|---|---|---|---|---|---|---|
| Comparison | |||||||
| Experiment Setup | Name of the Group | Name of the Group | Mean Difference | t | ptukey | Cohen’s d * | |
| Fixation method, dorsal load (N) | Crosswire | - | Parallel Wire | 3.13 | 0.51 | 0.867 | 0.24 |
| - | Single Wire | 133.63 | 21.765 | <0.001 | 10.26 | ||
| Parallel Wire | - | Single Wire | 130.5 | 21.255 | <0.001 | 10.02 | |
| Fixation method, radial load (N) | Crosswire | - | Parallel Wire | 47.8 | 9.18 | <0.001 | 4.33 |
| - | Single Wire | 189.4 | 36.36 | <0.001 | 17.14 | ||
| Parallel Wire | - | Single Wire | 141.6 | 27.17 | <0.001 | 12.81 | |
| Single wire fixation with shifted exit point, dorsal load (N) | D10 | - | D5 | 7.94 | 2.093 | 0.243 | 0.987 |
| - | Single Wire | 2.19 | 0.578 | 0.978 | 0.273 | ||
| - | P5 | −4.6 | −1.211 | 0.745 | −0.571 | ||
| - | P10 | −14.18 | −3.738 | 0.005 | −1.762 | ||
| D5 | - | Single Wire | −5.75 | −1.515 | 0.559 | −0.714 | |
| - | P5 | −12.53 | −3.305 | 0.016 | −1.558 | ||
| - | P10 | −22.12 | −5.831 | <0.001 | −2.749 | ||
| Single Wire | - | P5 | −6.79 | −1.79 | 0.394 | −0.844 | |
| - | P10 | −16.37 | −4.316 | <0.001 | −2.034 | ||
| P5 | - | P10 | −9.58 | −2.526 | 0.105 | −1.191 | |
| Single wire fixation with shifted exit point, radial load (N) | D10 | - | D5 | 0.0441 | 0.0119 | 1 | 0.00561 |
| - | Single Wire | 9.2868 | 2.503 | 0.11 | 1.17993 | ||
| - | P5 | 15.6805 | 4.2263 | 0.001 | 1.99229 | ||
| - | P10 | 13.4185 | 3.6166 | 0.007 | 1.70489 | ||
| D5 | - | Single Wire | 9.2427 | 2.4911 | 0.113 | 1.17433 | |
| - | P5 | 15.6364 | 4.2144 | 0.001 | 1.98668 | ||
| - | P10 | 13.3744 | 3.6047 | 0.007 | 1.69929 | ||
| Single Wire | - | P5 | 6.3937 | 1.7233 | 0.432 | 0.81235 | |
| - | P10 | 4.1318 | 1.1136 | 0.798 | 0.52496 | ||
| P5 | - | P10 | −2.2619 | −0.61 | 0.973 | −0.28739 | |
| Single wire fixation with shifted exit point, Torsion 5° (Nm) | D10 | - | D5 | 0.03055 | 2.443 | 0.125 | 1.151 |
| - | Single Wire | 0.03406 | 2.724 | 0.068 | 1.284 | ||
| - | P5 | 0.00935 | 0.747 | 0.944 | 0.352 | ||
| - | P10 | 0.04015 | 3.211 | 0.021 | 1.513 | ||
| D5 | - | Single Wire | 0.00352 | 0.281 | 0.999 | 0.132 | |
| - | P5 | −0.0212 | −1.695 | 0.448 | −0.799 | ||
| - | P10 | 0.0096 | 0.768 | 0.938 | 0.362 | ||
| Single Wire | - | P5 | −0.02472 | −1.976 | 0.296 | −0.932 | |
| - | P10 | 0.00609 | 0.487 | 0.988 | 0.23 | ||
| P5 | - | P10 | 0.03081 | 2.463 | 0.12 | 1.161 | |
| Single wire fixation with shifted exit point, Torsion 10° (Nm) | D10 | - | D5 | 0.03689 | 3.032 | 0.033 | 1.429 |
| - | Single Wire | 0.04135 | 3.4 | 0.013 | 1.603 | ||
| - | P5 | 0.04482 | 3.684 | 0.006 | 1.737 | ||
| - | P10 | 0.07615 | 6.26 | <0.001 | 2.951 | ||
