Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater
Abstract
1. Introduction
2. Experiments
2.1. Materials
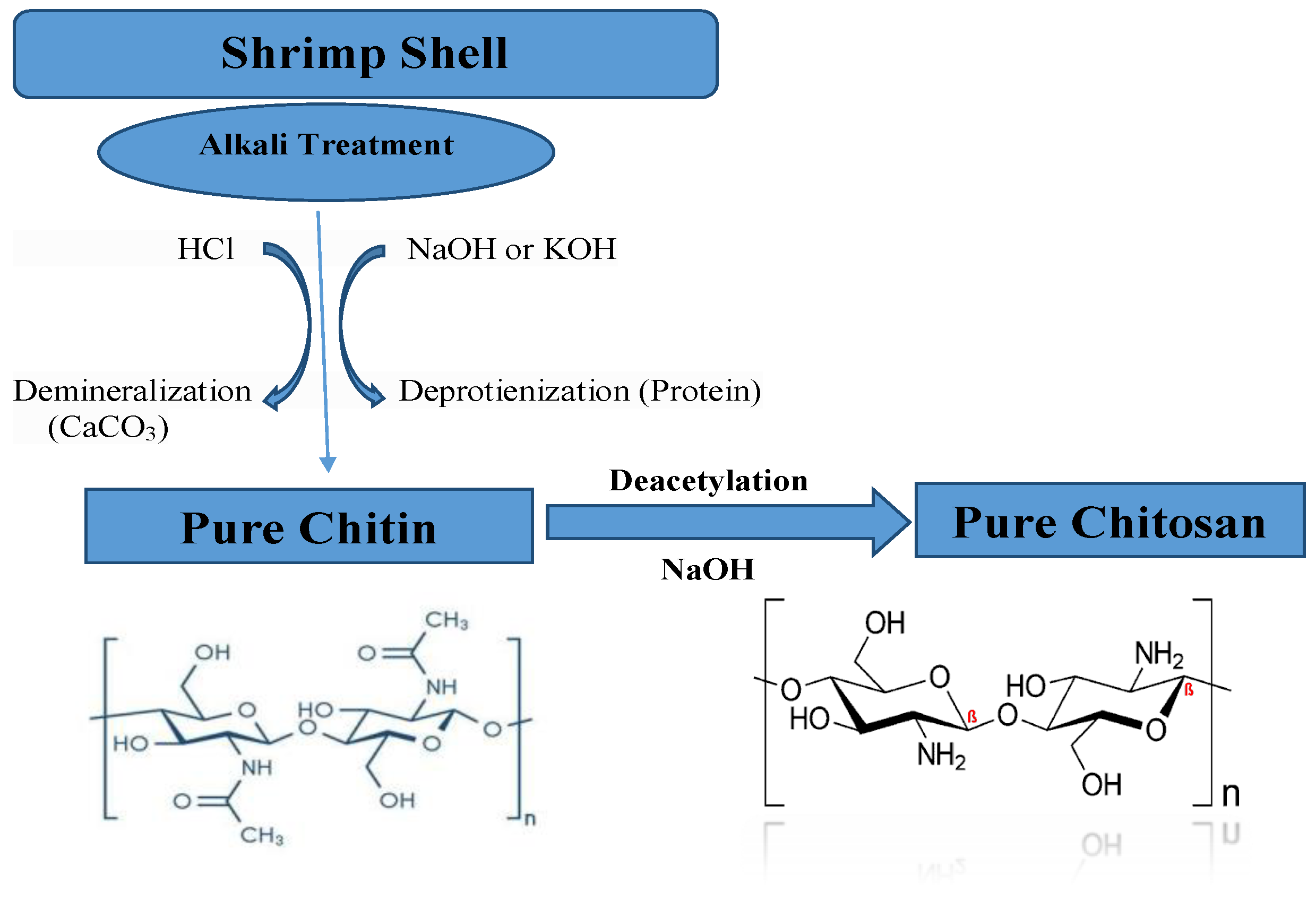
2.2. Extraction of Chitosan
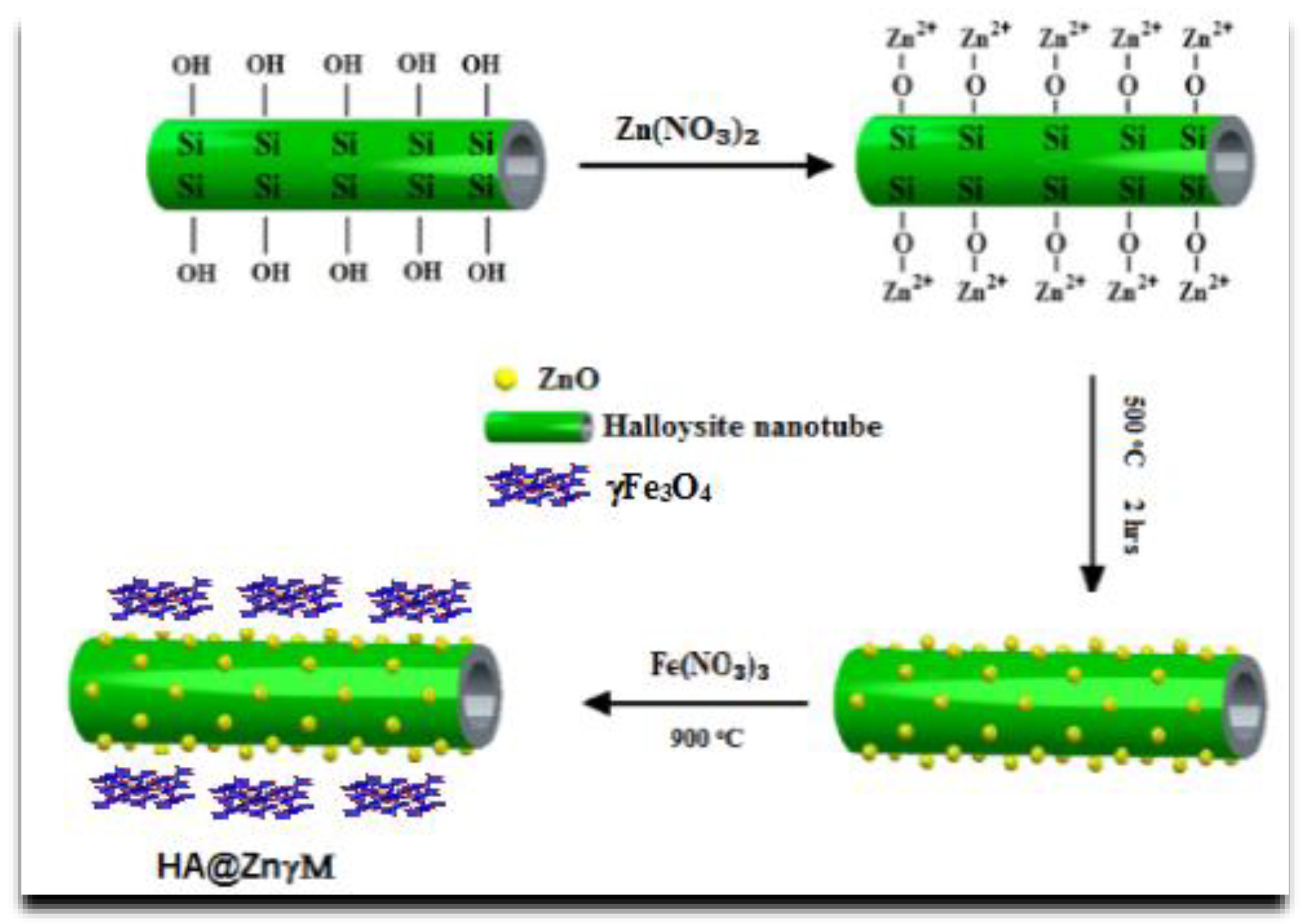
2.3. Synthesis of Halloysite Nanotubes/ZnγFe3O4 Core/Shell (HNPs/ZnγM)
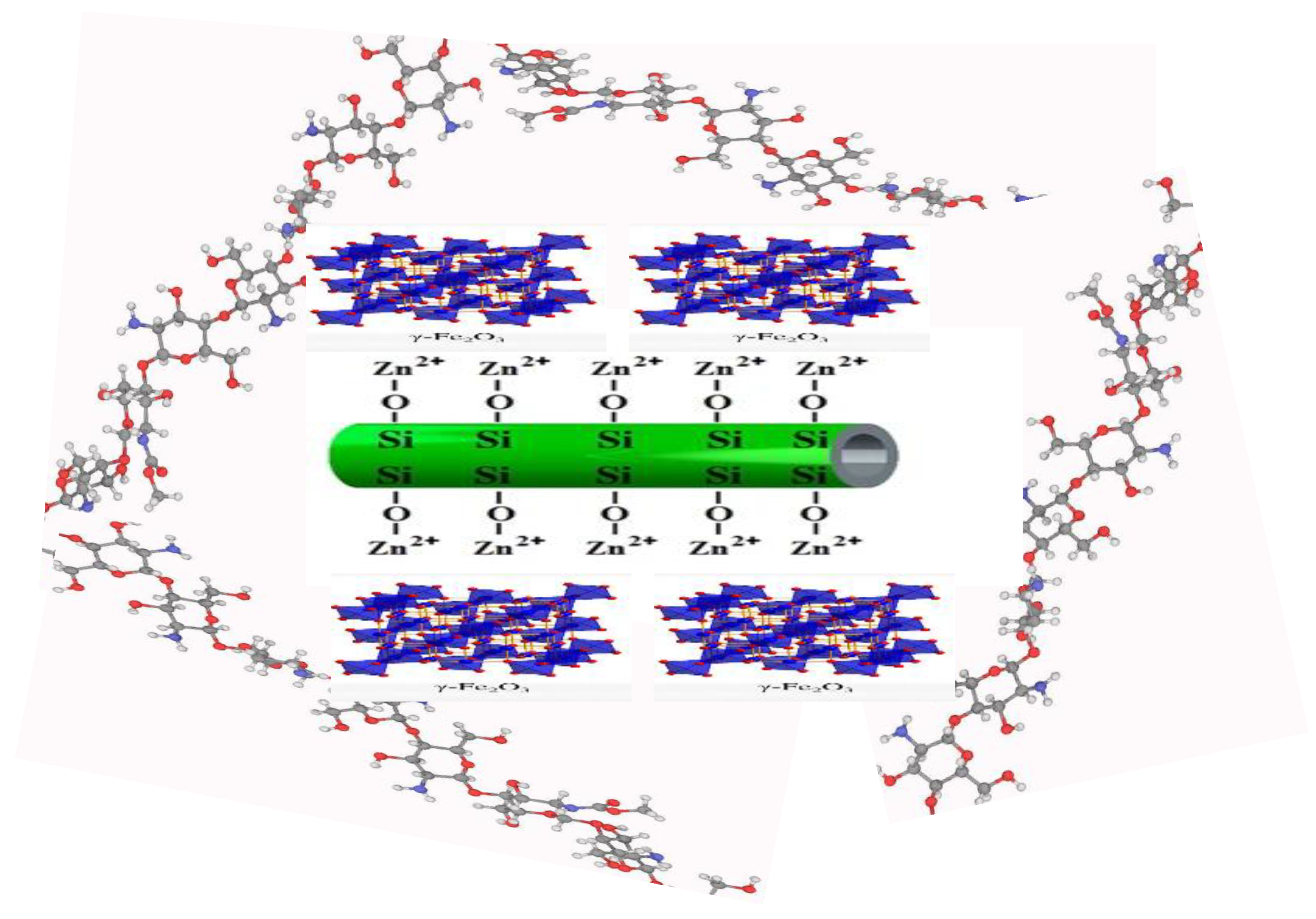
2.4. Synthesis of a Novel Chitosan/Grafted Halloysite@ZnγFe3O4 Tetra-Nanocomposite (Ch/g-HA@ZnγM)
2.5. Characterization of Materials
2.6. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of Materials
3.1.1. Physical and Chemical Characterization of Prepared Chitin and Chitosan
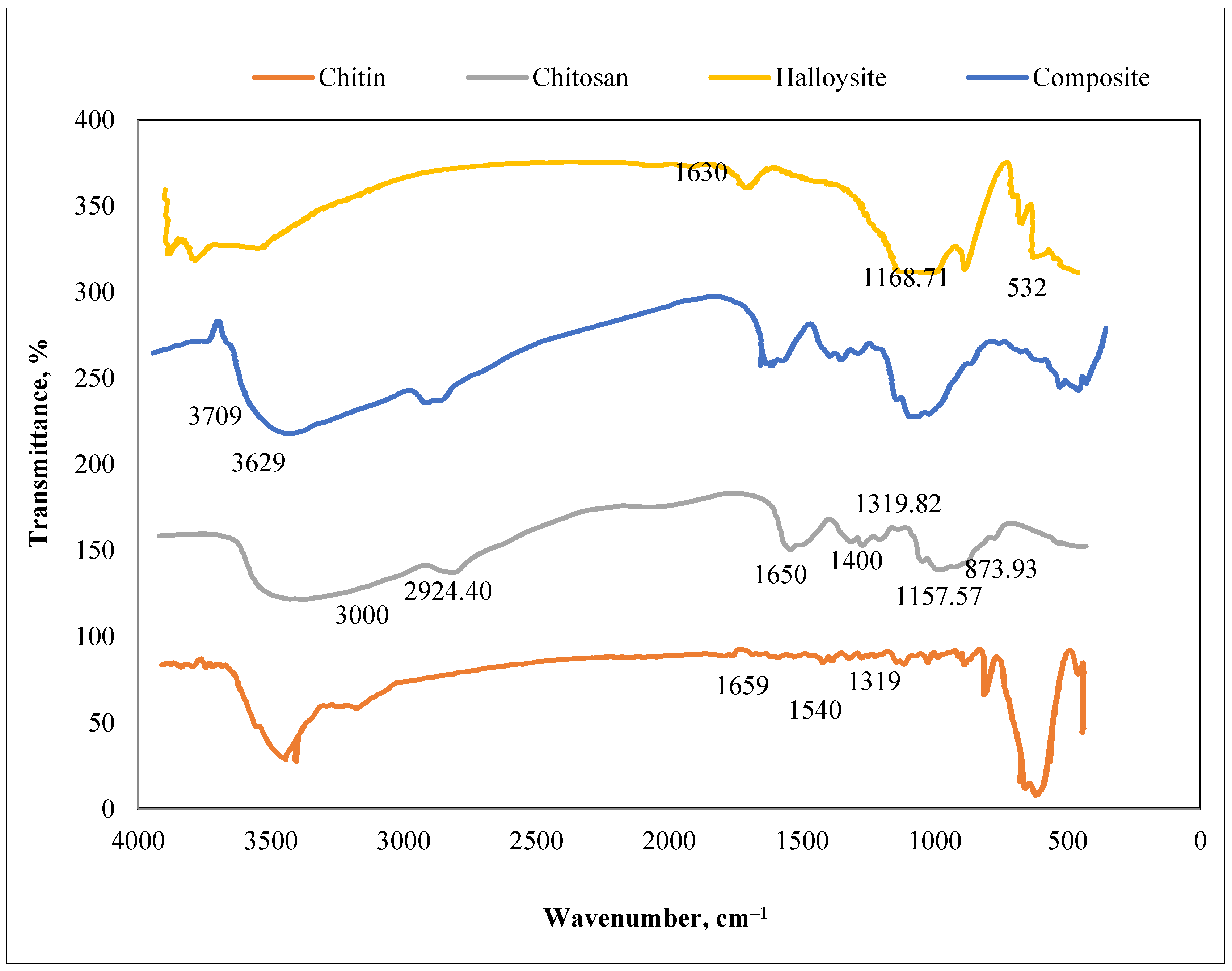
3.1.2. Fourier Transform Infrared (FTIR) Spectra
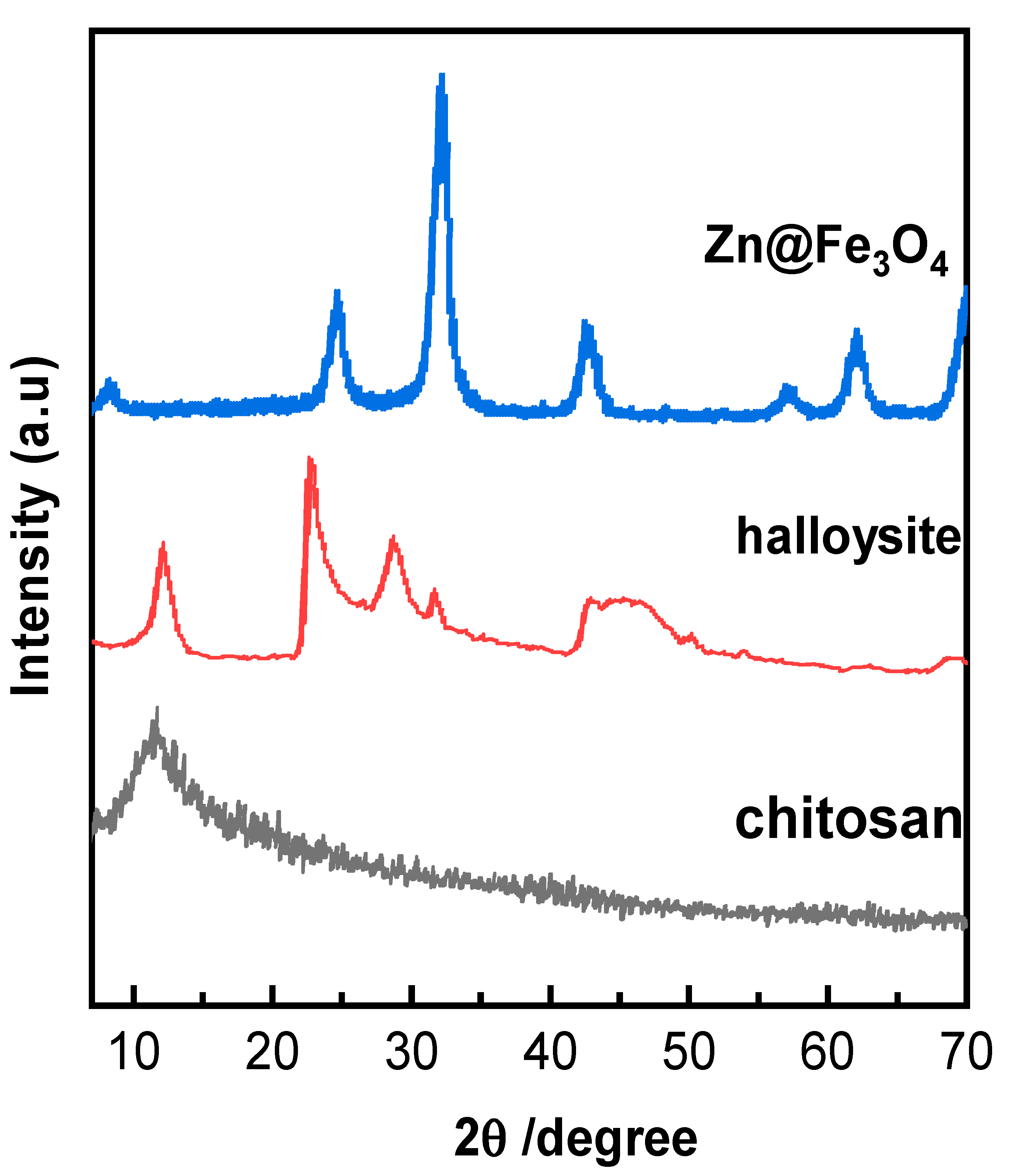
3.1.3. X-Ray Diffraction (XRD)
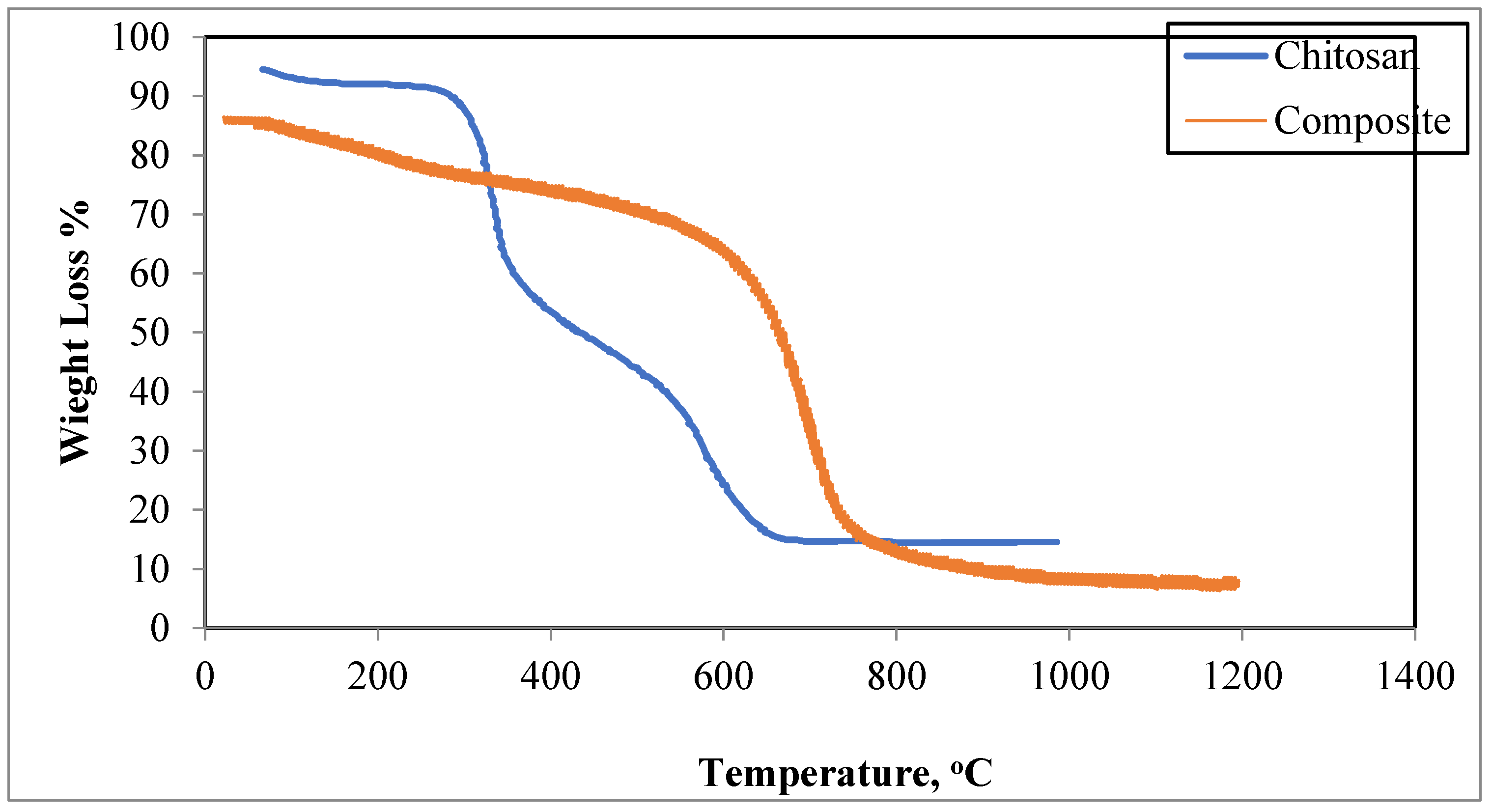
3.1.4. Thermal Analysis
3.1.5. Gel Permeation Chromatography (GPC)
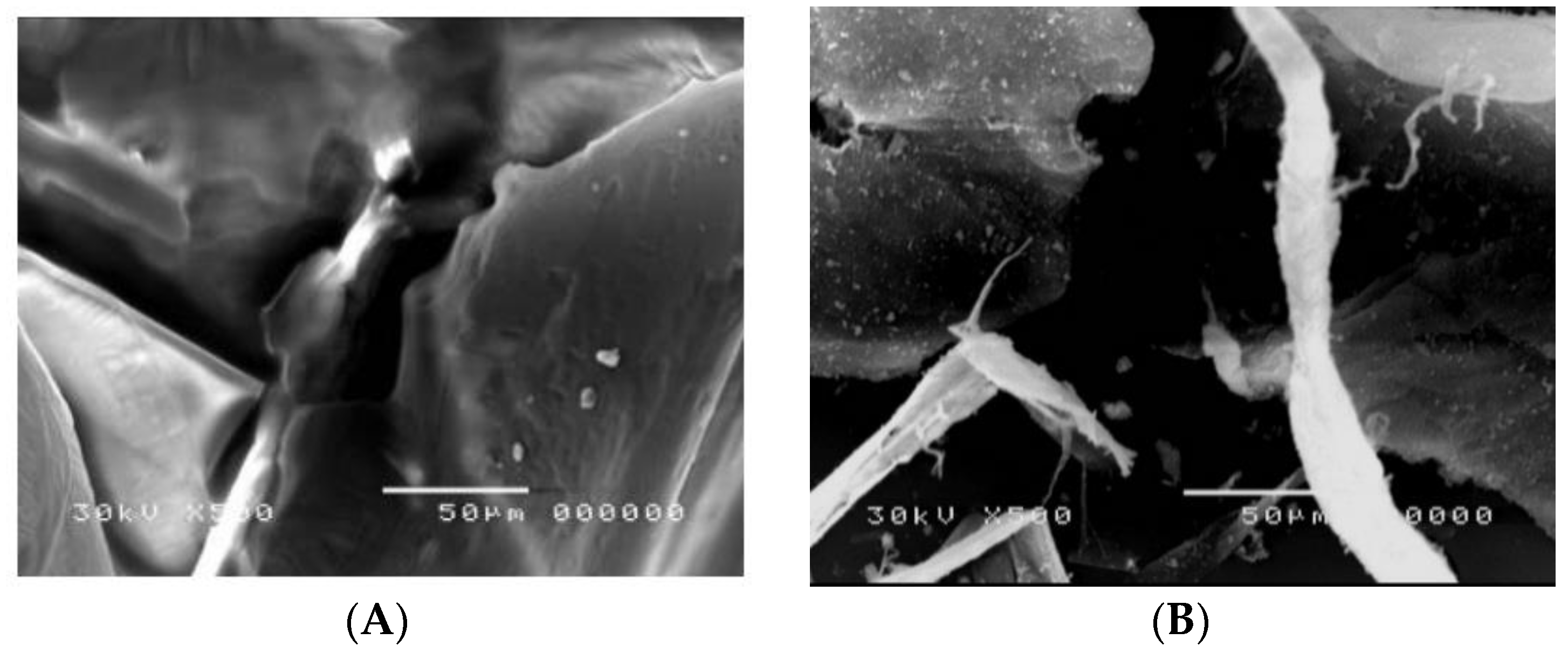
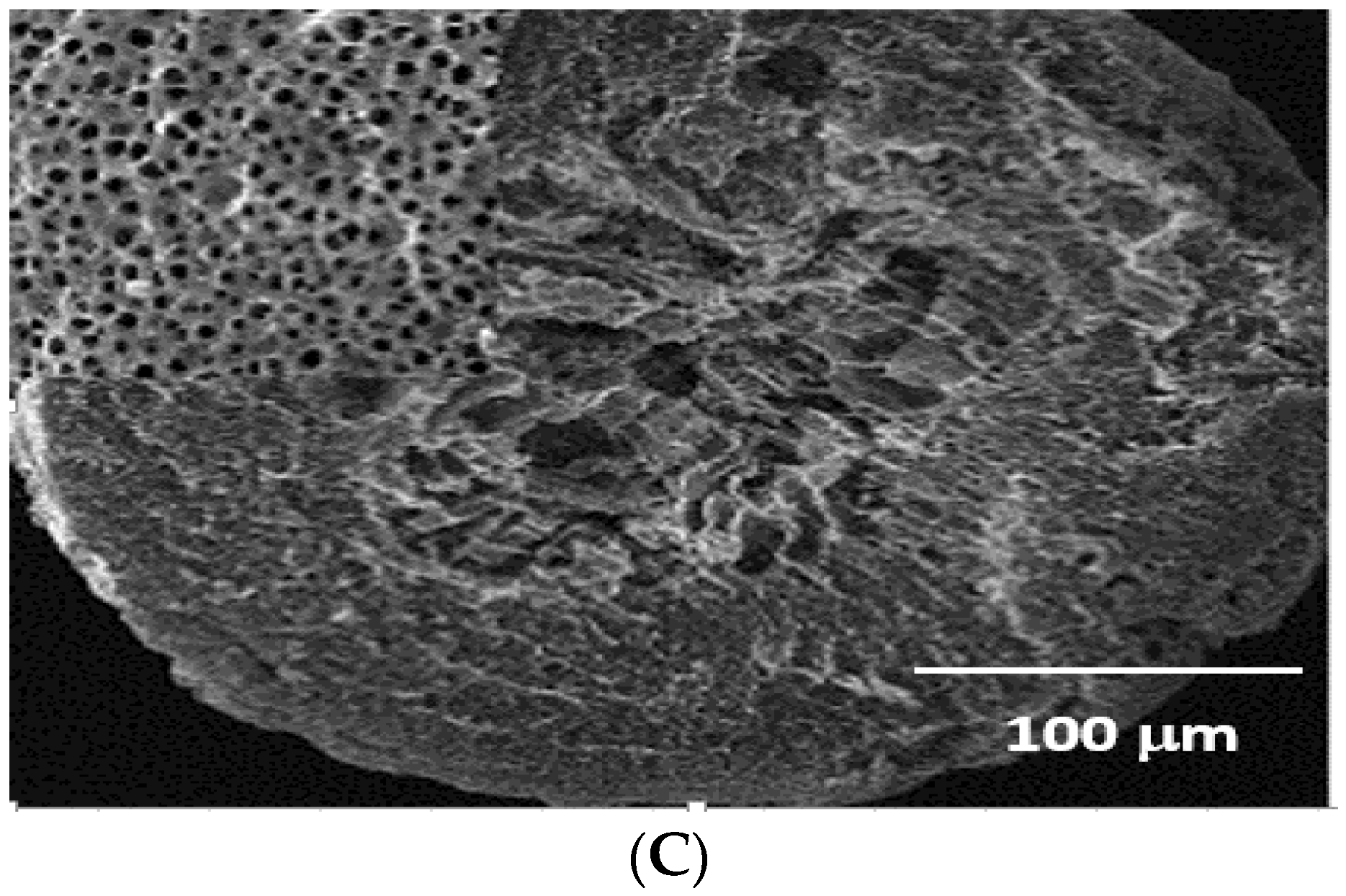
3.1.6. Scanning Electron Microscope (SEM)
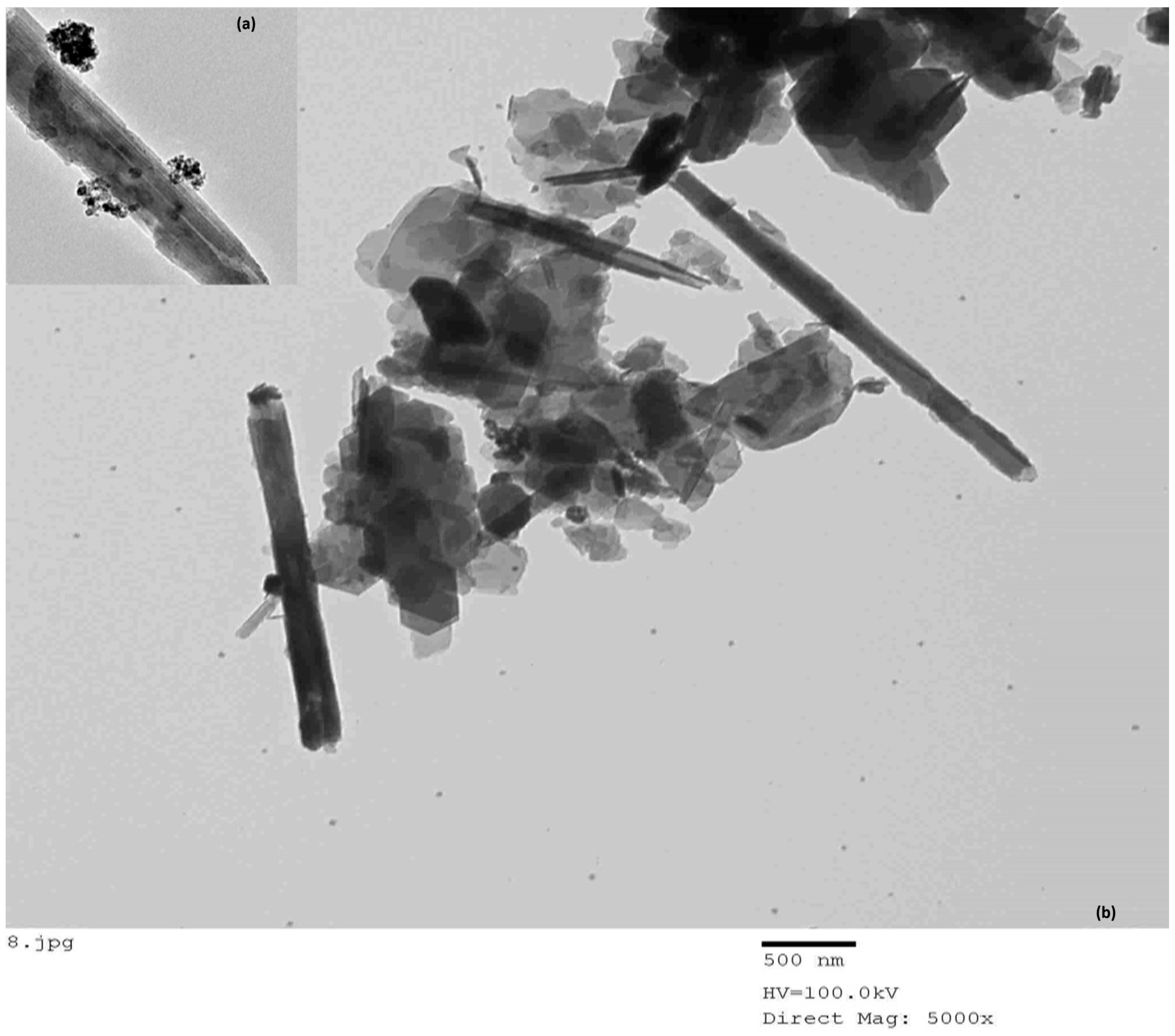
3.1.7. TEM
3.1.8. Ch/g-HNTs@ZnγM Zero Charge Point (pHpzc)
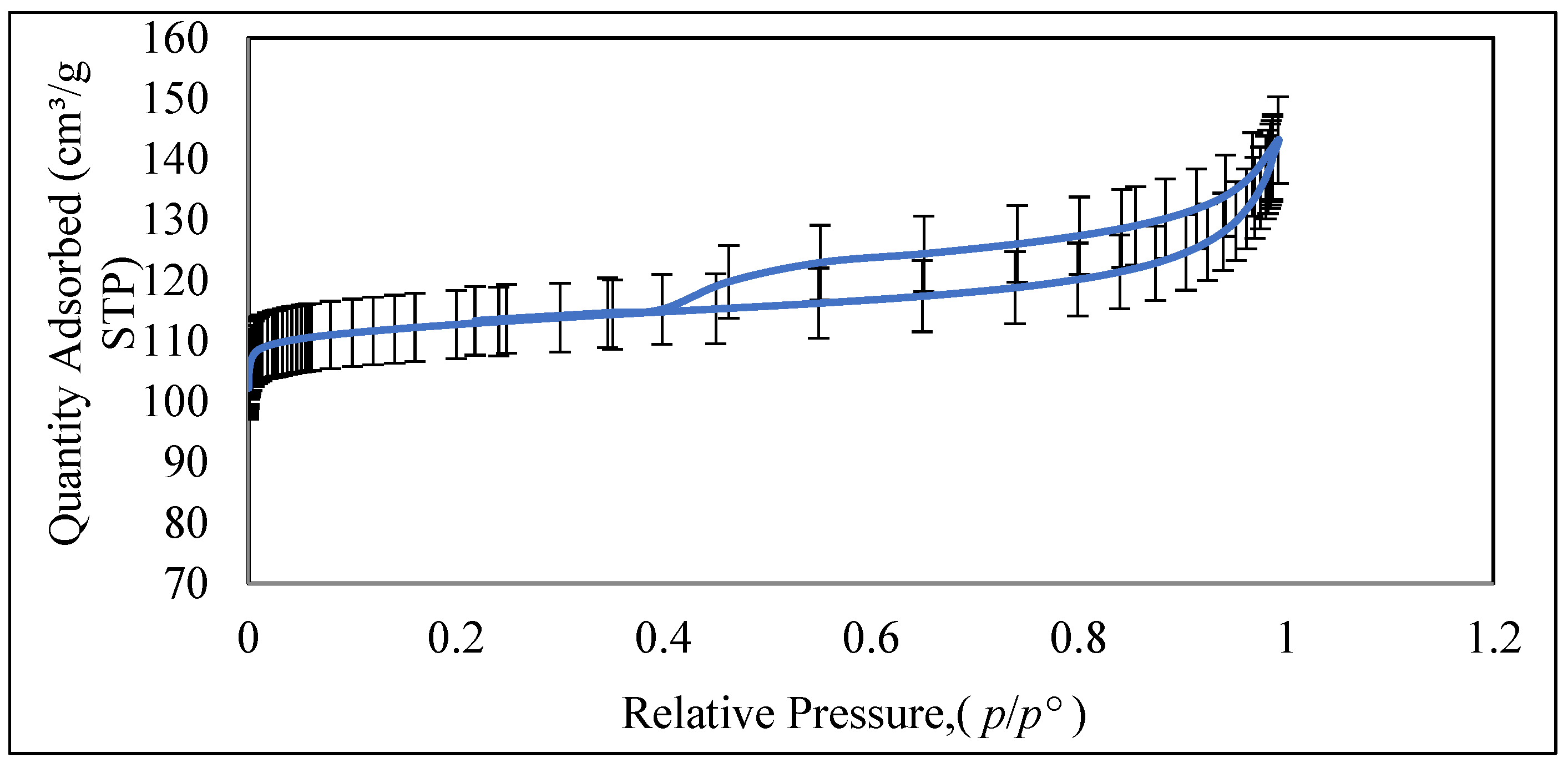
3.1.9. N2 Adsorption-Desorption
3.2. Effect of Parameters on Ions Removal
3.2.1. Effect of Concentrations, at Constant Temperature = 30 °C and pH = 9
3.2.2. Effect of pH at Constant Concentration = 20 mg/L and Temperature = 30 °C
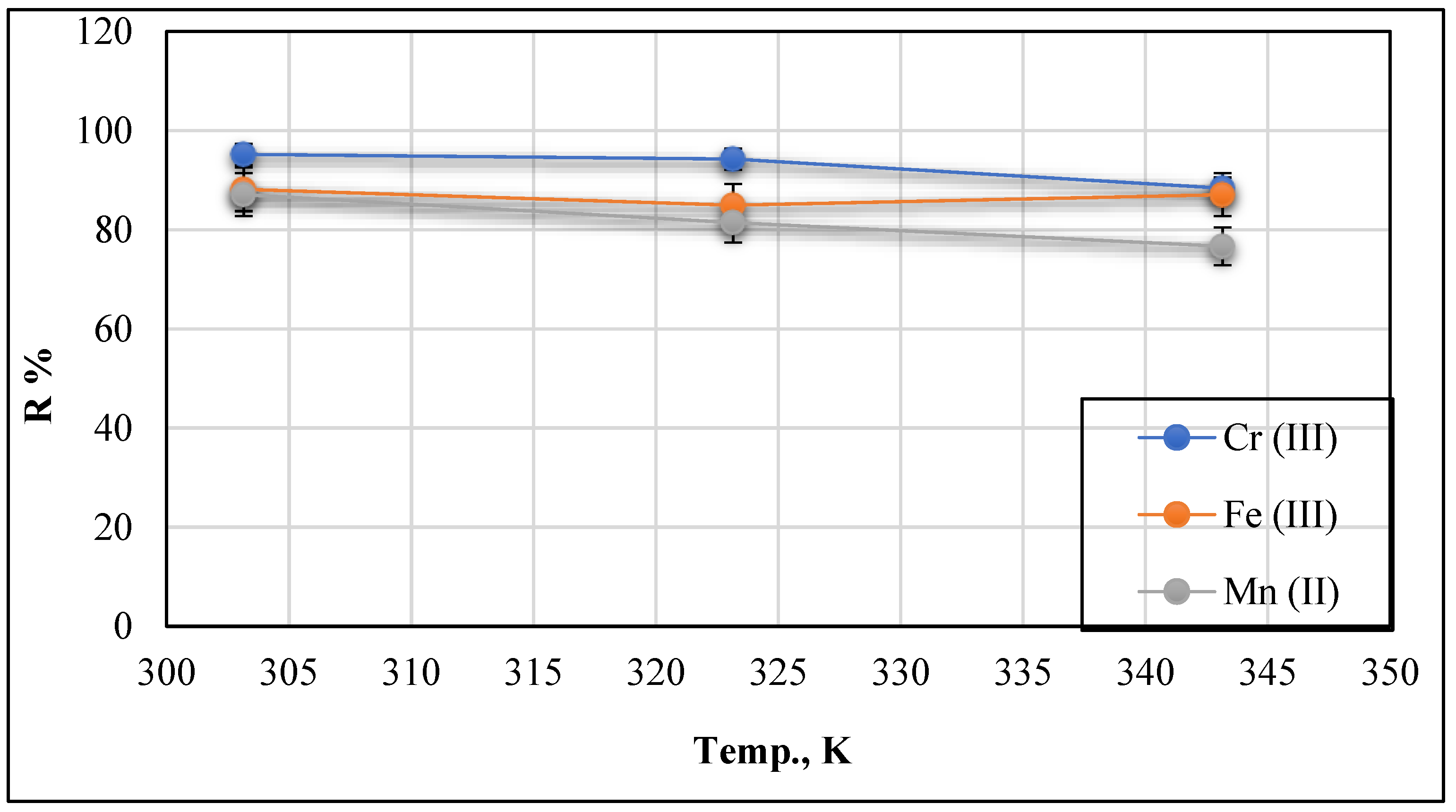
3.2.3. Effect of Temperatures at Co = 20 mg/L and pH = 9
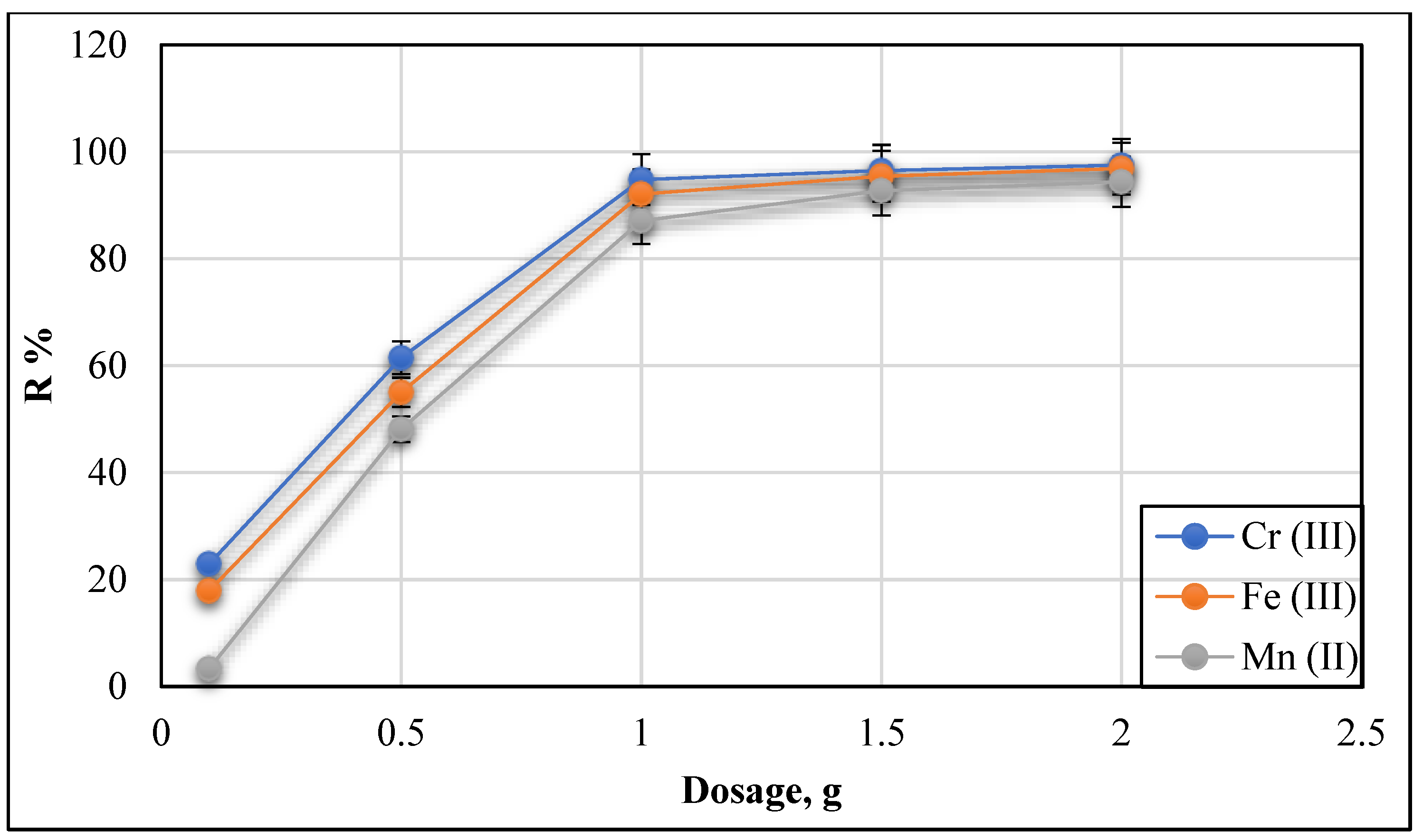
