Expandable Graphite for Flame Retardant PA6 Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Preparation and Testing
2.1.1. Microscopy
2.1.2. Thermal Analysis
2.1.3. Fire Testing
2.1.4. Electrical, Heat and Thermal Conductivity, Heat Capacity and Density
2.1.5. Viscosity Measurements
3. Results
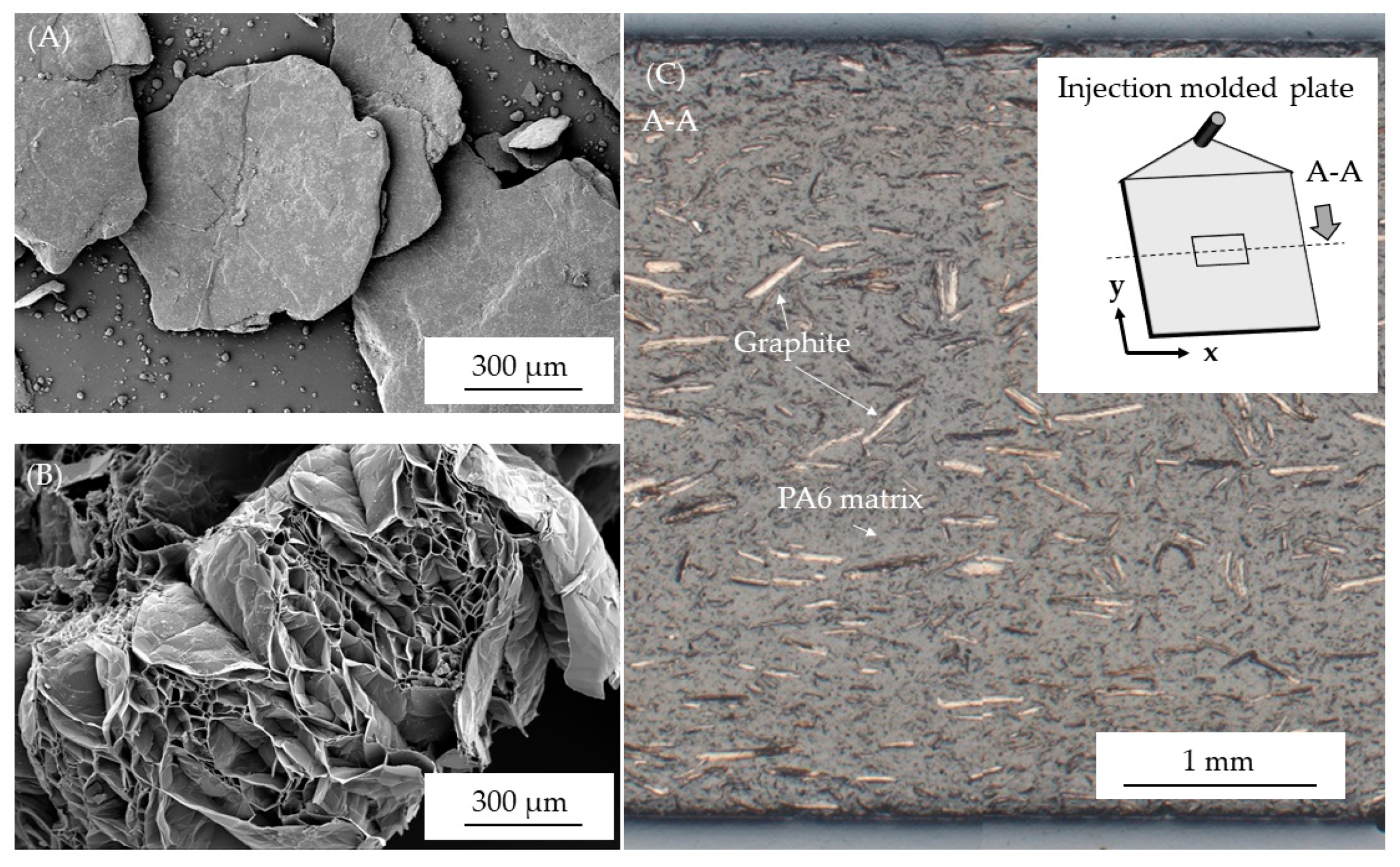
3.1. Microscopy
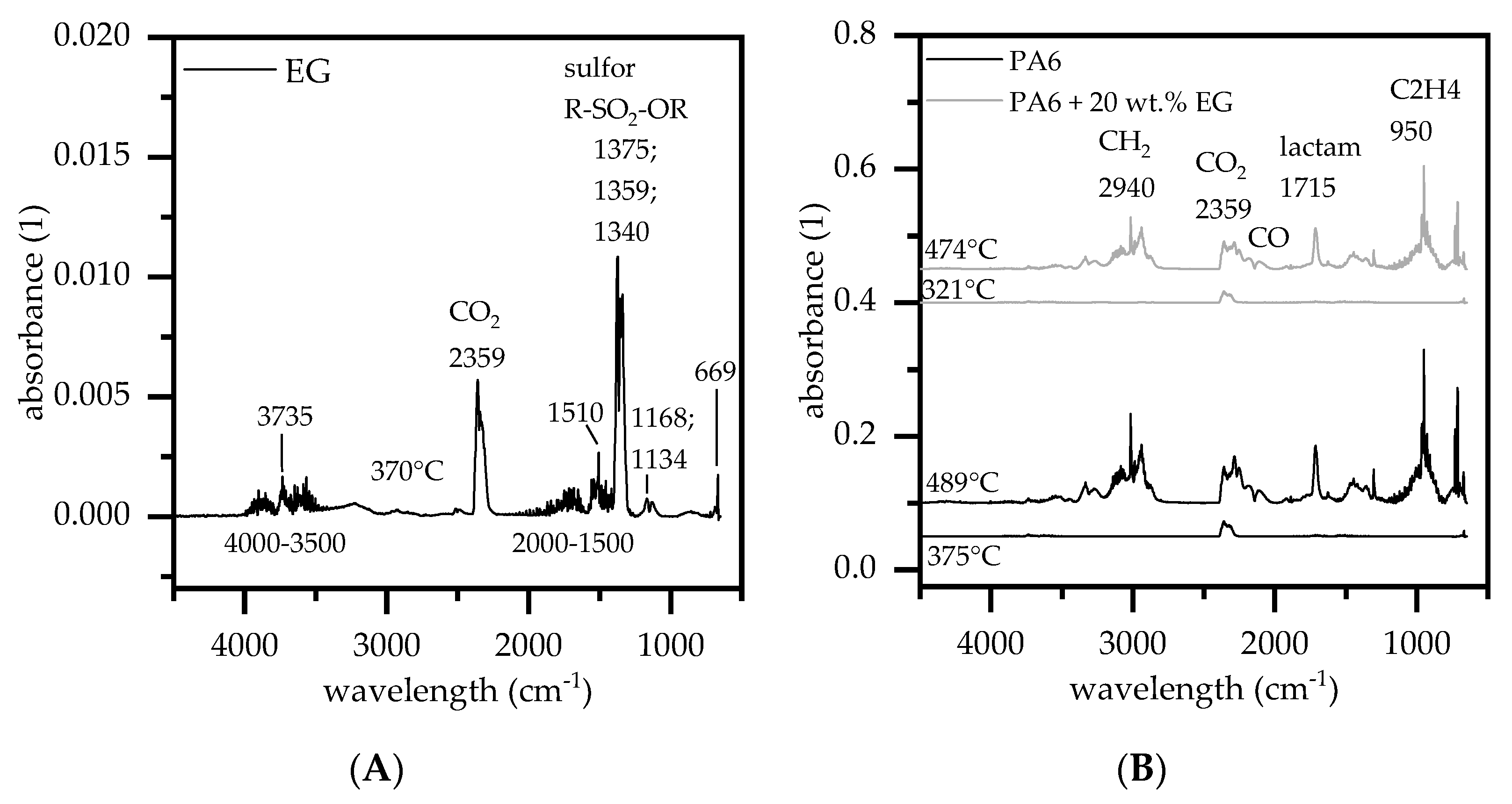
3.2. Thermal Decomposition and Gas Analysis