| D5 | - | Single Wire | 0.00447 | 0.367 | 0.996 | 0.173 | |
| - | P5 | 0.00793 | 0.652 | 0.965 | 0.307 | ||
| - | P10 | 0.03927 | 3.228 | 0.02 | 1.522 | ||
| Single Wire | - | P5 | 0.00346 | 0.285 | 0.999 | 0.134 | |
| - | P10 | 0.0348 | 2.861 | 0.049 | 1.348 | ||
| P5 | - | P10 | 0.03134 | 2.576 | 0.094 | 1.214 | |
Appendix E
| Kruskal–Wallis | ||||
|---|---|---|---|---|
| χ2 | df | p | ε2 | |
| Torsion 5° (Nm) | 15.6 | 2 | <0.001 | 0.601 |
| Torsion 10° (Nm) | 17.7 | 2 | <0.001 | 0.681 |
Appendix F
| Post Hoc Tests—Results | |||||
|---|---|---|---|---|---|
| W | p | ||||
| Torsion 5° (Nm) | Crosswire | - | Parallel Wire | 3.06 | 0.078 |
| Crosswire | - | Single Wire | −3.43 | 0.04 | |
| Parallel Wire | - | Single Wire | −5.06 | 0.001 | |
| Torsion 10° (Nm) | Crosswire | - | Parallel Wire | 2.19 | 0.27 |
| Crosswire | - | Single Wire | −4.81 | 0.002 | |
| Parallel Wire | - | Single Wire | −5.06 | 0.001 | |
Appendix G
| Descriptive Statistics | |||||
|---|---|---|---|---|---|
| Fixation Method | Dorsal Load (N) | Radial Load (N) | Torsion 5° (Nm) | Torsion 10° (Nm) | |
| Mean | D10 | 118 | 176 | 0.192 | 0.263 |
| D5 | 110 | 176 | 0.162 | 0.226 | |
| Single wire | 116 | 166 | 0.158 | 0.222 | |
| P5 | 123 | 160 | 0.183 | 0.219 | |
| P10 | 132 | 162 | 0.152 | 0.187 | |
| Median | D10 | 120 | 175 | 0.192 | 0.265 |
| D5 | 111 | 175 | 0.163 | 0.226 | |
| Single wire | 116 | 162 | 0.155 | 0.218 | |
| P5 | 121 | 160 | 0.172 | 0.226 | |
| P10 | 134 | 162 | 0.146 | 0.177 | |
| Standard deviation | D10 | 9.03 | 7.98 | 0.0152 | 0.0133 |
| D5 | 9.69 | 10.4 | 0.019 | 0.0199 | |
| Single wire | 5.15 | 9.45 | 0.0303 | 0.0272 | |
| P5 | 3.19 | 6.28 | 0.0283 | 0.0294 | |
| P10 | 10.6 | 3.15 | 0.0347 | 0.0339 | |
| Variance | D10 | 81.6 | 63.7 | 2.30 × 10−4 | 1.77 × 10−4 |
| D5 | 93.9 | 107 | 3.62 × 10−4 | 3.98 × 10−4 | |
| Single wire | 26.5 | 89.2 | 9.21 × 10−4 | 7.37 × 10−4 | |
| P5 | 10.2 | 39.5 | 8.01 × 10−4 | 8.65 × 10−4 | |
| P10 | 112 | 9.95 | 0.00121 | 0.00115 | |
| Shapiro–Wilk W | D10 | 0.912 | 0.945 | 0.886 | 0.929 |
| D5 | 0.956 | 0.956 | 0.962 | 0.963 | |
| Single wire | 0.933 | 0.781 | 0.974 | 0.96 | |
| P5 | 0.858 | 0.924 | 0.844 | 0.927 | |
| P10 | 0.836 | 0.907 | 0.97 | 0.98 | |
| Shapiro–Wilk p | D10 | 0.331 | 0.636 | 0.183 | 0.476 |
| D5 | 0.754 | 0.755 | 0.816 | 0.833 | |
| Single wire | 0.509 | 0.012 | 0.927 | 0.796 | |
| P5 | 0.091 | 0.424 | 0.063 | 0.451 | |
| P10 | 0.052 | 0.295 | 0.899 | 0.964 | |
References
- Beekman, F.; Sullivan, J.E. Some observations on fractures of long bones in children. Am. J. Surg. 1941, 51, 722–738. [Google Scholar] [CrossRef]
