A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-)Polyphospinate and Melamine Polyphosphate for Polyamide 6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.1.1. Fire Testing
2.1.2. Decomposition Analytics
2.1.3. Microscopy
3. Results
3.1. Thermal Decomposition
3.2. Evolved Gas Analysis—TGA-FTIR and TGA-GC/MS
3.3. Burning Behavior—Cone Calorimeter, LOI and UL-94
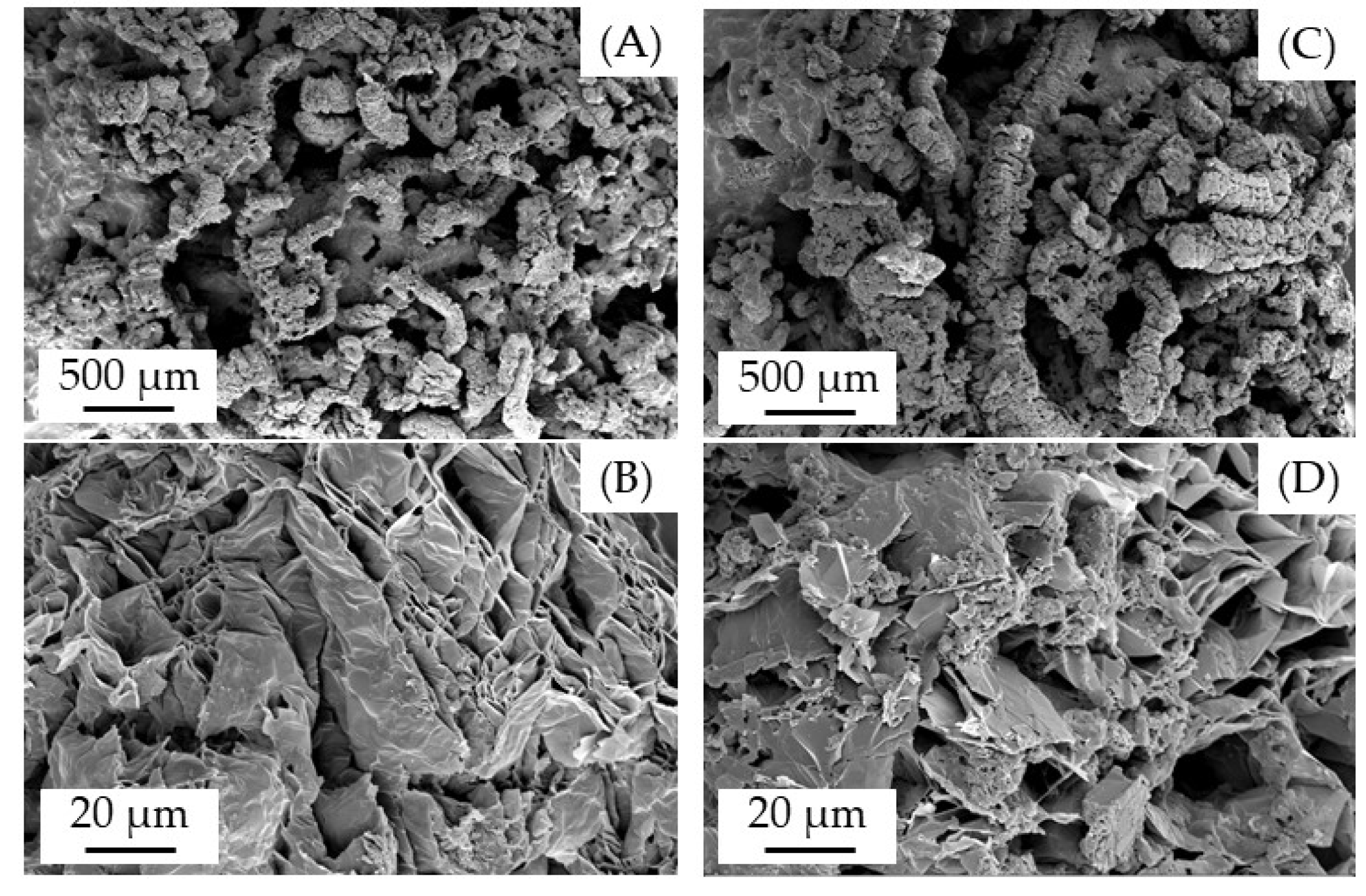
3.4. Char Residue Analysis
4. Discussion
5. Conclusions
- PA6 + 20 wt.% EG/5 wt.% AlPi/MPP (3:2) formulations were found to improve the LOI to 46%, whereas only minor performance compromises in cone calorimeter tests must be accepted.