3.2.4. Effect of Adsorbent Dosage (Conc. 20 mg/L and pH 9)
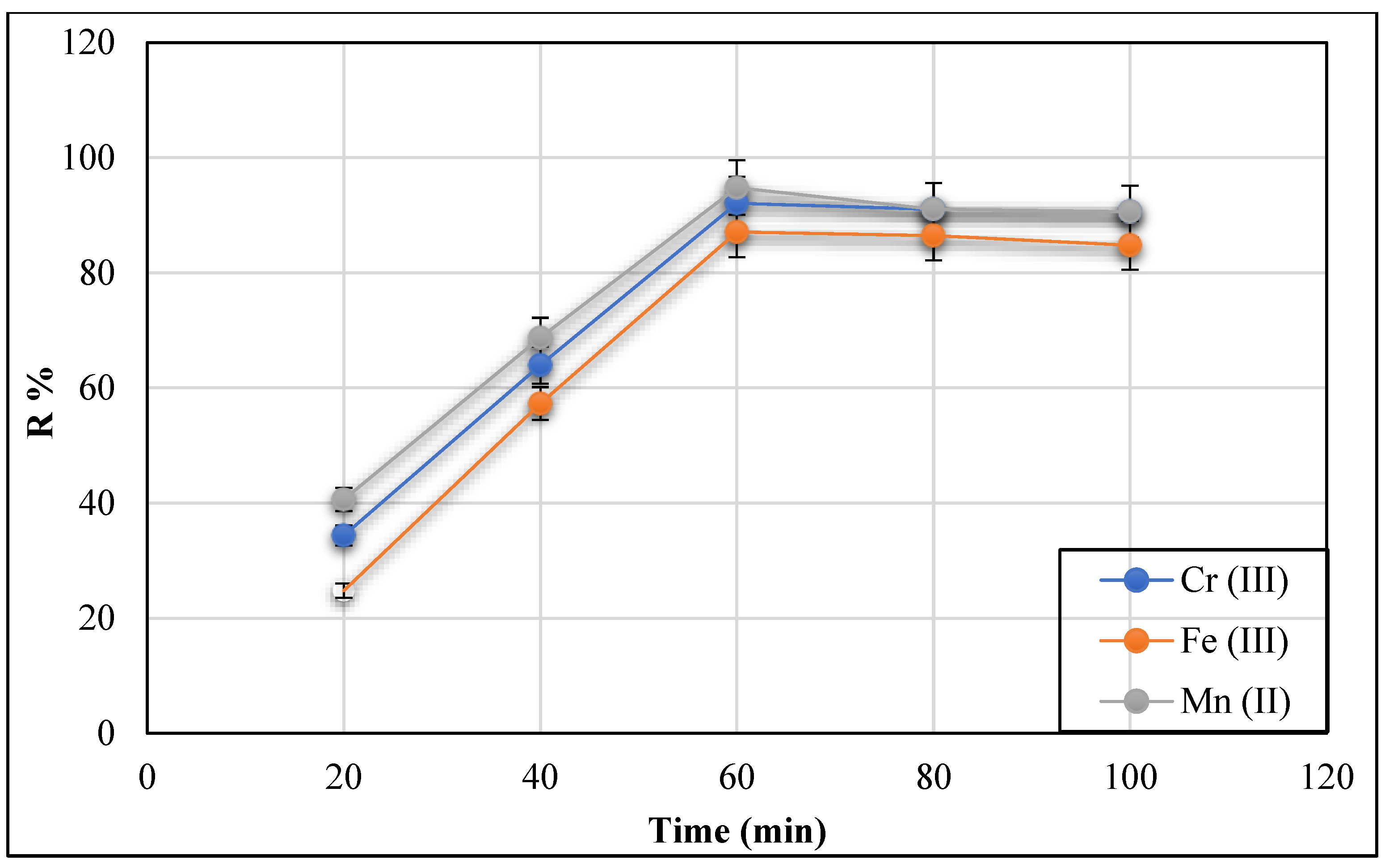
3.2.5. Effect of Mixing Time
3.2.6. Kinetics Study and Adsorption Modelling
3.2.7. Adsorption Isotherm Modeling
3.2.8. Thermodynamic Adsorption Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| Ch | Chitosan |
| g-HNTs | grafted halloysite nanotubes |
| NaOH | sodium hydroxide |
| HCl | hydrochloric acid |
| CaCO3 | calcium carbonate |
| KOH | potassium hydroxide |
| DI | deionized water |
| SDS | an aqueous solution of sodium dodecyl sulfate |
| EDTA | ethylenediaminetetraacetic acid |
| Fe(NO3)3 | ferric nitrate |
| Zn(NO3)2 | zinc nitrate |
| ZnγFe3O4 | Alpha iron zincate |
| FTIR | Fourier transform infrared |
| TGA | Thermogravimetric analysis |
| GPC | Gel permeation chromatography |
| XRD | X-ray diffraction |
| pHpzc | Point of zero charge |
| SEM | scanning electron microscope |
| TEM | Transmission electron microscopy |
References
- Singh, R.P.; Singh, P.; Araujo, A.S.F.; Hakimi Ibrahim, M.; Sulaiman, O. Management of urban solid waste: Vermicomposting a sustainable option. Resour. Conserv. Recycl. 2011, 55, 719–729. [Google Scholar] [CrossRef]
- Agamuthu, P.; Fauziah, S. Waste management technologies in Malaysia: The future prospect. In Proceedings of the ICAST Future: An Integrated Approach towards Science and Technology for Sustainable Development, Pahang, Malaysia, 13–15 June 2008; pp. 1–3. [Google Scholar]