- Crawford, A.H. Pitfalls and complications of fractures of the distal radius and ulna in childhood. Hand Clin. 1988, 4, 403–413. [Google Scholar] [CrossRef]
- Blount, W.P.; Schaefer, A.A.; Johnson, J.H. Fractures of the Forearm in Children. J. Am. Med. Assoc. 1942, 120, 111–116. [Google Scholar] [CrossRef]
- Voto, S.J.; Weiner, D.S.; Leighley, B. Redisplacement after closed reduction of forearm fractures in children. J. Pediatr. Orthop. 1990, 10, 79–84. [Google Scholar] [CrossRef]
- Gandhi, R.K.; Wilson, P.; Mason Brown, J.J.; Macleod, W. Spontaneous correction of deformity following fractures of the forearm in children. Br. J. Surg. 1962, 50, 5–10. [Google Scholar] [CrossRef]
- Wendling-Keim, D.S.; Wieser, B.; Dietz, H.G. Closed reduction and immobilization of displaced distal radial fractures. Method of choice for the treatment of children? Eur. J. Trauma Emerg. Surg. 2015, 41, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.S.; Williams, R.L. Forearm fractures in children: Avoiding redisplacement. Injury 1995, 26, 691–692. [Google Scholar] [CrossRef]
- Voto, S.J.; Weiner, D.S.; Leighley, B. Use of pins and plaster in the treatment of unstable pediatric forearm fractures. J. Pediatr. Orthop. 1990, 10, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Satish, B.R.; Vinodkumar, M.; Suresh, M.; Seetharam, P.Y.; Jaikumar, K. Closed reduction and K-wiring with the Kapandji technique for completely displaced pediatric distal radial fractures. Orthopedics 2014, 37, e810–e816. [Google Scholar] [CrossRef]
- Parikh, S.N.; Jain, V.V.; Youngquist, J. Intrafocal pinning for distal radius metaphyseal fractures in children. Orthopedics 2013, 36, 783–788. [Google Scholar] [CrossRef]
- Lieber, J.; Schmid, E.; Schmittenbecher, P.P. Unstable diametaphyseal forearm fractures: Transepiphyseal intramedullary Kirschner-wire fixation as a treatment option in children. Eur. J. Pediatr. Surg. 2010, 20, 395–398. [Google Scholar] [CrossRef]
- Hargreaves, D.G.; Drew, S.J.; Eckersley, R. Kirschner wire pin tract infection rates: A randomized controlled trial between percutaneous and buried wires. J. Hand Surg. Br. 2004, 29, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Kantharuban, S.; Shilston, S.; Pearce, O.J. Complications of Kirschner-wire fixation in distal radius fractures. Tech. Hand Up Extrem. Surg. 2012, 16, 120–123. [Google Scholar] [CrossRef]
- Miller, B.S.; Taylor, B.; Widmann, R.F.; Bae, D.S.; Snyder, B.D.; Waters, P.M. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: A prospective, randomized study. J. Pediatric Orthop. 2005, 25, 490–494. [Google Scholar] [CrossRef]