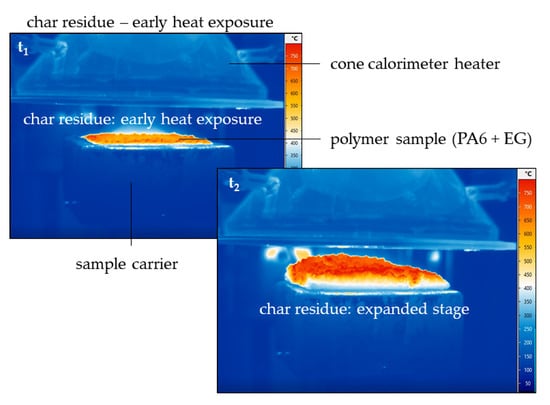
3.3. Fire Testing
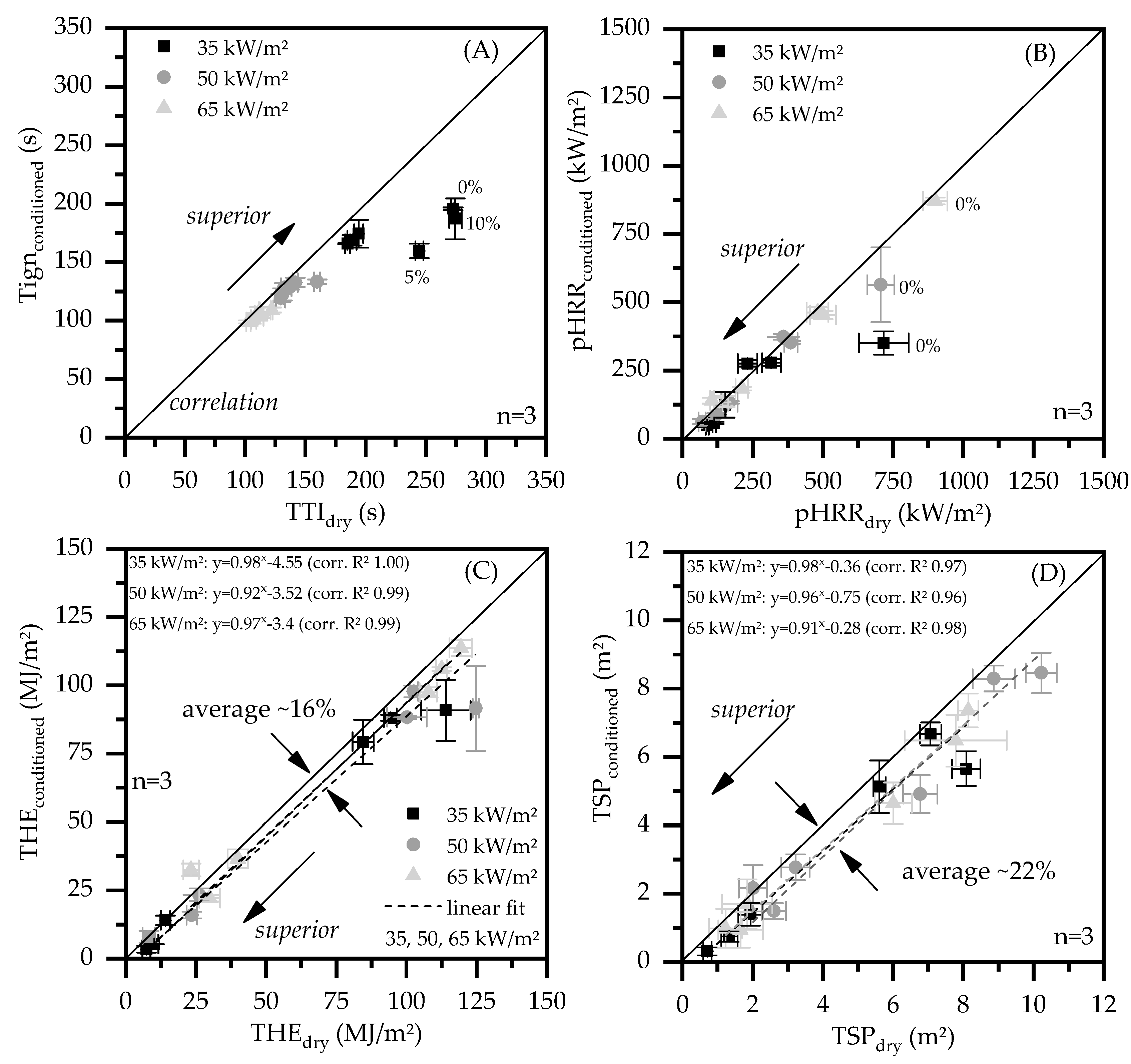
3.4. Correlation Analysis
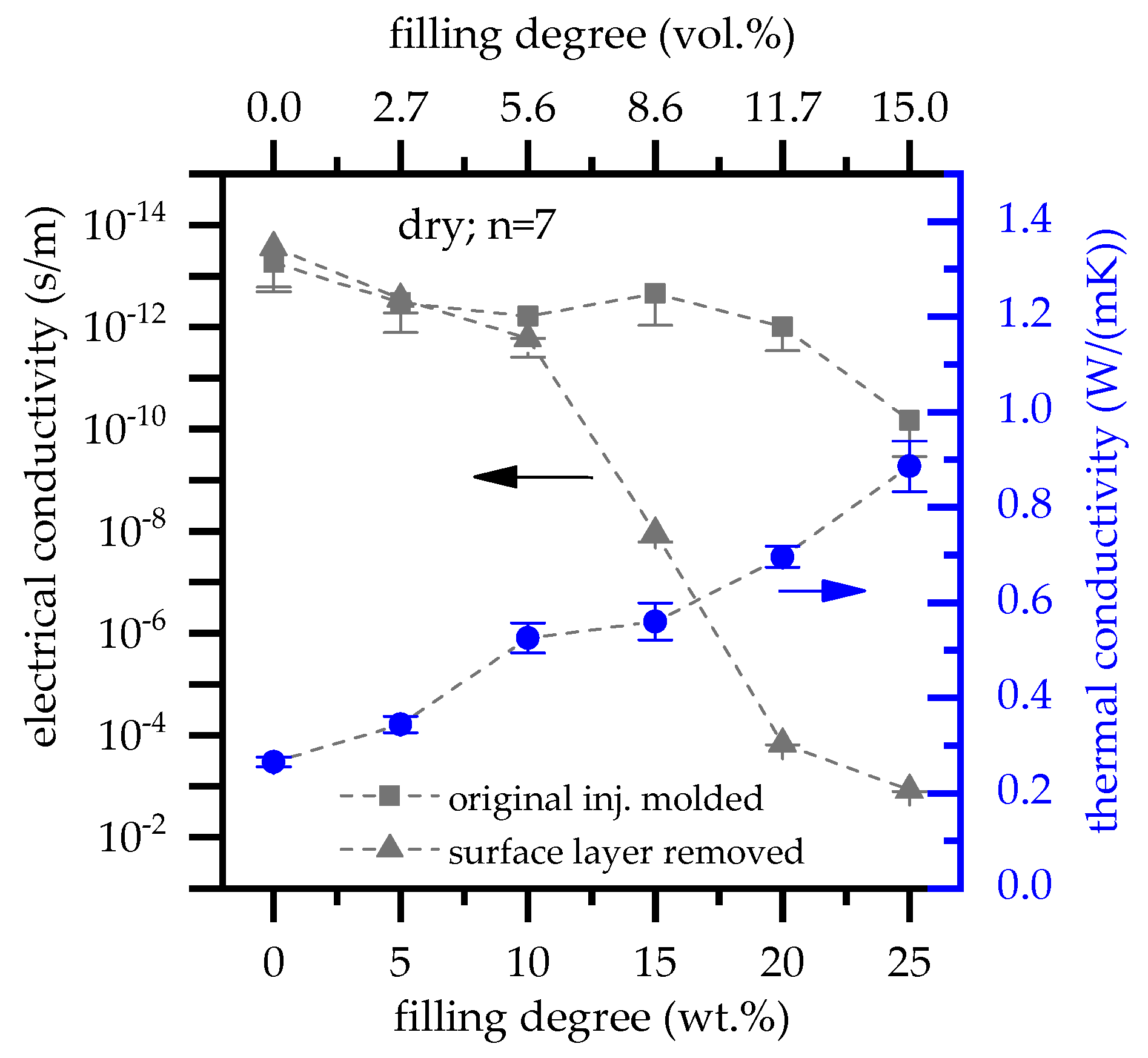
3.5. Electrical and Thermal Conductivity
3.6. Melt Viskosity
4. Discussion
5. Conclusions
- No expansion occurred for temperatures below 290 °C. Process stability is assumed up to this temperature.
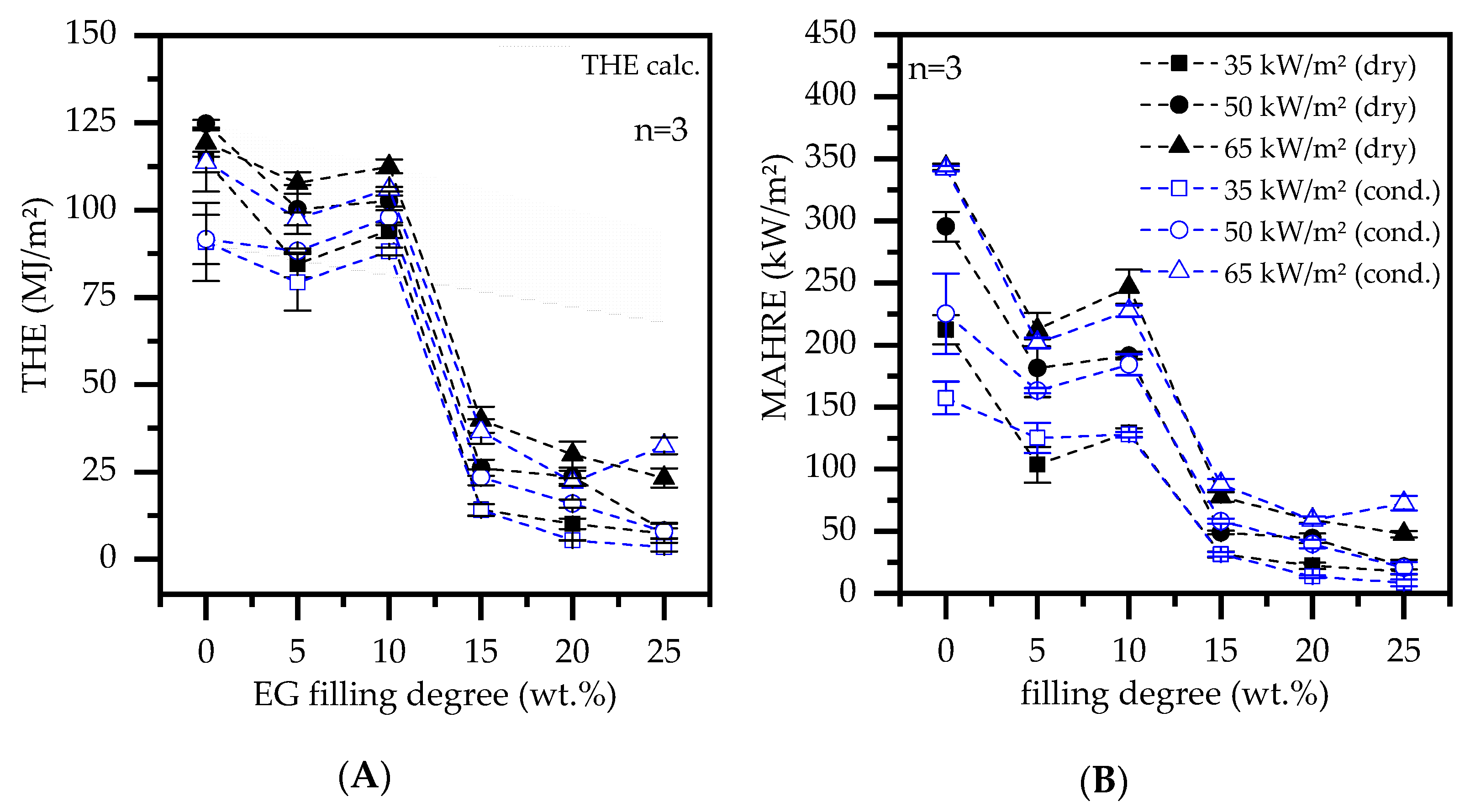
- PA6/ EG formulations were found to perform specifically well in cone calorimeter tests. It was found that a minimum filling level of 15 wt.% is required to achieve adequate results.
- In cone calorimeter tests, sample conditions were found to predominantly effect the parameters total heat emitted (THE) and total smoke production (TSP). As an average over all tests conducted, humid-conditioned samples were found to reduce the THE by 16% and the TSP by 22%. Sensitivity of the time to ignition (Tign) and the peak heat relelase rate (pHRR) were specifically found for net PA6 as well as when low heat fluxes were applied.
- PA6/ EG formulations were found to work less sufficient in flammability tests. Filling degrees of 20 wt. for humid-conditioned samples and 25 wt.% for dried samples achieved a V-0 UL-94 classification.
- Higher filling degrees were found to increase the heat conductivity of net PA6 versus PA6 containing 25 wt.% EG from 0.27 W/(m*K) to 0.89 W/(m*K).
- The percolation threshold was found to be dependent on the preparation method. Injection molded plates did not exceed electrical conductivity, whereas plates which were processed to remove the surface layers achieved percolation for filling degrees between 10 wt.% (5.6 vol.%) and 20 wt.% (11.7 vol.%).
