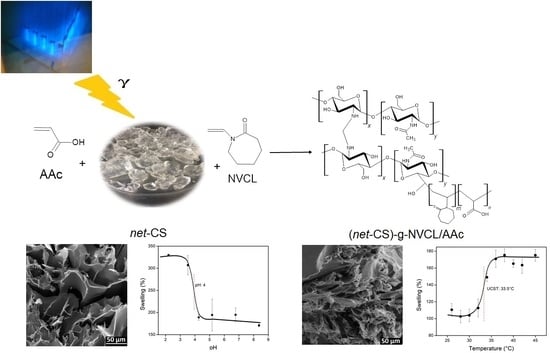
Binary Graft of Poly(N-vinylcaprolactam) and Poly(acrylic acid) onto Chitosan Hydrogels Using Ionizing Radiation for the Retention and Controlled Release of Therapeutic Compounds
Abstract
:1. Introduction
2. Materials and Methods
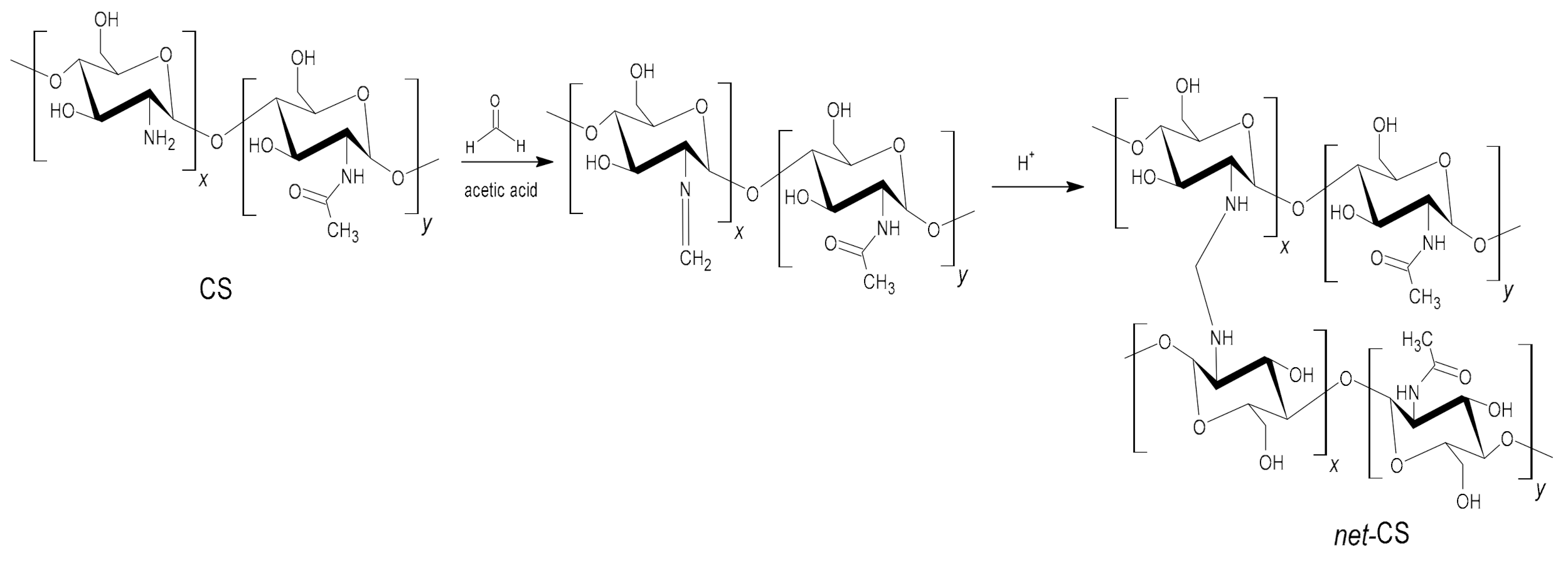
2.1. Crosslinking of CS
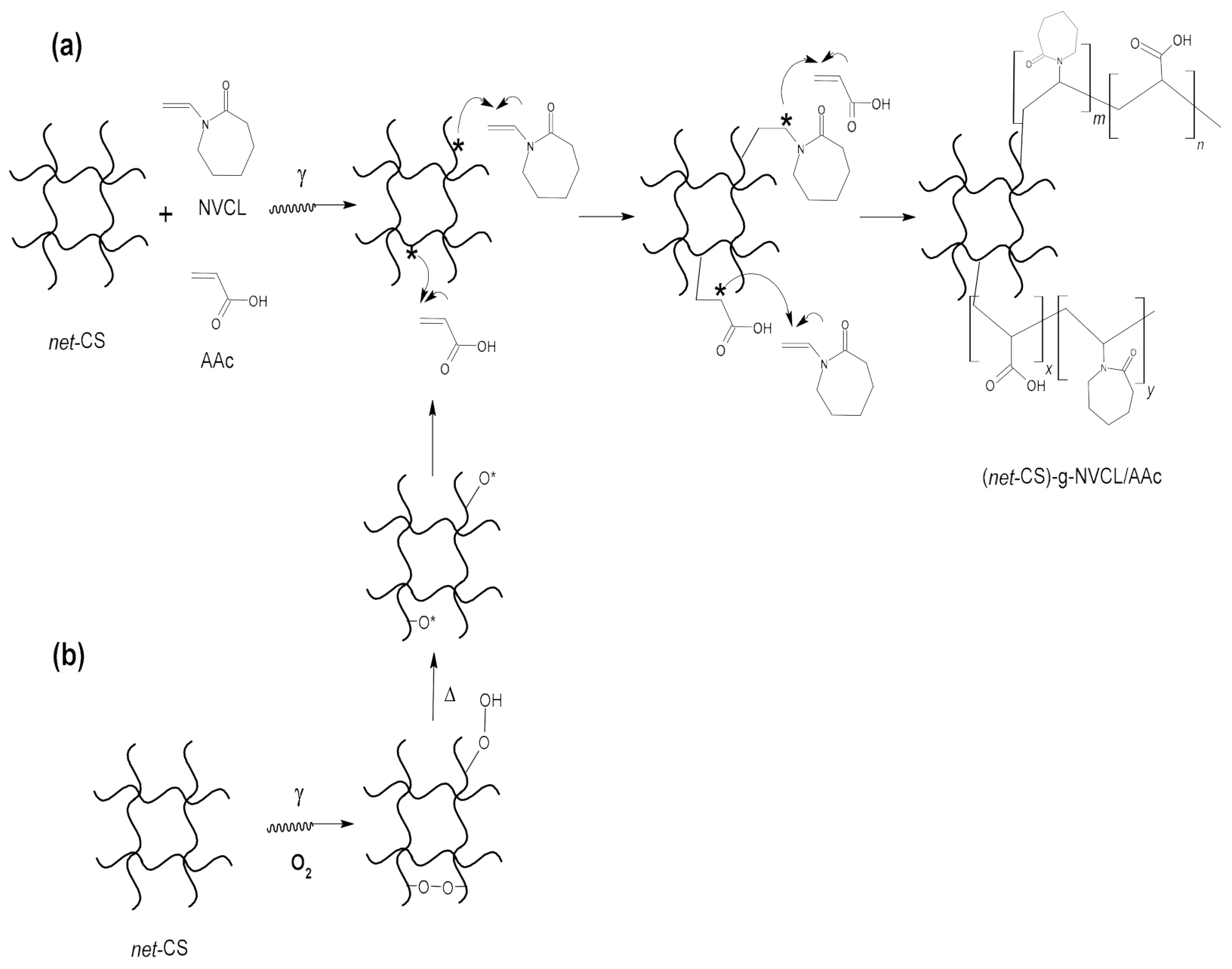
2.2. Grafting of NVCL/AAc onto net-CS
2.2.1. Direct Method
2.2.2. Oxidative Pre-Irradiation Method
2.3. Characterization
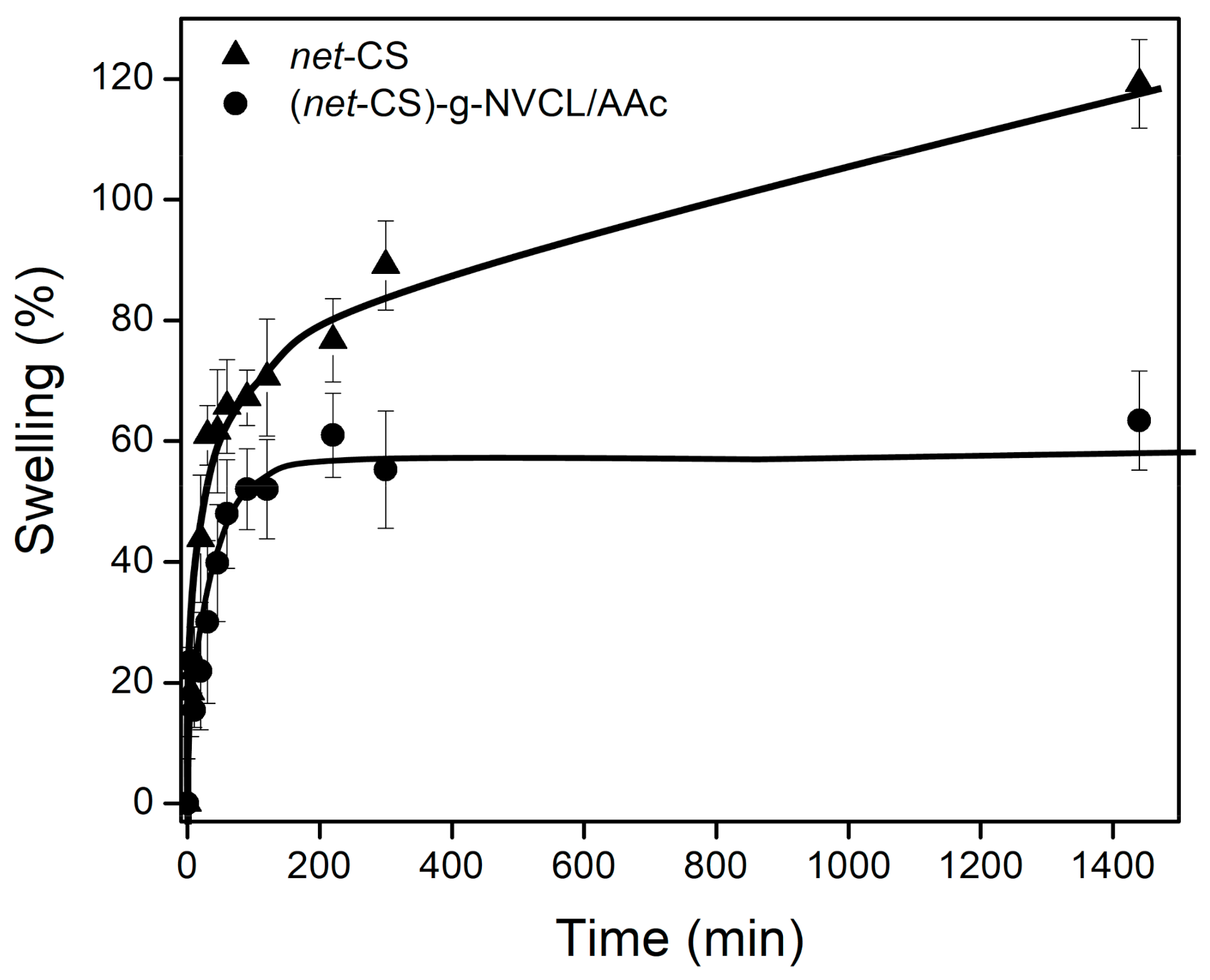
2.3.1. Equilibrium Swelling Time
2.3.2. Critical Points (Temperature and pH)