- McLauchlan, G.J.; Cowan, B.; Annan, I.H.; Robb, J.E. Management of completely displaced metaphyseal fractures of the distal radius in children. A prospective, randomised controlled trial. J. Bone Joint Surg. Br. 2002, 84, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Rastogi, V.; Ginn, M. A review on 3D printing techniques for medical applications. Curr. Opin. Chem. Eng. 2020, 28, 152–157. [Google Scholar] [CrossRef]
- Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3D Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Ujfalusi, Z.; Pentek, A.; Told, R.; Schiffer, A.; Nyitrai, M.; Maroti, P. Detailed Thermal Characterization of Acrylonitrile Butadiene Styrene and Polylactic Acid Based Carbon Composites Used in Additive Manufacturing. Polymers 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Toth, L.; Schiffer, A.; Nyitrai, M.; Pentek, A.; Told, R.; Maroti, P. Developing an anti-spastic orthosis for daily home-use of stroke patients using smart memory alloys and 3D printing technologies. Mater. Des. 2020, 195, 109029. [Google Scholar] [CrossRef]
- Tilton, M.; Lewis, G.S.; Bok Wee, H.; Armstrong, A.; Hast, M.W.; Manogharan, G. Additive manufacturing of fracture fixation implants: Design, material characterization, biomechanical modeling and experimentation. Addit. Manuf. 2020, 33, 101137. [Google Scholar] [CrossRef]
- Longfield, E.A.; Brickman, T.M.; Jeyakumar, A. 3D Printed Pediatric Temporal Bone: A Novel Training Model. Otol. Neurotol. 2015, 36, 793–795. [Google Scholar] [CrossRef]
- Hochman, J.B.; Kraut, J.; Kazmerik, K.; Unger, B.J. Generation of a 3D printed temporal bone model with internal fidelity and validation of the mechanical construct. Otolaryngol. Head Neck Surg. 2014, 150, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Mamelle, E.; De Seta, D.; Sterkers, O.; Bernardeschi, D.; Torres, R. Modifications to a 3D-printed temporal bone model for augmented stapes fixation surgery teaching. Eur Arch. Otorhinolaryngol. 2017, 274, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M. 3D printed medical parts with different materials using additive manufacturing. Clin. Epidemiol. Glob. Health 2020, 8, 215–223. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Bagheri, A.; Petit-Rojo, O. 3D Printing of Porous Scaffolds with Controlled Porosity and Pore Size Values. Materials 2018, 11, 1532. [Google Scholar] [CrossRef]
- Pepelnjak, T.; Karimi, A.; Maček, A.; Mole, N. Altering the Elastic Properties of 3D Printed Poly-Lactic Acid (PLA) Parts by Compressive Cyclic Loading. Materials 2020, 13, 4456. [Google Scholar] [CrossRef]