- Two effects have been concluded to work synergistically: (1) A complementary CO2 evaporation given by PA6/AlPi/MPP decomposition works efficiently by gas phase dilution, while expandable graphite is still in early expansion stages giving non or little fire barrier. (2) Solid PA6/AlPi/MPP decomposition products also work as “glue” in between expanded graphite, significantly stabilizing the residual char.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. e-Polymers 2010, 10, 1. [Google Scholar] [CrossRef]
- Seefeldt, H.; Duemichen, E.; Braun, U. Flame retardancy of glass fiber reinforced high temperature polyamide by use of aluminum diethylphosphinate: Thermal and thermo-oxidative effects. Polym. Int. 2013, 62, 1608–1616. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Levchik, S.V.; Camino, G.; Costa, L.; Levchik, G.F. Mechanism of action of phosphorus-based flame retardants in nylon 6. I. Ammonium polyphosphate. Fire Mater. 1995, 19, 1–10. [Google Scholar] [CrossRef]
- Levchik, S.V.; Levchik, G.F.; Camino, G.; Costa, L.; Lesnikovich, A.I. Mechanism of Action of Phosphorus-based Flame Retardants in Nylon 6. III. Ammonium Polyphosphate/Manganese Dioxide. Fire Mater. 1996, 20, 183–190. [Google Scholar] [CrossRef]
- Tai, Q.; Yuen, R.K.K.; Yang, W.; Qiao, Z.; Song, L.; Hu, Y. Iron-montmorillonite and zinc borate as synergistic agents in flame-retardant glass fiber reinforced polyamide 6 composites in combination with melamine polyphosphate. Compos. Part A Appl. Sci. Manuf. 2012, 43, 415–422. [Google Scholar] [CrossRef]
- Batistella, M.A.; Sonnier, R.; Otazaghine, B.; Petter, C.O.; Lopez-Cuesta, J.-M. Interactions between kaolinite and phosphinate-based flame retardant in Polyamide 6. Appl. Clay Sci. 2018, 157, 248–256. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the scientific approach to polymer flame retardancy: Part 2—Concepts. J. Fire Sci. 2017, 35, 3–20. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the scientific approach to polymer flame retardancy: Part 1—Scientific terms and methods. J. Fire Sci. 2016, 34, 447–467. [Google Scholar] [CrossRef]