2.3.3. Drug Loading and Release
3. Results and Discussion
3.1. Crosslinking of CS
3.2. Effect of Dose on Grafting of NVCL/AAc onto net-CS
3.3. Characterization
3.3.1. FTIR
3.3.2. Equilibrium Swelling
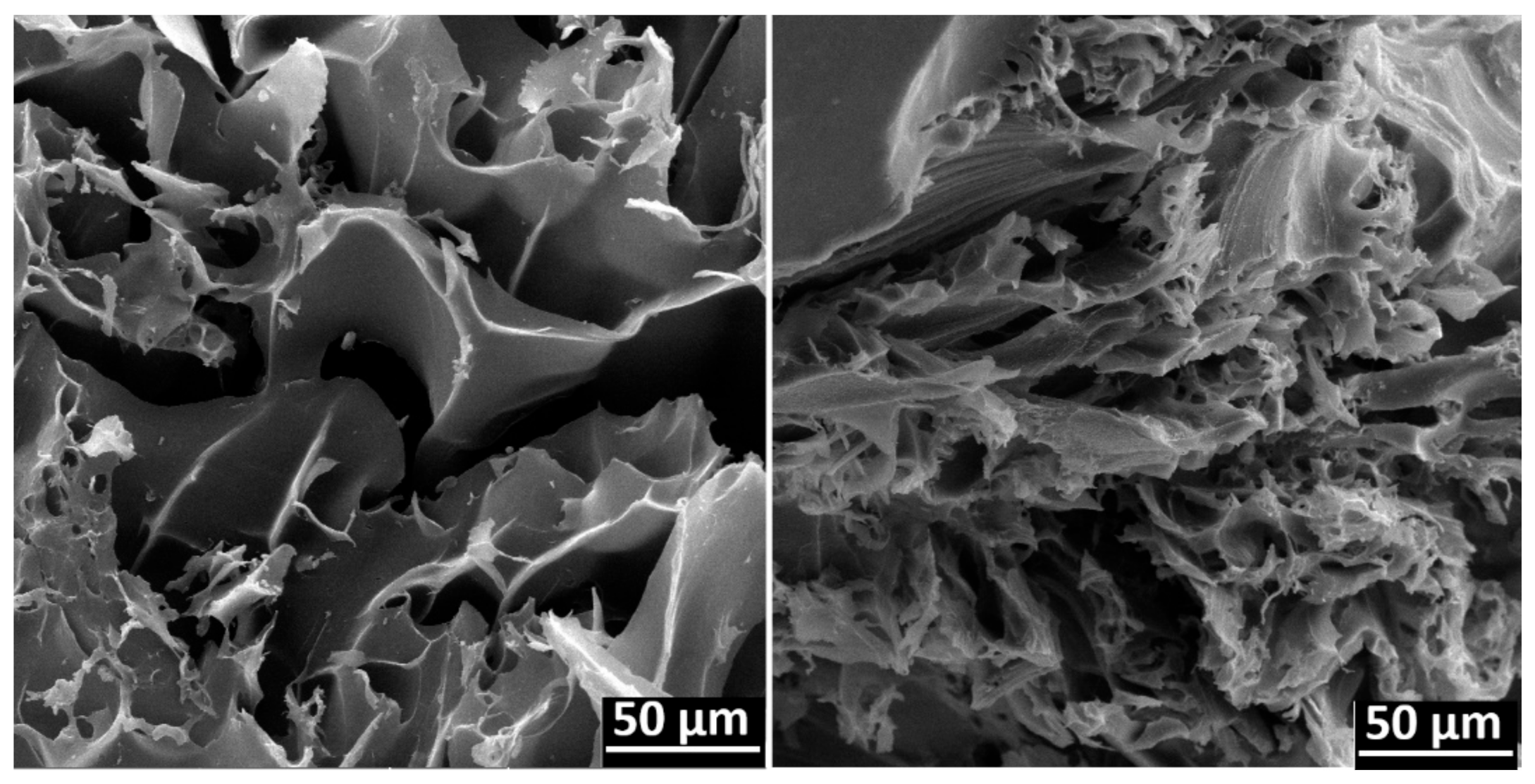
3.3.3. SEM
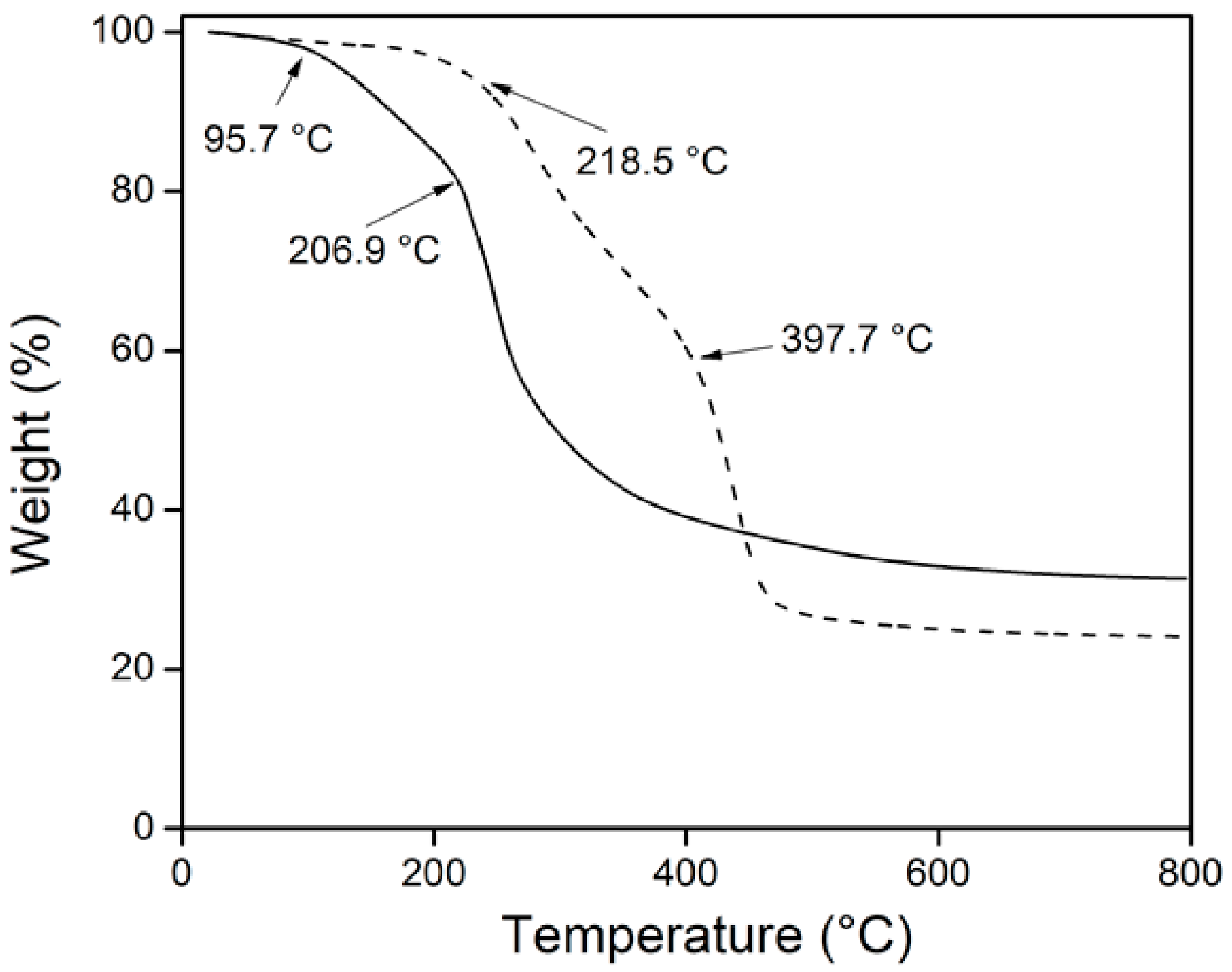
3.3.4. Thermogravimetric Analysis
3.3.5. Critical Temperature Determination
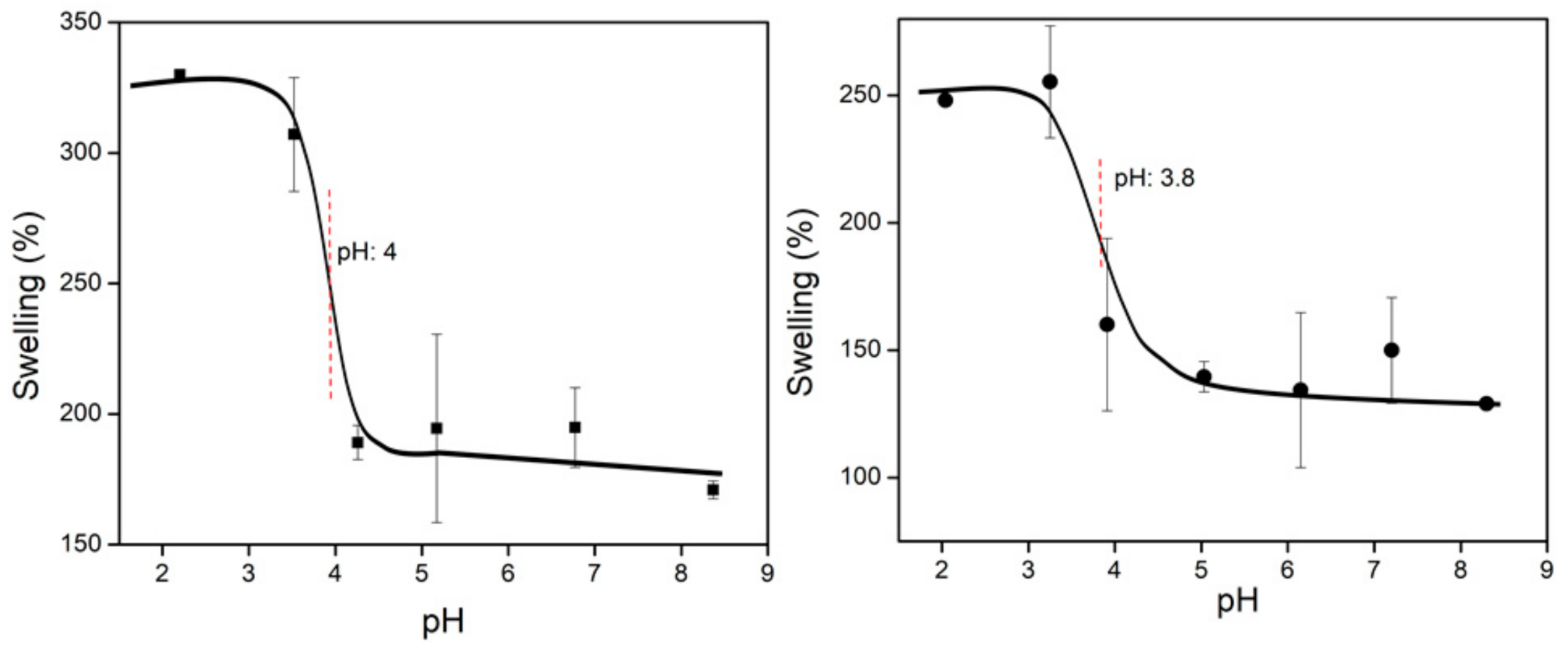
3.3.6. Determination of the Critical pH of the Systems
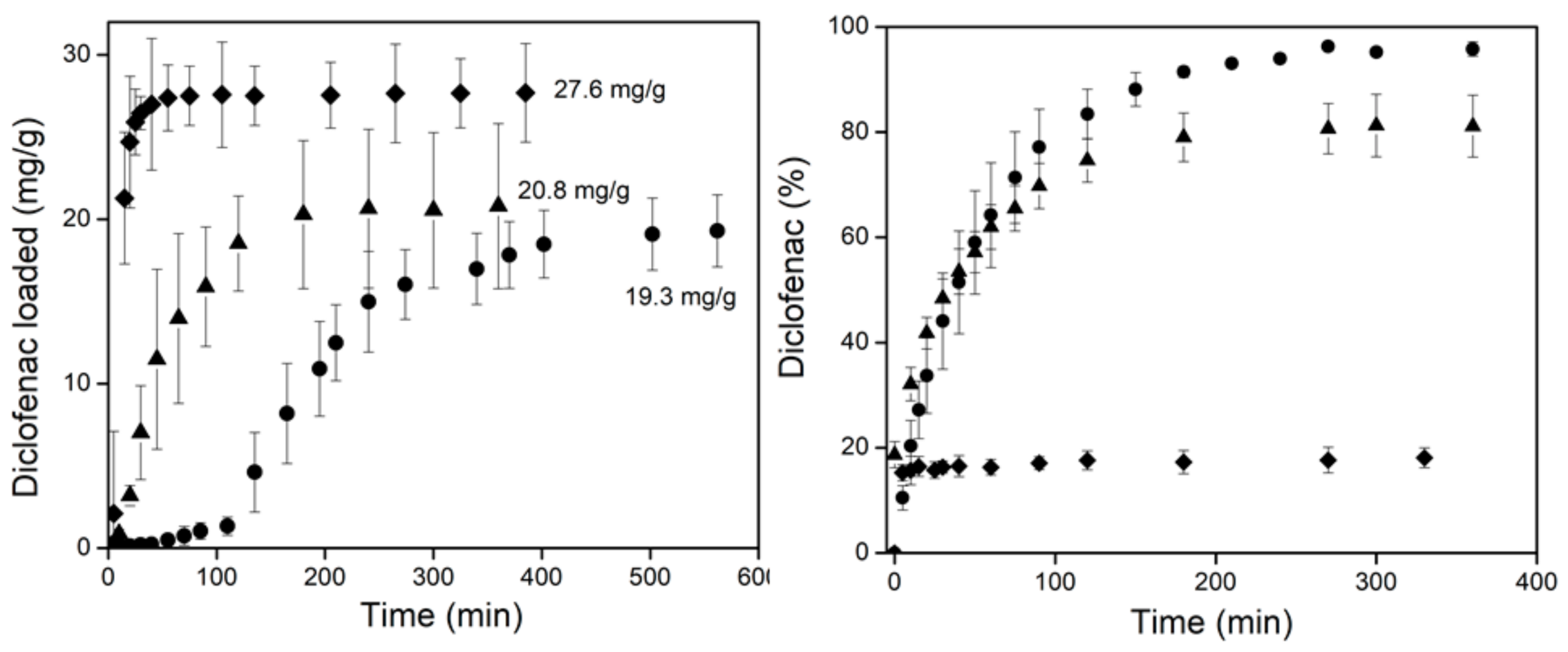
3.4. Drug Loading and Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dash, M.; Chiellini, M.