- Gadaleta, D.J.; Huang, D.; Rankin, N.; Hsue, V.; Sakkal, M.; Bovenzi, C.; Huntley, C.T.; Willcox, T.; Pelosi, S.; Pugliese, R.; et al. 3D printed temporal bone as a tool for otologic surgery simulation. Am. J. Otolaryngol. 2020, 41, 102273. [Google Scholar] [CrossRef]
- Haffner, M.; Quinn, A.; Hsieh, T.Y.; Strong, E.B.; Steele, T. Optimization of 3D Print Material for the Recreation of Patient-Specific Temporal Bone Models. Ann. Otol. Rhinol. Laryngol. 2018, 127, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Goudie, C.; Kinnin, J.; Bartellas, M.; Gullipalli, R.; Dubrowski, A. The Use of 3D Printed Vasculature for Simulation-based Medical Education Within Interventional Radiology. Cureus 2019, 11, e4381. [Google Scholar] [CrossRef]
- Told, R.; Marada, G.; Rendeki, S.; Pentek, A.; Nagy, B.; Molnar, F.J.; Maroti, P. Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide. Polymers 2021, 13, 2647. [Google Scholar] [CrossRef]
- Johnson, J.P.; Borenstein, T.R.; Waryasz, G.R.; Klinge, S.A.; McClure, P.K.; Chambers, A.B.; Hayda, R.A.; Born, C.T. Vertically Oriented Femoral Neck Fractures: A Biomechanical Comparison of 3 Fixation Constructs. J. Orthop. Trauma 2017, 31, 363–368. [Google Scholar] [CrossRef]
- Bizzotto, N.; Tami, I.; Tami, A.; Spiegel, A.; Romani, D.; Corain, M.; Adani, R.; Magnan, B. 3D Printed models of distal radius fractures. Injury 2016, 47, 976–978. [Google Scholar] [CrossRef]
- Caiti, G.; Dobbe, J.G.G.; Strijkers, G.J.; Strackee, S.D.; Streekstra, G.J. Positioning error of custom 3D-printed surgical guides for the radius: Influence of fitting location and guide design. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 507–518. [Google Scholar] [CrossRef]
- Wang, X.; Sanyal, A.; Cawthon, P.M.; Palermo, L.; Jekir, M.; Christensen, J.; Ensrud, K.E.; Cummings, S.R.; Orwoll, E.; Black, D.M.; et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J. Bone Miner. Res. 2012, 27, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Pentek, A.; Nyitrai, M.; Schiffer, A.; Abraham, H.; Bene, M.; Molnar, E.; Told, R.; Maroti, P. The Effect of Printing Parameters on Electrical Conductivity and Mechanical Properties of PLA and ABS Based Carbon Composites in Additive Manufacturing of Upper Limb Prosthetics. Crystals 2020, 10, 398. [Google Scholar] [CrossRef]
- Kyoung, S.U.S.; Panxi, Z.; Byoung-Chul, S.; Sung-Uk, Z. Infill Print Parameters for Mechanical Properties of 3D Printed PLA Parts. Korean Soc. Manuf. Process. Eng. 2018, 17, 9–16. [Google Scholar]
- Gonabadi, H.; Chen, Y.; Yadav, A.; Bull, S. Investigation of the effect of raster angle, build orientation, and infill density on the elastic response of 3D printed parts using finite element microstructural modeling and homogenization techniques. Int. J. Adv. Manuf. Technol. 2021, 1–26. [Google Scholar] [CrossRef]