- Ismail, M.; Akhtar, K.; Khan, M.; Kamal, T.; Khan, M.A.; M Asiri, A.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mishra, A. Heavy Metal Contamination. In Soil Contamination; IntechOpen: London, UK, 2021. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Blanusa, M.; Varnai, V.M.; Piasek, M.; Kostial, K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr. Med. Chem. 2005, 12, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, D.; Tsang, D.C.; Wang, L.; Ok, Y.S.; Feng, Y. A critical review of risks, characteristics, and treatment strategies for potentially toxic elements in wastewater from shale gas extraction. Environ. Int. 2019, 125, 452–469. [Google Scholar] [CrossRef]
- Taleb, M.A.; Kumar, R.; Al-Rashdi, A.A.; Seliem, M.K.; Barakat, M. Fabrication of SiO2/CuFe2O4/polyaniline composite: A highly efficient adsorbent for heavy metals removal from aquatic environment. Arab. J. Chem. 2020, 13, 7533–7543. [Google Scholar] [CrossRef]
- Gunatilake, S. Methods of removing heavy metals from industrial wastewater. Methods 2015, 1, 14. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Cellulose based grafted biosorbents-Journey from lignocellulose biomass to toxic metal ions sorption applications-A review. J. Mol. Liq. 2017, 232, 62–93. [Google Scholar] [CrossRef]
- Magdy, A.; Wassel, M.; Hosny, R.; Desouky, A.; Mahmod, A. Study the removal of cupper ions from textile effluent using cross linked chitosan. In Proceedings of the 8th International Conferences of Textile Research Division, Cairo, Egypt, 25–27 September 2017. [Google Scholar]