- Focke, W.W.; Kruger, H.J.; Mhike, W.; Taute, A.; Roberson, A.; Ofosu, O. Polyethylene flame retarded with expandable graphite and a novel intumescent additive. J. Appl. Polym. Sci. 2014, 131, 13. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Pang, X.; Shi, X.; Xu, J. Expandable Graphite in Polyethylene: The Effect of Modification, Particle Size and the Synergistic Effect with Ammonium Polyphosphate on Flame Retardancy, Thermal Stability and Mechanical Properties. Combust. Sci. Technol. 2020, 192, 575–591. [Google Scholar] [CrossRef]
- Wang, H.; Cao, J.; Luo, F.; Cao, C.; Qian, Q.; Huang, B.; Xiao, L.; Chen, Q. Hugely enhanced flame retardancy and smoke suppression properties of UHMWPE composites with silicone-coated expandable graphite. Polym. Adv. Technol. 2019, 30, 1673–1683. [Google Scholar] [CrossRef]
- Xie, R.; Qu, B. Synergistic effects of expandable graphite with some halogen-free flame retardants in polyolefin blends. Polym. Degrad. Stab. 2001, 71, 375–380. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. The influence of layered, spherical, and tubular carbon nanomaterials’ concentration on the flame retardancy of polypropylene. Polym. Compos. 2015, 36, 1230–1241. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Xu, Y.; Chen, X.; Chen, M.; Yu, J.; Hu, S.; Zhang, Z. Effect of the particle size of expandable graphite on the thermal stability, flammability, and mechanical properties of high-density polyethylene/ethylene vinyl-acetate/expandable graphite composites. Polym. Eng. Sci. 2014, 54, 1162–1169. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. J. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Focke, W.W.; Muiambo, H.; Mhike, W.; Kruger, H.J.; Ofosu, O. Flexible PVC flame retarded with expandable graphite. Polym. Degrad. Stab. 2014, 100, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.