- Viscosity measurements showed an increase in MVR for higher filling degrees, achieving 114 cm3/10 min for PA6 containing 25 wt.%. Rotational viscosity measurements indicate a sheer thinning effect by EG incorporation. For filling degrees of 15 wt.% EG the complex viscosity increases, whereas PA6 containing 20 wt.% and 25 wt.% subsequently decrease the complex viscosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, A.B. The Future of Flame Retardant Polymers—Unmet Needs and Likely New Approaches. Polym. Rev. 2019, 59, 25–54. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms-Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [Green Version]
- Focke, W.W.; Badenhorst, H.; Mhike, W.; Kruger, H.J.; Lombaard, D. Characterization of commercial expandable graphite fire retardants. Thermochim. Acta 2014, 584, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Tang, Q.; Liao, W.; Wang, G.; Wei, W.; Li, C. Green Preparation of Expandable Graphite and Its Application in Flame-Resistance Polymer Elastomer. Ind. Eng. Chem. Res. 2017, 56, 5253–5261. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame Retardants in Commercial Use or Development for Polyolefins. J. Fire Sci. 2008, 26, 5–43. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Luo, Z.; Yang, B.; Guo, S.; Yu, J. Synergistic effects of expandable graphite with magnesium hydroxide on the flame retardancy and thermal properties of polypropylene. Polym Eng. Sci 2007, 47, 1756–1760. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Carbon black, multiwall carbon nanotubes, expanded graphite and functionalized graphene flame retarded polypropylene nanocomposites. Polym. Adv. Technol. 2013, 24, 916–926. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. The influence of layered, spherical, and tubular carbon nanomaterials’ concentration on the flame retardancy of polypropylene. Polym. Compos. 2015, 36, 1230–1241. [Google Scholar] [CrossRef]
- Mattausch, H.; Laske, S.; Hohenwarter, D.; Holzer, C. The effect of mineral fillers on the rheological, mechanical and thermal properties of halogen-free flame-retardant polypropylene/expandable graphite compounds. In Proceedings of the AIP Conference Proceedings, Rhodes, Greece, 23–29 September 2015. [Google Scholar] [CrossRef] [Green Version]
- Schartel, B.; Braun, U.; Schwarz, U.; Reinemann, S. Fire retardancy of polypropylene/flax blends. Polymer 2003, 44, 6241–6250. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, M.; Ning, X.; Chen, X.; Sun, Z.; Ma, Y.; Qin, J.; Yu, J.; Zhang, Z.; Le Yang Bo, X.; et al. The Thermal Stability and Flammability of Expandable Graphite-Filled Polypropylene/Thermoplastic Polyurethane Blends. J. Macromol. Sci. Part B 2014, 53, 756–768. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Focke, W.W.; Kruger, H.J.; Mhike, W.; Taute, A.; Roberson, A.; Ofosu, O. Polyethylene flame retarded with expandable graphite and a novel intumescent additive. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Ma, Y.; Xu, Y.; Chen, X.; Chen, M.; Yu, J.; Hu, S.; Zhang, Z. Effect of the particle size of expandable graphite on the thermal stability, flammability, and mechanical properties of high-density polyethylene/ethylene vinyl-acetate/expandable graphite composites. Polym. Eng. Sci. 2014, 54, 1162–1169. [Google Scholar] [CrossRef]
- Tang, M.; Qi, F.; Chen, M.; Sun, Z.; Xu, Y.; Chen, X.; Zhang, Z.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on flammability and thermal properties of HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Wang, H.; Cao, J.; Luo, F.; Cao, C.; Qian, Q.; Huang, B.; Xiao, L.; Chen, Q. Hugely enhanced flame retardancy and smoke suppression properties of UHMWPE composites with silicone-coated expandable graphite. Polym. Adv. Technol. 2019, 30, 1673–1683. [Google Scholar] [CrossRef]
- Ge, L.-L.; Duan, H.-J.; Zhang, X.-G.; Chen, C.; Tang, J.-H.; Li, Z.-M. Synergistic effect of ammonium polyphosphate and expandable graphite on flame-retardant properties of acrylonitrile-butadiene-styrene. J. Appl. Polym. Sci. 2012, 126, 1337–1343. [Google Scholar] [CrossRef]