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Sousa, A.I.; Carvalho-Silva, J.; Ferreira, I.M.M.; Novoc, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.V.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Ingberr, D.E. Manufacturing of large-scale functional objects using biodegradable chitosan bioplastic. Macromol. Matter. Eng. 2014, 299, 932–938. [Google Scholar] [CrossRef]
- Singh, A.; Narvi, S.S.; Dutta, P.K.; Pandey, N.D. External stimuli response on a novel chitosan hydrogel crosslinked with formaldehyde. Bull. Mater. Sci. 2016, 29, 233–238. [Google Scholar] [CrossRef]
- Cruz, A.; Garcia, L.; Ortega, A.; Isoshima, T.; Burillo, G. Radiation grafting of N-vinylcaprolactam onto nano and macrogels of Chitosan: Synthesis and Characterization. Carbohydr. Polym. 2017, 155, 303–312. [Google Scholar] [CrossRef]
- Sokker, H.H.; Ghaffar, A.M.A.; Gad, Y.H.; Aly, A.S. Synthesis and characterization of hydrogels based on grafted chitosan for the controlled drug release. Carbohydr. Polym. 2009, 75, 222–229. [Google Scholar] [CrossRef]
- Nataraj, D.; Sakkara, S.; Meghwal, M.; Reddy, N. Crosslinked chitosan films with controllable properties for commercial applications. Int. J. Biol. Macromol. 2018, 120, 1256–1264. [Google Scholar] [CrossRef]