- Burakov, A.; Burakova, I.; Galunin, E.; Kucherova, A. New carbon nanomaterials for water purification from heavy metals. In Handbook of Ecomaterials; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Perrich, J.R. Activated Carbon Adsorption for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Husnain, S.M.; Um, W.; Chang, Y.-S. Magnetite-based adsorbents for sequestration of radionuclides: A review. RSC Adv. 2018, 8, 2521–2540. [Google Scholar] [CrossRef]
- Fathy, M.; Hosny, R.; Keshawy, M.; Gaffer, A. Green synthesis of graphene oxide from oil palm leaves as novel adsorbent for removal of Cu(II) ions from synthetic wastewater. Graphene Technol. 2019, 4, 33–40. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2020, 9, 104901. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.; Morin-Crini, N. Green adsorbents for pollutant removal. Environ. Chem. A Sustain. World 2018, 18, 23–71. [Google Scholar]
- Hosny, R.; Abdel-Moghny, T.; Ramzi, M.; Desouky, S.; Shama, S. Preparation and Characterization of Natural Polymer for Treatment Oily Produced Water. Int. J. Curr. Res. 2014, 6, 5413–5418. [Google Scholar]
- Luo, J.; Yu, D.; Hristovski, K.D.; Fu, K.; Shen, Y.; Westerhoff, P.; Crittenden, J.C. Critical Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Mechanism Identification and Engineering Design. Environ. Sci. Technol. 2021, 55, 4287–4304. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Aravind, J.; Kamaraj, M.; Sureshbabu, P.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Ali, M.A.; Mubarak, M.F.; Keshawy, M.; Zayed, M.A.; Ataalla, M. Adsorption of Tartrazine anionic dye by novel fixed bed Core-Shell- polystyrene Divinylbenzene/Magnetite nanocomposite. Alexandria Eng. J. 2021. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan–hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Nizamuddin, S.; Karri, R.R. Magnetic nanoadsorbents’ potential route for heavy metals removal—A review. Environ. Sci. Pollut. Res. 2020, 27, 24342–24356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Marotta, A.; Luzzi, E.; Salzano de Luna, M.; Aprea, P.; Ambrogi, V.; Filippone, G. Chitosan/zeolite composite aerogels for a fast and effective removal of both anionic and cationic dyes from water. Polymers 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides–A review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Luo, J.; Fu, K.; Yu, D.; Hristovski, K.D.; Westerhoff, P.; Crittenden, J.C. Review of advances in engineering nanomaterial adsorbents for metal removal and recovery from water: Synthesis and microstructure impacts. ACS EST Eng. 2021, 1, 623–661. [Google Scholar] [CrossRef]
- Adegoke, H.I.; Adekola, F.A.; Fatoki, O.S.; Ximba, B.J. Sorptive Interaction of Oxyanions with Iron Oxides: A Review. Pol. J. Environ. Stud. 2013, 22, 7–24. [Google Scholar]
- Hosny, R.; Fathy, M.; Abdelraheem, O.H.; Zayed, m.A. Utilization of Cross-Linked Chitosan/ACTF Biocomposite for Softening Hard Water: Optimization by Adsorption Modeling. Egypt. J. Chem. 2019, 62, 437–456. [Google Scholar] [CrossRef]
- Saliu, T.; Oladoja, N. Assessing the suitability of solid aggregates for nutrient recovery from aqua systems. J. Water Process. Eng. 2020, 33, 101000. [Google Scholar] [CrossRef]