- Li, Z.; Qu, B. Flammability characterization and synergistic effects of expandable graphite with magnesium hydroxide in halogen-free flame-retardant EVA blends. Polym. Degrad. Stab. 2003, 81, 401–408. [Google Scholar] [CrossRef]
- Pang, X.-Y.; Tian, Y.; Shi, X.-Z. Synergism between hydrotalcite and silicate-modified expandable graphite on ethylene vinyl acetate copolymer combustion behavior. J. Appl. Polym. Sci. 2017, 134, 863. [Google Scholar] [CrossRef]
- Sover, A.; Marzynkevitsch, S.; Munack, B. Processing Conditions of Expandable Graphite in PP andPA Matrix and their Performance. Mater. Plast. 2018, 55, 507–510. [Google Scholar] [CrossRef]
- Alongi, J.; Frache, A.; Gioffredi, E. Fire-retardant poly(ethylene terephthalate) by combination of expandable graphite and layered clays for plastics and textiles. Fire Mater. 2011, 35, 383–396. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. J. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Focke, W.W.; Muiambo, H.; Mhike, W.; Kruger, H.J.; Ofosu, O. Flexible PVC flame retarded with expandable graphite. Polym. Degrad. Stab. 2014, 100, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Chung DD, L. Review Graphite. J. Mater. Sci 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Yang, X.; Yang, W.; Fan, J.; Wu, J.; Zhang, K. Effects of molding on property of thermally conductive and electrically insulating polyamide 6–based composite. J. Thermoplast. Compos. Mater. 2019, 32, 1190–1203. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- Babrauskas, V. The Cone Calorimeter. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D.T., Hall, J.R., Jr., Harada, K., Kuligowski, E.D., Puchovsky, M., Torero, J.L., Watts, J.M., Jr., Wieczorek, C.J., Eds.; Springer: New York, NY, USA, 2016; pp. 952–980. [Google Scholar]
- Babrauskas, V. Heat Release Rate. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D.T., Hall, J.R., Jr., Harada, K., Kuligowski, E.D., Puchovsky, M., Torero, J.L., Watts, J.M., Jr., Wieczorek, C.J., Eds.; Springer: New York, NY, USA, 2016; pp. 799–904. [Google Scholar]
- Herrera, M.; Matuschek, G.; Kettrup, A. Comparative studies of polymers using TA–MS, macro TA–MS and TA–FTIR. Thermochim. Acta 2000, 361, 69–76. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere 2001, 42, 601–607. [Google Scholar] [CrossRef]
- Hornsby, P.R.; Wang, J.; Rothon, R.; Jackson, G.; Wilkinson, G.; Cossick, K. Thermal decomposition behaviour of polyamide fire-retardant compositions containing magnesium hydroxide filler. Polym. Degrad. Stab. 1996, 51, 235–249. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G. Intercalation compounds of graphite. Adv. Phys. 1981, 30, 139–326. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.; Zou, H.; Liang, M. Study of different-sized sulfur-free expandable graphite on morphology and properties of water-blown semi-rigid polyurethane foams. RSC Adv. 2014, 4, 37302–37310. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the scientific approach to polymer flame retardancy: Part 2—Concepts. J. Fire Sci. 2017, 35, 3–20. [Google Scholar] [CrossRef]
- Afanasov, I.M.; Savchenko, D.V.; Ionov, S.G.; Rusakov, D.A.; Seleznev, A.N.; Avdeev, V.V. Thermal conductivity and mechanical properties of expanded graphite. Inorg Mater. 2009, 45, 486–490. [Google Scholar] [CrossRef]
- Jia, Y.; He, H.; Yu, P.; Chen, J.; Tian, S. Preparation and characterization of synergistically improved thermally conductive polyamide 6 with low melting point metal and low-temperature expandable graphite. Polym. Compos. 2018, 39, 1818–1826. [Google Scholar] [CrossRef]
- Fang, K.; Chen, Y.F.; Zhang, S.C.; Sun, H.R.; Wang, G.H.; Sun, X.K. The Effect of Particle Size of Expandable Graphite on the Properties of an Expandable Thermal Insulation Material. KEM 2016, 697, 441–444. [Google Scholar] [CrossRef]