- Kovan, V.; Tezel, T.; Topal, E.S.; Çamurlu, H.E. Printing Parameters Effect on Surface Characteristics of 3D Printed Pla Materials. Mach. Technol. Mater. 2018, 12, 266–269. [Google Scholar]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J. Uses and abuses of analysis of variance. Br. J. Clin. Pharmacol. 1983, 15, 629–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rea, L.M.; Parker, R.A. Designing and Conducting Survey Research: A Comprehensive Guide; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zemel, B.; Bass, S.; Binkley, T.; Ducher, G.; Macdonald, H.; McKay, H.; Moyer-Mileur, L.; Shepherd, J.; Specker, B.; Ward, K.; et al. Peripheral quantitative computed tomography in children and adolescents: The 2007 ISCD Pediatric Official Positions. J. Clin. Densitom. 2008, 11, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Mischler, D.; Wee, H.; Reid, J.; Varga, P. Finite Element Analysis of Fracture Fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [CrossRef] [PubMed]



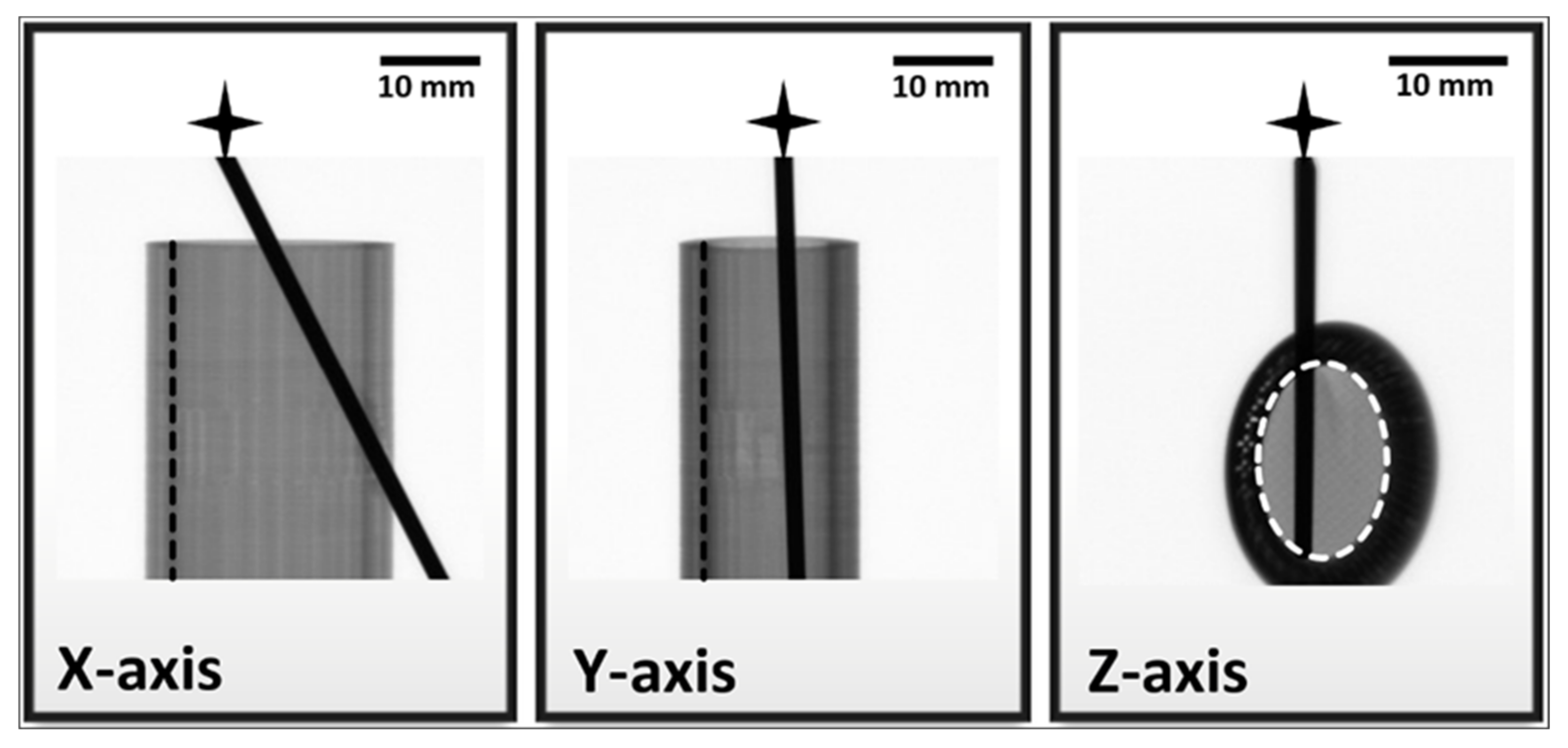
| Numeric Values of Grayscale Shades | |||
|---|---|---|---|
| Cortical | Medullar | K-wire | |
| X-axis | 105 ± 1 | 129 ± 3 | 5 ± 1 |
| Y-axis | 69 ± 1 | 116 ± 9 | 5 ± 1 |
| Z-axis | 17 ± 2 | 133 ± 4 | 4 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, A.G.; Ujfalusi, Z.; Told, R.; Hanna, D.; Józsa, G.; Maróti, P. Development of a Novel X-ray Compatible 3D-Printed Bone Model to Characterize Different K-Wire Fixation Methods in Support of the Treatment of Pediatric Radius Fractures. Polymers 2021, 13, 4179. https://doi.org/10.3390/polym13234179
Lamberti AG, Ujfalusi Z, Told R, Hanna D, Józsa G, Maróti P. Development of a Novel X-ray Compatible 3D-Printed Bone Model to Characterize Different K-Wire Fixation Methods in Support of the Treatment of Pediatric Radius Fractures. Polymers. 2021; 13(23):4179. https://doi.org/10.3390/polym13234179
Chicago/Turabian StyleLamberti, Anna Gabriella, Zoltan Ujfalusi, Roland Told, Dániel Hanna, Gergő Józsa, and Péter Maróti. 2021. "Development of a Novel X-ray Compatible 3D-Printed Bone Model to Characterize Different K-Wire Fixation Methods in Support of the Treatment of Pediatric Radius Fractures" Polymers 13, no. 23: 4179. https://doi.org/10.3390/polym13234179
APA StyleLamberti, A. G., Ujfalusi, Z., Told, R., Hanna, D., Józsa, G., & Maróti, P. (2021). Development of a Novel X-ray Compatible 3D-Printed Bone Model to Characterize Different K-Wire Fixation Methods in Support of the Treatment of Pediatric Radius Fractures. Polymers, 13(23), 4179. https://doi.org/10.3390/polym13234179









