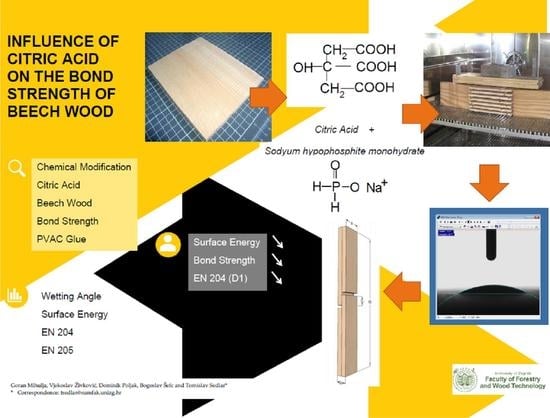
Influence of Citric Acid on the Bond Strength of Beech Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Wetting Angle and Surface Energy
2.3. Bond Strength (EN 204)
3. Results
3.1. Wetting Angle and Surface Energy
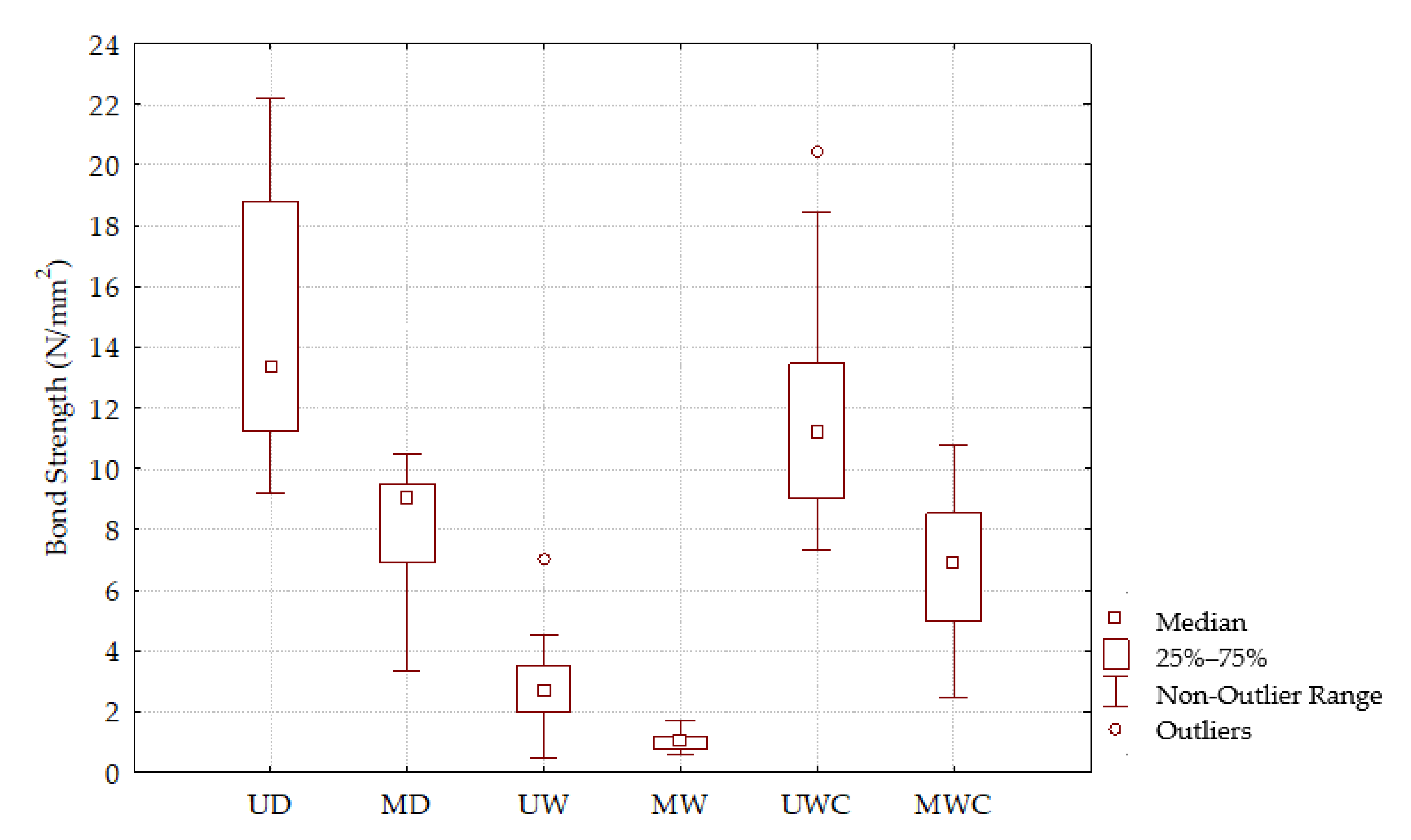
3.2. Bond Strength
3.2.1. Group 1—(Unmodified Dry (UD) and Modified Dry (MD))
3.2.2. Group 2—(Unmodified Wet (UW) and Modified Wet (MW))
3.2.3. Group 3—(Unmodified Wet Conditioned (UWC) and Modified Wet Conditioned (MWC))
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowell, R.M.; Konkol, P. Treatments that Enhance Physical Properties of Wood; Forest Products Laboratory General Technical Report FPL-GTR-55; United States Department of Agriculture, Forest Service: Washington, DC, USA, 1987.