- Toan, N.V. Production of Chitin and Chitosan from Partially Autolyzed Shrimp Shell Materials. Open Biomater. J. 2009, 1, 21–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. J. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Rawtani, D. Future Aspects of Halloysite Nanotubes in Forensic Investigations. J. Nanomed. Res. 2017, 6. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Bahar, S.; Ziarani, G.M.; Faridbod, F.; Ganjali, M.R. A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J. Magn. Magn. Mater. 2017, 441, 193–203. [Google Scholar] [CrossRef]
- Chatterjee, S.; Adhya, M.; Guha, A.K.; Chatterjee, B.P. Chitosan from Mucorrouxii: Production and Physico-Chemical Characterization. J. Process. Biochem. 2005, 40, 395–400. [Google Scholar] [CrossRef]
- Rawtani, D.; Pandey, G.; Tharmavaram, M.; Pathak, P.; Akkireddy, S.; Agrawal, Y.K. Development of a novel ‘nanocarrier’ system based on Halloysite Nanotubes to overcome the complexation of ciprofloxacin with iron: An in vitro approach. Appl. Clay Sci. 2017, 150, 293–302. [Google Scholar] [CrossRef]
- El-Dakkony, S.R.; Mubarak, M.F.; Ali, H.R.; Gaffer, A.; Moustafa, Y.M.; Abdel-Rahman, A.-H. Composite thin-film membrane of an assembled activated carbon thin film with autoself-healing and high-efficiency water desalination. Environ Dev Sustain 2021. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Mubarak, M.F. Adsorption of Cationic Dye Using A newly Synthesized CaNiFe2O4/Chitosan Magnetic Nanocomposite: Kinetic and Isotherm Studies. J. Polym. Environ. 2021, 29, 1835–1851. [Google Scholar] [CrossRef]