- Liu, J.; Pang, X.; Shi, X.; Xu, J. Expandable Graphite in Polyethylene: The Effect of Modification, Particle Size and the Synergistic Effect with Ammonium Polyphosphate on Flame Retardancy, Thermal Stability and Mechanical Properties. Combust. Sci. Technol. 2020, 192, 575–591. [Google Scholar] [CrossRef]
- Salmeia, K.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef] [Green Version]
- Naoshi, S.; Chihong, L. Suppression Effect of Water Vapor on Flammability Limits of Hydrocarbon Fuels—A study on fire suppression by water mist. In Proceedings of the 6th Asia-Oceania Symposium on Fire Science and Technology, Daegu, Korea, 17–20 March 2004. [Google Scholar]





| No. | PA6/EG wt.% | tonset (99%) °C | DTG-Peak °C/min | Residue % | Residue Calc. % |
|---|---|---|---|---|---|
| 1 | 0/100 | 307 ± 1 | 349 ± 3 | 83 | - |
| 2 | 100/0 | 393 ± 2 | 477 ± 2 | 0.8 ± 0.1 | - |
| 3 | 95/5 | 373 ± 1 | 476 ± 2 | 5.7 ± 0.2 | 4.9 |
| 4 | 90/10 | 338 ± 5 | 475 ± 1 | 10.1 ± 0.2 | 9.0 |
| 5 | 85/15 | 332 ± 0 | 477 ± 1 | 12.7 ± 0.1 | 13.1 |
| 6 | 80/20 | 333 ± 4 | 471 ± 2 | 18.7 ± 1.1 | 17.2 |
| 7 | 75/25 | 316 ± 3 | 471 ± 1 | 21.7 ± 0.5 | 21.4 |
| net PA6 | 5 wt.% | 10 wt.% | 15 wt.% | 20 wt.% | 25 wt.% | |
|---|---|---|---|---|---|---|
| dry | HB | HB | HB | HB | V2 | V0 |
| t1: 5 ± 5 s | t1: 28 ± 4 s | t1: 17 ± 1 s | t1: 1 ± 0 s | t1: 0 ± 0 s | t1: 0 ± 0 s | |
| t2: 50 ± 7 s | t2: −5 ± 5 s | t2: 3 ± 1 | t2: 9 ± 4 s | t2: 3 ± 1 s | t2: 0 ± 0 s | |
| Cign*: yes | Cign*: yes | Cign *: yes | Cign*: yes | Cign*: yes | Cign*: no | |
| humid cond. | V0 | HB | HB | V2 | V0 | V0 |
| t1: 1 ± 0 s | t1: 47 ± 7 | t1: 7 ± 7 s | t1: 7 s | t1: 0 ± 0 s | t1: 0 ± 0 s | |
| t2: −2 ± 0 s | t2: - | t2: 4 ± 2 | t2: 13 s | t2: 4 ± 1 s | t2: 0 ± 0 s | |
| Cign *: no | Cign *: yes | Cign *: yes | Cign *: yes | Cign *: no | Cign *: no |
| ID. | PA6/EG(wt.%) | Tign (s) | pHRR (kW/m2) | THR (MJ/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| dry | 35 | 50 | 65 | 35 | 50 | 65 | 35 | 50 | 65 | |
| 100/0 | 273 ± 5 | 160 ± 3 | 122 ± 1 | 716 ± 34 | 706 ± 28 | 899 ± 43 | 114 ± 8 | 125 ± 1 | 119 ± 4 | |
| 95/5 | 245 ± 15 | 129 ± 4 | 103 ± 2 | 231 ± 15 | 386 ± 16 | 500 ± 41 | 85 ± 4 | 100 ± 7 | 108 ± 3 | |
| 90/10 | 233 ± 11 | 131 ± 1 | 109 ± 1 | 316 ± 12 | 359 ± 14 | 480 ± 39 | 94 ± 2 | 103 ± 2 | 113 ± 2 | |
| 85/15 | 194 ± 9 | 132 ± 3 | 107 ± 2 | 151 ± 20 | 167 ± 12 | 211 ± 22 | 14 ± 2 | 26 ± 2 | 40 ± 4 | |
| 80/20 | 185 ± 4 | 137 ± 1 | 111 ± 1 | 113 ± 12 | 122 ± 3 | 152 ± 10 | 10 ± 2 | 24 ± 2 | 30 ± 4 | |
| 75/25 | 188 ± 6 | 142 ± 2 | 112 ± 3 | 88 ± 7 | 70 ± 7 | 105 ± 7 | 7 ± 1 | 8 ± 2 | 23 ± 3 | |
| cond. | ||||||||||
| 100/0 | 196 ± 1 | 133 ± 2 | 109 ± 2 | 351 ± 21 | 564 ± 34 | 871 ± 12 | 91 ± 7 | 92 ± 6 | 113 ± 3 | |
| 95/5 | 160 ± 6 | 119 ± 3 | 99 ± 1 | 276 ± 11 | 353 ± 5 | 453 ± 15 | 79 ± 8 | 88 ± 1 | 97 ± 2 | |
| 90/10 | 187 ± 0 | 122 ± 5 | 105 ± 2 | 278 ± 12 | 373 ± 11 | 462 ± 19 | 88 ± 1 | 98 ± 2 | 106 ± 1 | |
| 85/15 | 174 ± 6 | 128 ± 4 | 101 ± 0 | 124 ± 5 | 133 ± 5 | 183 ± 7 | 14 ± 2 | 23 ± 2 | 37 ± 4 | |
| 80/20 | 166 ± 1 | 129 ± 2 | 108 ± 4 | 58 ± 4 | 88 ± 5 | 136 ± 10 | 5 ± 0 | 16 ± 1 | 22 ± 1 | |
| 75/25 | 168 ± 5 | 133 ± 4 | 104 ± 1 | 49 ± 8 | 62 ± 10 | 140 ± 9 | 4 ± 1 | 8 ± 2 | 16 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomiak, F.; Rathberger, K.; Schöffel, A.; Drummer, D. Expandable Graphite for Flame Retardant PA6 Applications. Polymers 2021, 13, 2733. https://doi.org/10.3390/polym13162733
Tomiak F, Rathberger K, Schöffel A, Drummer D. Expandable Graphite for Flame Retardant PA6 Applications. Polymers. 2021; 13(16):2733. https://doi.org/10.3390/polym13162733
Chicago/Turabian StyleTomiak, Florian, Klaus Rathberger, Angelina Schöffel, and Dietmar Drummer. 2021. "Expandable Graphite for Flame Retardant PA6 Applications" Polymers 13, no. 16: 2733. https://doi.org/10.3390/polym13162733
APA StyleTomiak, F., Rathberger, K., Schöffel, A., & Drummer, D. (2021). Expandable Graphite for Flame Retardant PA6 Applications. Polymers, 13(16), 2733. https://doi.org/10.3390/polym13162733