- Militz, H.; Beckers, E.P.J.; Homan, W.J. Modification of Solid Wood: Research and Practical Potential; Document No.: IRG/WP 97-40098; The International Research Group on Wood Preservation: Stockholm, Sweden, 1997. [Google Scholar]
- Yildiz, S.; Gumuskaya, E. The effects of thermal modification on crystalline structure of cellulose in soft and hardwood. Build. Environ. 2007, 41, 62–67. [Google Scholar] [CrossRef]
- Cai, Z.; Ji, B.; Yan, K.; Zhu, Q. Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy. Polymers 2019, 11, 2071. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. Chemical modification of wood. Wood Fiber Sci. 1994, 26, 270–280. [Google Scholar]
- Hill, C.A.S. Wood Modification—Chemical, Thermal, and Other Processes; John Wiley & Sons: Chichester, UK; Hoboken, NJ, USA, 2006; p. 260. [Google Scholar]
- Despot, R.; Hasan, M.; Šefc, B.; Jug, M. Biological durability of wood modified by citric acid. Drv. Ind. 2008, 59, 55–59. [Google Scholar]
- Šefc, B.; Trajković, J.; Sinković, T.; Hasan, M.; Ištok, I. Compression Strength of Fir and Beech Wood Modified by Citric Acid. Drvna inudstrija 2012, 63, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Bischof Vukušić, S.; Katović, D.; Schramm, C.; Trajković, J.; Šefc, B. Polycarboxylic acids as non-formaldehyde antiswelling agents for wood. Holzforschung 2006, 60, 439–444. [Google Scholar] [CrossRef]
- Hasan, M.; Despot, R.; Katović, D.; Bischof Vukusic, S.; Bogner, A.; Jambreković, V. Citric Acid—Promising Agent for Increased Biological Effectiveness of Wood. In Proceedings of the 3rd European Conference on Wood Modification, Cardiff, UK, 15–16 October 2007. [Google Scholar]
- Šefc, B. Utjecaj Obrade Drva Limunskom Kiselinom na Njegova Svojstva. Ph.D. Thesis, University of Zagreb, Faculty of Forestry, Zagreb, Croatia, 2006. [Google Scholar]
- Šefc, B.; Trajković, J.; Hasan, M.; Katović, D.; Bischof Vukušić, S.; Frančić, M. Dimensional stability of wood modified by citric acid using different catalysts. Drvna Ind. 2009, 60, 23–26. [Google Scholar]
- Yuan, J.; Hu, Y.; Li, L.; Cheng, F. The mechanical strength change of wood modified with DMDHEU. BioResources 2013, 8, 1076–1088. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Li, X. Comparison of physical-mechanical and mould-proof properties of furfurylated and DMDHEU-modified wood. BioResources 2019, 14, 9628–9644. [Google Scholar] [CrossRef]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech wood modification based on in situ esterification with sorbitol and citric acid. Wood Sci. Technol. 2020, 54, 79–502. [Google Scholar] [CrossRef] [Green Version]
- Emmerich, L.; Militz, H.; Brischke, C. Long-term performance of DMDHEU-treated wood installed in different test set-ups in ground, above ground and in the marine environment. Int. Wood Prod. J. 2020, 11, 27–37. [Google Scholar] [CrossRef]