- Athapaththu, S. A Comprehensive Study of Cd (II) Removal from Aqueous Solution via Adsorption and Solar Photocatalysis. Master Thesis, The University of Western, London, ON, Canada, 12 December 2013. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]



| Retention Time | Mn | Mw | Mp | Mz | Mz+1 | Polydispersity |
|---|---|---|---|---|---|---|
| 27.220 | 50,015 | 86,380 | 50,493 | 144,904 | 208,744 | 1.727065 |
| Retention Time | Mn | Mw | Mp | Mz | Mz+1 | Polydispersity |
|---|---|---|---|---|---|---|
| 26.112 | 51,518 | 110,534 | 90,177 | 193,865 | 260,533 | 2.145541 |
| Kinetic Order | Parameters | Metal Ions | ||
|---|---|---|---|---|
| Cr (III) | Fe (III) | Mn (II) | ||
| Pseudo-first-order | qe (mg/g) | 1.0234 | 1.0268 | 1.01547 |
| R2 | 0.85433 | 0.29425 | 0.89897 | |
| K1 (min−1) | 0.01478 | 0.0019 | 0.01023 | |
| Pseudo-second-order | qe (mg/g) | 2.4078 | 2.40781 | 2.63299 |
| R2 | 0.98978 | 0.96695 | 0.9878 | |
| K2 (min−1) | 1.626199 | 1.23978 | 0.70769 | |
| Model | Constants | Metal Ions | ||
|---|---|---|---|---|
| Cr (III) | Fe (III) | Mn (II) | ||
| Langmuir | qm (mg/g) | 0.893395 | 0.4467 | 0.23182 |
| KL (L/mg) | 0.231614 | 0.115807 | 0.107141 | |
| R2 | 0.999989 | 0.999989 | 0.999700 | |
| Freundlich | ||||
| Kf | 0.753768 | 0.351077 | 0.283438 | |
| n | 0.408312 | 0.41059 | 0.57226 | |
| R2 | 0.99261 | 0.98584 | 0.994095 | |
| Cr (III) | |||
| T (K) | ΔH (J/mol) | ΔS (J/mol.K) | ΔG (J/mol) |