-L.; Duan, H.-J.; Zhang, X.-G.; Chen, C.; Tang, J.-H.; Li, Z.-M. Synergistic effect of ammonium polyphosphate and expandable graphite on flame-retardant properties of acrylonitrile-butadiene-styrene. J. Appl. Polym. Sci. 2012, 126, 1337–1343. [Google Scholar] [CrossRef]
- Jia, Y.; He, H.; Yu, P.; Chen, J.; Tian, S. Preparation and characterization of synergistically improved thermally conductive polyamide 6 with low melting point metal and low-temperature expandable graphite. Polym. Compos. 2018, 39, 1818–1826. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S. Current Practice and Recent Commercial Developments in Flame Retardancy of Polyamides. J. Fire Sci. 2004, 22, 251–264. [Google Scholar] [CrossRef]
- Morgan, A.B.; Wilkie, C.A. Non-Halogenated Flame Retardant Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Schartel, B.; Weiß, A.; Sturm, H.; Kleemeier, M.; Hartwig, A.; Vogt, C.; Fischer, R.X. Layered silicate epoxy nanocomposites: Formation of the inorganic-carbonaceous fire protection layer. Polym. Adv. Technol. 2011, 22, 1581–1592. [Google Scholar] [CrossRef]
- Wu, G.M.; Schartel, B.; Bahr, H.; Kleemeier, M.; Yu, D.; Hartwig, A. Experimental and quantitative assessment of flame retardancy by the shielding effect in layered silicate epoxy nanocomposites. Combust. Flame 2012, 159, 3616–3623. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Braun, E.; Levin, B.C. Nylons: A review of the literature on products of combustion and toxicity. Fire Mater. 1987, 11, 71–88. [Google Scholar] [CrossRef]
- Price, D.; Horrocks, A.R. Polymer Degradation and the Matching of FR Chemistry to Degradation. In Fire Retardancy of Polymeric Materials; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 15–43. [Google Scholar]