- Mamiński, M.; Kozakiewicz, P.; Jaskółowski, W.; Chin, K.L.; H’ng, P.S.; Toczyłowska-Mamińska, R. Enhancement of technical value of oil palm (Elaeis guineensis Jacq.) waste trunk through modification with 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU). Eur. J. Wood Prod. 2016, 74, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, R.; Minato, K. Chemical Modification of Wood by Non-Formaldehyde Cross-linking Reagents, Part 1. Wood Sci. Technol. 1994, 28, 101–110. [Google Scholar] [CrossRef]
- Peyer, S.M.; Wolcott, M.P.; Fenoglio, D.J. Reducing moisture swell of densified wood with polycarboxylicacid resin. Wood Fiber Sci. 2007, 32, 520–526. [Google Scholar]
- Militz, H. Treatment of timber with water soluble dimethylol resins to improve their dimensional stability and durability. Wood Sci. Technol. 1993, 27, 347–355. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wang, X.; Kang, I.S. Ester Crosslinking of Cotton Fabric by Polymeric Carboxylic Acids and Citric Acid. Text. Res. J. 1997, 67, 334–342. [Google Scholar] [CrossRef]
- Katović, D.; Bischof-Vukušić, S.; Štefanić, G. Istraživanja mehanizama esterifikacije polikarboksilnih kiselina sa celuloznim materijalom. Tekstil 2000, 49, 551–554. [Google Scholar]
- Follrich, J.; Müller, U.; Gindl, W. Effects of thermal modification on the adhesion between spruce wood (Picea abies Karst.) and athermoplastic polymer. Holz als Roh-und Werksoff 2006, 64, 373–376. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Wood chemical modification based on bio-based polycarboxylic acid and polyols—status quo and future perspectives. Wood Mater. Sci. Eng. 2021, 1–15. [Google Scholar] [CrossRef]
- Katović, D.; Bischof-Vukušić, S.; Trajković, J.; Šefc, B. Alternativna sredstva i postupci kemijske modifikacije drva. Drvna Ind. 2004, 55, 175–180. [Google Scholar]
- Ando, D.; Umemura, K. Bond Structures between Wood Components and Citric Acid in Wood-Based Molding. Polymers 2021, 13, 58. [Google Scholar] [CrossRef]
- Miklečić, J.; Jirouš Rajković, V. Accelerated weathering of coated and uncoated beech wood modified with citric acid. Drvna Ind. 2011, 62, 277–282. [Google Scholar] [CrossRef]
- Lee, S.H.; Md Tahir, P.; Lum, W.C.; Tan, L.P.; Bawon, P.; Park, B.-D.; Osman Al Edrus, S.S.; Abdullah, U.H. A Review on Citric Acid as Green Modifying Agent and Binder for Wood. Polymers 2020, 12, 1692. [Google Scholar] [CrossRef]
- Blomquist, R.F. 1963 Edgar Marburg Lecture: Adhesives—Past, Present, and Future. In Adhesion; ASTM International: West Conshohocken, PA, USA, 1964; pp. 179–212. [Google Scholar] [CrossRef]
- Bogner, A. Kvašenje drva i adhezija. Drvna Ind. 1993, 44, 139–143. [Google Scholar]
- Bryant, B.S. Interaction of Wood Surface, and Adhesive Variables. For. Prod. J. 1968, 18, 57–62. [Google Scholar]