| 303.15 | −35,703.1 | 110.688 | −69,258.1 |
| 323.15 | −35,703.1 | 110.688 | −71,471.9 |
| 343.15 | −35,703.1 | 110.688 | −73,685.6 |
| Fe (III) | |||
| T (K) | ΔH (J/mol) | ΔS (J/mol.K) | ΔG (J/mol) |
| 303.15 | −30,960.4 | 99.57985 | −61,148 |
| 323.15 | −30,960.4 | 99.57985 | −63,139.6 |
| 343.15 | −30,960.4 | 99.57985 | −65,131.2 |
| Mn (II) | |||
| T (K) | ΔH (J/mol) | ΔS (J/mol.K) | ΔG (J/mol) |
| 303.15 | −43,996.5 | 135.2966 | −85,011.6 |
| 323.15 | −43,996.5 | 135.2966 | −87,717.6 |
| 343.15 | −43,996.5 | 135.2966 | −90,423.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarak, M.F.; Ragab, A.H.; Hosny, R.; Ahmed, I.A.; Ahmed, H.A.; El-Bahy, S.M.; El Shahawy, A. Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater. Polymers 2021, 13, 2714. https://doi.org/10.3390/polym13162714
Mubarak MF, Ragab AH, Hosny R, Ahmed IA, Ahmed HA, El-Bahy SM, El Shahawy A. Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater. Polymers. 2021; 13(16):2714. https://doi.org/10.3390/polym13162714
Chicago/Turabian StyleMubarak, Mahmoud F., Ahmed H. Ragab, Rasha Hosny, Inas A. Ahmed, Hanan A. Ahmed, Salah M. El-Bahy, and Abeer El Shahawy. 2021. "Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater" Polymers 13, no. 16: 2714. https://doi.org/10.3390/polym13162714
APA StyleMubarak, M. F., Ragab, A. H., Hosny, R., Ahmed, I. A., Ahmed, H. A., El-Bahy, S. M., & El Shahawy, A. (2021). Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater. Polymers, 13(16), 2714. https://doi.org/10.3390/polym13162714