- Bhuiyan, A.L. Some aspects of the thermal stability action of the structure in aliphatic polyamides and polyacrylamides. Polymer 1984, 25, 1699–1710. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Comparative studies of polymers using TA–MS, macro TA–MS and TA–FTIR. Thermochim. Acta 2000, 361, 69–76. [Google Scholar] [CrossRef]
- Herrera, M.; Wilhelm, M.; Matuschek, G.; Kettrup, A. Thermoanalytical and pyrolysis studies of nitrogen containing polymers. J. Anal. Appl. Pyrolysis 2001, 58–59, 173–188. [Google Scholar] [CrossRef]
- Herrera, M. Dissertation—Untersuchung Flüchtiger Verbindungen bei der Thermischen Zersetzung von Stickstoffhaltigen Polymerwerkstoffen; Universitätsbibliothek der TU München: Munich, Germany, 2000. [Google Scholar]
- Nagase, Y.; Komatsu, T.; Sumiya, Y.; Ikeda, K.; Sekine, Y. Thermal Degradation of Polyamides. Nippon. Kagaku Kaishi 1979, 11, 1560–1568. [Google Scholar] [CrossRef]
- Kondo, S.; Takizawa, K.; Takahashi, A.; Tokuhashi, K. Extended Le Chatelier’s formula for carbon dioxide dilution effect on flammability limits. J. Hazard. Mater. 2006, 138, 1–8. [Google Scholar] [CrossRef]
- Lyon, E.R. Chapter 3—Plastics and Rubbers. In Handbook of Building Materials for Fire Protection; Harper, C.A., Ed.; McGraw-Hill Handbooks: New York, NY, USA, 2004; pp. 121–171. [Google Scholar]
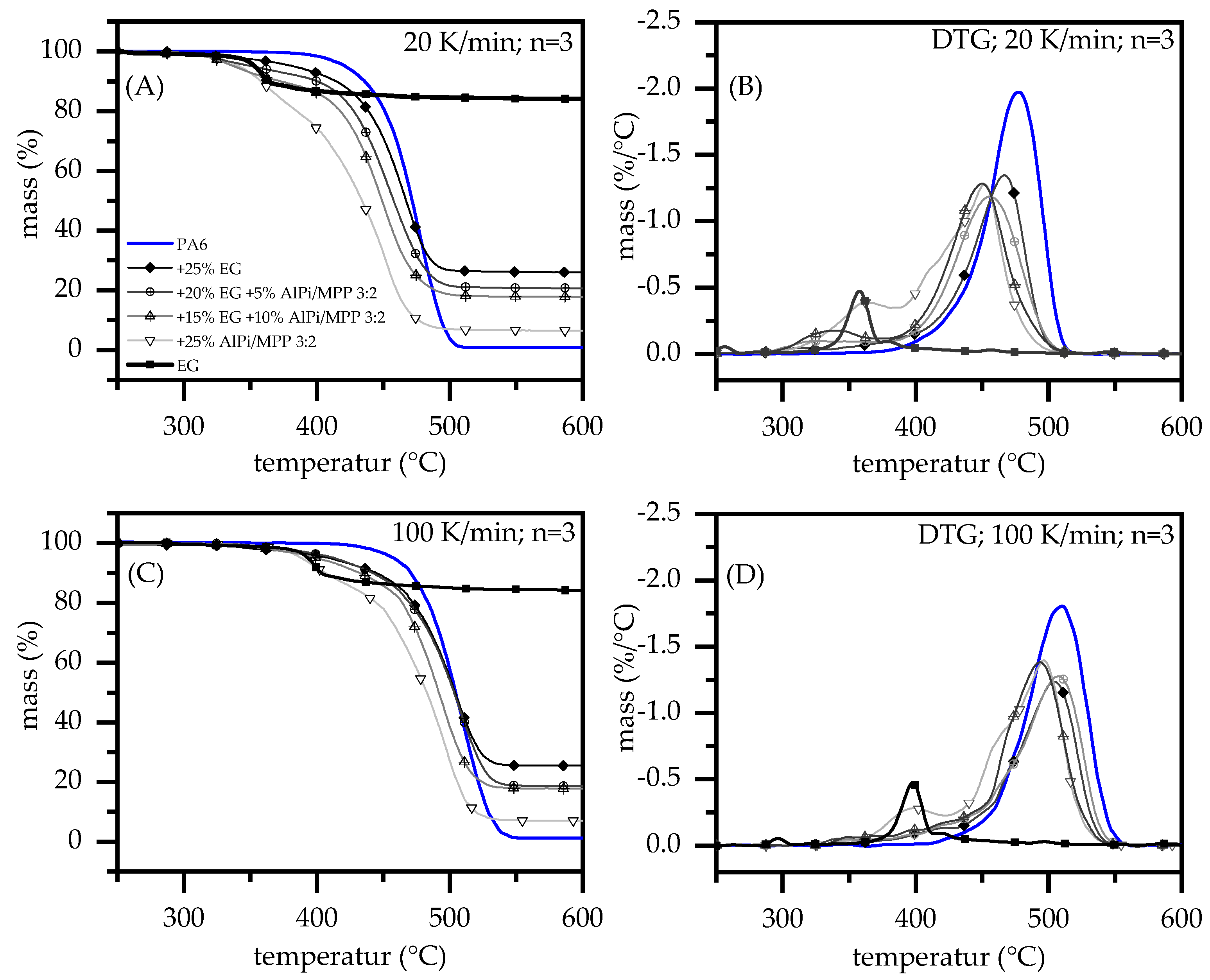


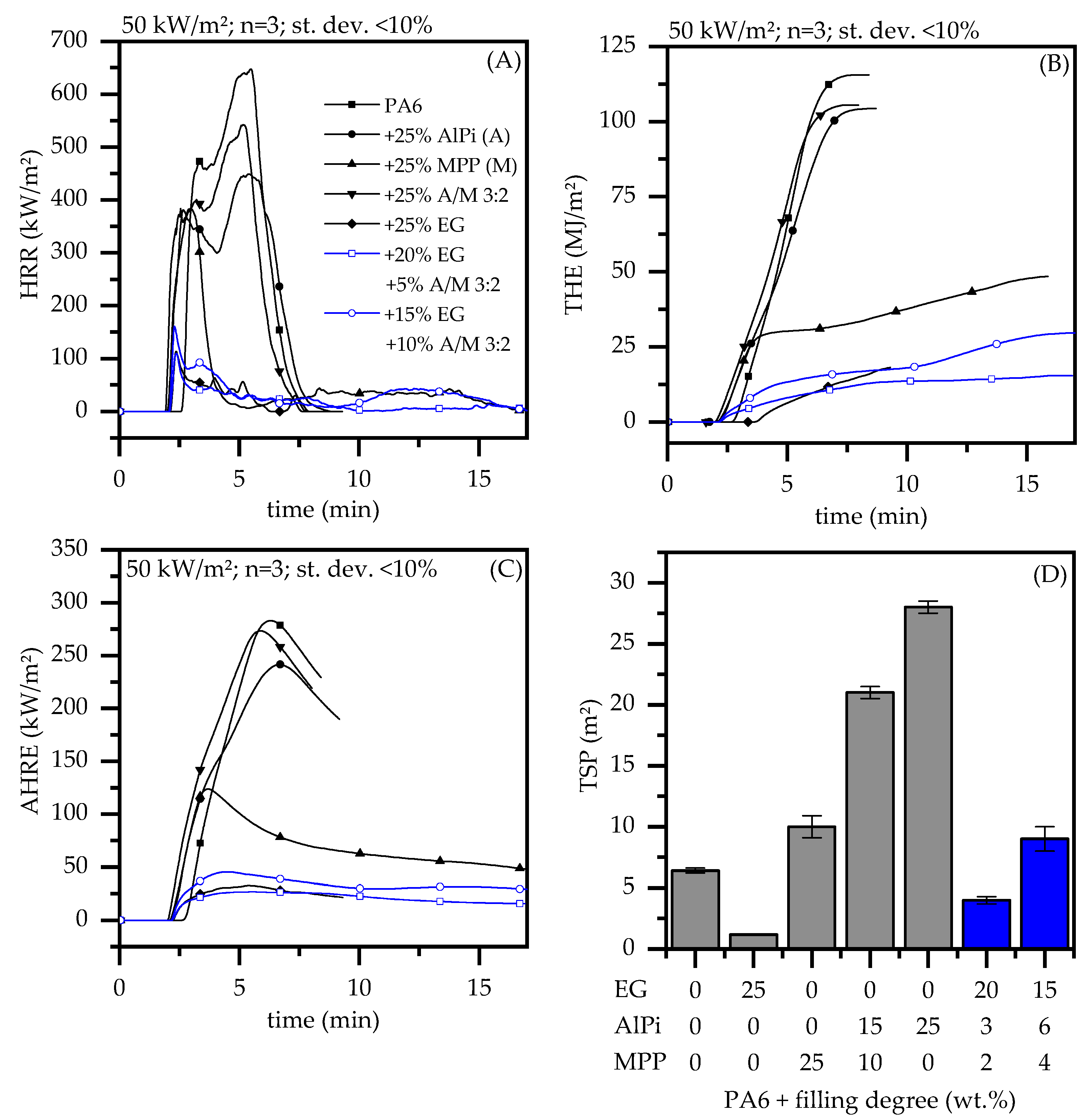



| 20 K/min | 100 K/min | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | PA6 wt.% | EG/AlPi/MPP wt.% | tonset (99%) °C | DTG-Peak °C/min | Residue % | tonset (99%) °C | DTG-Peak °C/min | Residue % |
| 1 | 100 | 0/0/0 | 393 ± 2 | 477 ± 2 | 0.8 ± 0.1 | 433 ± 0 | 510 ± 0 | 0.9 ± 0.4 |
| 0 | 100/0/0 | 307 ± 2 | 357 ± 2 | 84.0 ± 0.5 | 348 ± 2 | 398 ± 2 | 84.0 ± 0.5 | |
| 2 | 75 | 25/0/0 | 313 ± 5 | 466 ± 5 | 25.5 ± 0.6 | 338 ± 2 | 503 ± 2 | 25.0 ± 0.5 |
| 3 | 75 | 0/25/0 | 383 ± 2 | 466 ± 2 | 3.8 ± 0.1 | 425 ± 0 | 486 ± 0 | 5.0 ± 0.0 |