- Qiao, P.Z.; Trimble, B.S. Fiber-reinforced composite, and wood bonded interfaces: Part 1. Durability and shear strength. J. Compos. Technol. Res. 2000, 22, 224–231. [Google Scholar]
- Laidlaw, R.A.; Paxton, B.H. The effect of moisture content and wood preservatives on the assembly gluing of timber. In Building Research Establishment; Current Paper CP; Princes Risborough Laboratory: Aylesbury, UK, 1974. [Google Scholar]
- Lavisci, P.; Berti, S.; Pizzo, B.; Triboulot, P.; Zanuttini, R. A delamination test for structural wood adhesives used in thick joints. Holz als Roh-und Werkstoff 2001, 59, 153–154. [Google Scholar] [CrossRef]
- Stehr, M.; Östlund, S. An Investigation of the Crack Tendency on Wood Surfaces After Different Machining Operations. Holzforschung 2000, 54, 427–436. [Google Scholar] [CrossRef]
- Mihulja, G.; Bogner, A.; Banči, D. A new method for the determination of the proportion of wood failure area on the glue bond. Drvna Ind. 2008, 58, 193–197. [Google Scholar]
- Sun, S.; Zhao, Z.; Umemura, K. Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Huang, C.; Sun, S.; Zhang, M.; Umemura, K.; Yong, Q. Further Exploration of Sucrose–Citric Acid Adhesive: Investigation of Optimal Hot-Pressing Conditions for Plywood and Curing Behavior. Polymers 2019, 11, 1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huaxu, Z.; Hua, L.S.; Tahir, P.M.; Ashaari, Z.; Al-Edrus, S.S.O.; Ibrahim, N.A.; Abdullah, L.C.; Mohamad, S.F. Physico-Mechanical and Biological Durability of Citric Acid-Bonded Rubberwood Particleboard. Polymers 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Standardization. European Standard. In EN 204 Classification of Thermoplastic Wood Adhesives for Non-Structural Applications; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- European Committee for Standardization. European Standard. In EN 205 Adhesives—Wood Adhesives for Non-Structural Applications—Determination of Tensile Shear Strength of Lap Joints; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- European Committee for Standardization. European Standard. In EN 828 Adhesives—Wettability—Determination by Measurement of Contact Angle and Surface Free Energy of Solid Surface; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- Gindl, M.; Reiterer, A.; Sinn, G.; Stanzl-Tschegg, S.E. Effect of Surface ageing on wettability, surface chemistry, and adhesion of wood. Holz als Roh-und Werkstoff 2004, 62, 237–280. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wu, S. Calculation of interfacial tension in polymer systems. J. Polym. Sci. Part C Polym. Symp. 1971, 34, 19–30. [Google Scholar] [CrossRef]


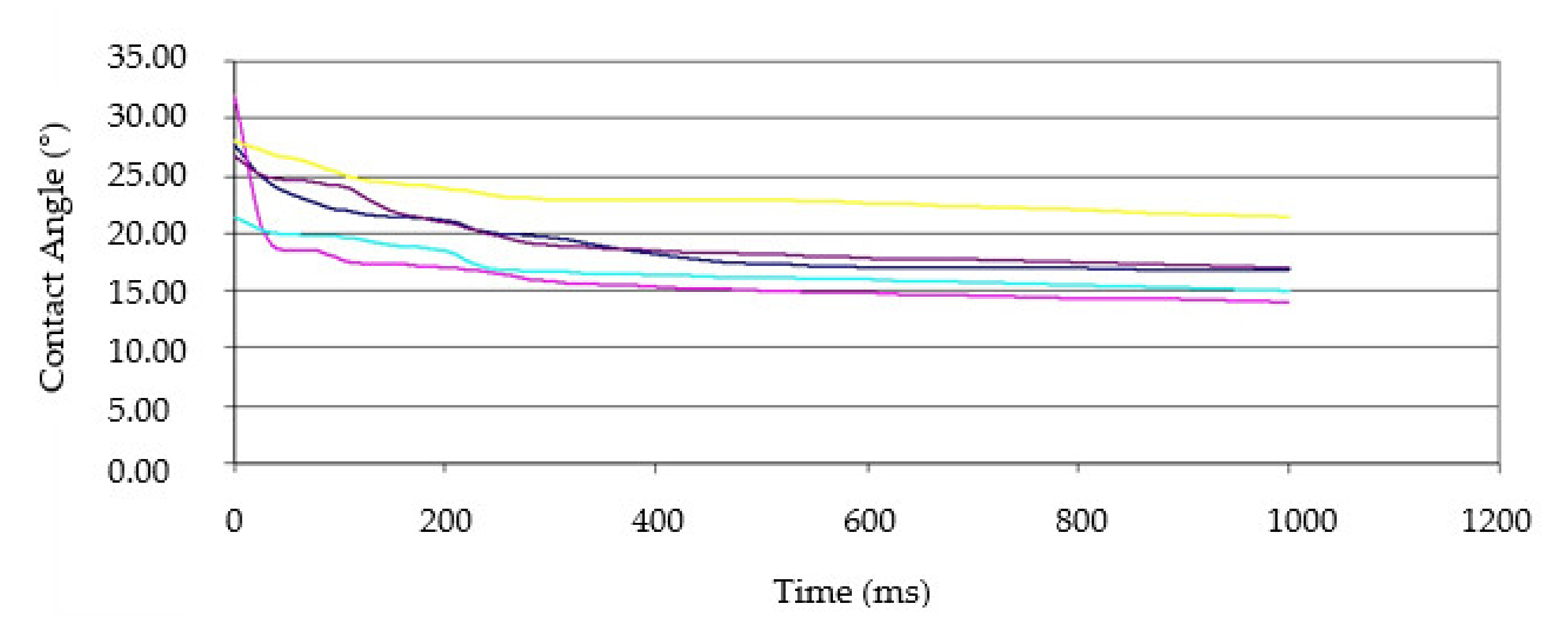


| Liquid | SFT (total) | SFT (LW 1) | SFT (Acid) | SFT (Base) | Modified Samples (sec.) | Control Samples (sec.) |
|---|---|---|---|---|---|---|