| 4 | 75 | 0/0/25 | 334 ± 5 | 391; 427 ± 5 | 11.7 ± 0.1 | 372 ± 0 | 424; 465 ± 0 | 11.5 ± 0.2 |
| 5 | 75 | 0/15/10 | 307 ± 5 | 362; 451 ± 5 | 6.3 ± 0.2 | 364 ± 7 | 400; 497 ± 7 | 6.7 ± 0.1 |
| 6 | 75 | 20/3/2 | 309 ± 2 | 456 ± 2 | 20.3 ± 0.6 | 354 ± 4 | 507 ± 4 | 18.2 ± 1.7 |
| 7 | 75 | 15/6/4 | 311 ± 1 | 339; 450 ± 1 | 17.4 ± 0.5 | 350 ± 0 | -; 493 ± 0 | 17.4 ± 1.7 |
| No. | MZ | 20 K/min; Peak Area % | 100 K/min; Peak Area % | |||||
|---|---|---|---|---|---|---|---|---|
| PA6 +25% EG | TGA: | ∆-7% 395 °C | ∆-15% 429 °C | ∆-71% 489 °C | ∆-7% 440 °C | ∆-15% 463 °C | ∆-71% 550 °C | |
| 1 | 44 | CO2 | 52 ± 4% | 21 ± 2% | 9 ± 3% | 28 ± 1% | 26 ± 1% | 11 ± 2% |
| 2 | 53 | C3H3N | 6 ± 1% | |||||
| 3 | 54 | C4H6 | 9 ± 0% | 8 ± 0% | 6 ± 0% | |||
| 4 | 64 | SO2 | 11 ± 0% | 3 ± 1% | 7 ± 1% | 3 ± 0% | 4 ± 0% | 2 ± 0% |
| 5 | 78 | C6H6 | 4 ± 0% | 5 ± 0% | 15 ± 1% | |||
| 6 | 113 | C6H11NO | 23 ± 2% | 66 ± 2% | 38 ± 2% | 28 ± 1% | 30 ± 0% | 13 ± 1% |
| PA6 +25% A/M | TGA: | ∆-7% 354 °C | ∆-15% 372 °C | ∆-92% 489 °C | ∆-7% 402 °C | ∆-15% 430 °C | ∆-92% 550 °C | |
| 1 | 41 | C3H6 | 6 ± 0% | 4 ± 0% | 4 ± 1% | |||
| 2 | 44 | CO2 | 50 ± 1% | 19 ± 2% | 6 ± 1% | 78 ± 3% | 27 ± 3% | 10 ± 1% |
| 3 | 54 | C4H6 | 5 ± 1% | 10 ± 2% | 7 ± 1% | |||
| 4 | 56 | C4H8 | 3 ± 0% | 10 ± 2% | ||||
| 5 | 78 | C6H6 | 4 ± 1% | 12 ± 1% | ||||
| 6 | 113 | C6H11NO | 33 ± 1% | 54 ± 4% | 44 ± 3% | 13 ± 2% | 21 ± 4% | 14 ± 1% |
| Nr. | PA6 wt.% | EG/AlPi/MPP wt.% | Mass g | tign s | pHRR kW/m2 | THE MJ/m2 | MAHRE kW/m2 | AMLR g/min | TSP m2 | FIGRA † kW/(m2*s) | FPI †† s*m2/kW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 0/0/0 | 44 ± 1 | 162 ± 2 | 648 ± 42 | 115 ± 10 | 289 ± 20 | 4.9 ± 0.3 | 6.4 ± 0.2 | 2.6 ± 0.10 | 0.2 ± 0.02 |
| 85 | 15/0/0 | 48 ± 1 | 143 ± 2 | 265 ± 10 | 84 ± 1 | 130 ± 3 | 2.7 ± 0.1 | 5.1 ± 0.1 | 1.6 ± 0.06 | 0.5 ± 0.03 | |
| 2 | 75 | 25/0/0 | 48 ± 1 | 134 ± 2 | 120 ± 12 | 12 ± 1 | 33 ± 3 | 1.4 ± 0.1 | 1.2 ± 0.0 | 0.9 ± 0.07 | 1.1 ± 0.01 |
| 3 | 75 | 0/25/0 | 45 ± 1 | 148 ± 2 | 464 ± 26 | 104 ± 3 | 242 ± 11 | 4.9 ± 0.3 | 28.0 ± 0.5 | 2.5 ± 0.07 | 0.3 ± 0.01 |
| 4 | 75 | 0/0/25 | 47 ± 1 | 126 ± 1 | 402 ± 15 | 49 ± 6 | 124 ± 8 | 2.2 ± 0.1 | 10.0 ± 0.9 | 2.3 ± 0.08 | 0.3 ± 0.01 |
| 5 | 75 | 0/15/10 | 46 ± 1 | 121 ± 1 | 563 ± 1 | 105 ± 2 | 274 ± 3 | 5.3 ± 0.0 | 21.0 ± 0.5 | 2.9 ± 0.20 | 0.2 ± 0.00 |
| 6 | 75 | 20/3/2 | 48 ± 1 | 134 ± 3 | 144 ± 4 | 15 ± 3 | 28 ± 3 | 1.6 ± 0.1 | 4.0 ± 0.3 | 1.0 ± 0.02 | 0.9 ± 0.02 |
| 7 | 75 | 15/6/4 | 48 ± 1 | 127 ± 3 | 169 ± 10 | 30 ± 2 | 47 ± 2 | 1.9 ± 0.1 | 9.0 ± 1.0 | 1.3 ± 0.04 | 0.8 ± 0.02 |
| Nr. | PA6 wt.% | EG/AlPi/MPP wt.% | LOI % | UL-94 |
|---|---|---|---|---|
| 1 | 100 | 0/0/0 | 26 | V-2 |
| 2 | 75 | 25/0/0 | 39 | V-0 |
| 3 | 75 | 0/25/0 | 42 | V-0 |
| 4 | 75 | 0/0/25 | 30 | HB |
| 5 | 75 | 0/15/10 | 25 | V-0 |
| 6 | 75 | 20/3/2 | 46 | V-0 |
| 7 | 75 | 15/6/4 | 41 | V-0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomiak, F.; Schoeffel, A.; Rathberger, K.; Drummer, D. A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-)Polyphospinate and Melamine Polyphosphate for Polyamide 6. Polymers 2021, 13, 2712. https://doi.org/10.3390/polym13162712
Tomiak F, Schoeffel A, Rathberger K, Drummer D. A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-)Polyphospinate and Melamine Polyphosphate for Polyamide 6. Polymers. 2021; 13(16):2712. https://doi.org/10.3390/polym13162712
Chicago/Turabian StyleTomiak, Florian, Angelina Schoeffel, Klaus Rathberger, and Dietmar Drummer. 2021. "A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-)Polyphospinate and Melamine Polyphosphate for Polyamide 6" Polymers 13, no. 16: 2712. https://doi.org/10.3390/polym13162712
APA StyleTomiak, F., Schoeffel, A., Rathberger, K., & Drummer, D. (2021). A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-)Polyphospinate and Melamine Polyphosphate for Polyamide 6. Polymers, 13(16), 2712. https://doi.org/10.3390/polym13162712