| Water [Ström et al.] | 72.80 | 21.80 | 25.50 | 25.50 | 4.50 | 5.00 |
| Formamide [Van Oss et al.] | 58.00 | 39.00 | 2.28 | 39.60 | 0.45 | 1.00 |
| Diiodomethane [Erbil] | 50.08 | 50.08 | 0.00 | 0.00 | 0.50 | 0.50 |
| Semple Type | Sample Mark | Number of Test Samples |
|---|---|---|
| Reference samples tested in dry condition (23 °C and 50%) | UD | 20 |
| Modified samples tested in dry condition (23 °C and 50%) | MD | 20 |
| Reference samples tested after immersed in water for 72 h | UW | 20 |
| Modified samples tested after immersed in water for 72 h | MW | 20 |
| Reference samples tested conditioned (23 °C and 50%) for seven days after soaking in water | UWC | 20 |
| Modified samples tested conditioned (23 °C and 50%) for seven days after soaking in water | MWC | 20 |
| Wetting Angle | Analysis of Variance-Marked Effects Are Significant at p < 0.05 | |||||||
|---|---|---|---|---|---|---|---|---|
| SS Effect | df Effect | MS Effect | SS Error | df Error | MS Error | F | p | |
| Water | 232.902 | 1 | 232.902 | 1309.630 | 29 | 45.159 | 5.157 | 0.0303 |
| Formamide | 38.002 | 1 | 38.002 | 250.081 | 28 | 8.931 | 4.254 | 0.0485 |
| Diiodomethane | 63.637 | 1 | 63.637 | 397.094 | 28 | 14.181 | 4.487 | 0.0431 |
| Method. | Work | WU | ||||
|---|---|---|---|---|---|---|
| Surface energy Total (mN/m) | Dispersive | Polar | Surface Energy Total (mN/m) | Dispersive | Polar | |
| Unmodified wood | 72.38 | 47.62 | 24.76 | 77.74 | 47.66 | 30.08 |
| Modified wood | 62.16 | 45.07 | 17.09 | 65.65 | 43.58 | 22.07 |
| Difference (%) | 14.12 | 15.55 | ||||
| Specimen Group | Mark | EN 204 Requirements (N/mm2) | BS (N/mm2) | SD (N/mm2) | C.V. (%) | BS Decrease (%) |
|---|---|---|---|---|---|---|
| Group 1 | UD | ≥10 | 14.92 | 4.269 | 28.61 | |
| MD | 8.12 | 2.040 | 25.12 | 45.58 | ||
| Group 2 | UW | ≥2 | 2.89 | 1.709 | 59.19 | |
| MW | 1.06 | 0.302 | 28.52 | 63.32 | ||
| Group 3 | UWC | ≥8 | 11.70 | 3.701 | 32.46 | |
| MWC | 6.77 | 2.565 | 37.87 | 42.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihulja, G.; Živković, V.; Poljak, D.; Šefc, B.; Sedlar, T. Influence of Citric Acid on the Bond Strength of Beech Wood. Polymers 2021, 13, 2801. https://doi.org/10.3390/polym13162801
Mihulja G, Živković V, Poljak D, Šefc B, Sedlar T. Influence of Citric Acid on the Bond Strength of Beech Wood. Polymers. 2021; 13(16):2801. https://doi.org/10.3390/polym13162801
Chicago/Turabian StyleMihulja, Goran, Vjekoslav Živković, Dominik Poljak, Bogoslav Šefc, and Tomislav Sedlar. 2021. "Influence of Citric Acid on the Bond Strength of Beech Wood" Polymers 13, no. 16: 2801. https://doi.org/10.3390/polym13162801
APA StyleMihulja, G., Živković, V., Poljak, D., Šefc, B., & Sedlar, T. (2021). Influence of Citric Acid on the Bond Strength of Beech Wood. Polymers, 13(16), 2801. https://doi.org/10.3390/polym13162801