- Hirsch, D.B.; Martinez-Alvarez, L.M.; Urtasun, N.B.; Maria, F.; Lazaro-Martinez, J.M.; Glisoni, R.J.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Lactoferrin purification and whey protein isolate recovery from cheese whey using chitosan mini-spheres. Int. Dairy J. 2020, 109, 104764. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Steven, C.V. Recent development in antibacterial and antifungal chitosan and itt derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Hirsch, D.B.; Baieli, M.F.; Urtasun, N.B.; Lazaro-Martinez, J.M.; Glisoni, R.J.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Sulfanilic Acid-Modified Chitosan Mini-Spheres and Their Application forLysozyme Purification from Egg White. Biotechnol. Prog. 2018, 34, 388–396. [Google Scholar] [CrossRef]
- Montes, J.A.; Ortega, A.; Burillo, G. Dual-stimuli responsive copolymers based on N-vinylcaprolactam/chitosan. J. Radioanal. Nucl. Chem. 2015, 303, 2143–2150. [Google Scholar] [CrossRef]
- Kudyshkin, V.O.; Futoryanskaya, A.M.; Milusheva, N.D.; Kareva, D.; Rashidova, S.H. Graft Copolymerization of N-vinyl Caprolactam onto Chitosan. Int. J. Mater. Chem. 2014, 4, 65–68. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J. Preliminary studies on radiation-induced changes in chitosan. Radiat. Phys. Chem. 1992, 39, 53–57. [Google Scholar] [CrossRef]
- Grycska, U.; Dondi, D.; Chmielewski, A.G.; Migdal, U.; Buttafava, A.; Faucitano, A. The mechanism of chitosan degradation by gamma and e-beam irradiation. Radiat. Phys. Chem. 2009, 78, 543–548. [Google Scholar] [CrossRef]
- Pengfei, L.; Maolin, Z.; Jilan, Z. Study on radiation-induced grafting of styrene onto chitin and chitosan. Radiat. Phys. Chem. 2001, 61, 149–153. [Google Scholar] [CrossRef]
- Wang, J.P.; Chen, Y.Z.; Zhang, S.J.; Yu, H.Q. A chitosan-based flocculant prepared with gamma-irradiation-induced grafting. Bioresour. Technol. 2008, 99, 3397–3402. [Google Scholar] [CrossRef]
- Hassan, M.S.; Haytham, M.M. Characterization and antimicrobial properties of metal complexes of polypropylene fibers grafted with acrylic acid using gamma irradiation. Polym. Adv. Technol. 2016, 27, 532–541. [Google Scholar] [CrossRef]
- Tinoco, D.; Ortega, A.; Burillo, G.; Islas, L.; Garcia-Uriostegui, L. Different hydrogel architectures synthesized by gamma radiation based on chitosan and N, N-dimethylacrylamide. MRS Comm. 2018, 8, 617–623. [Google Scholar] [CrossRef]
- Islas, L.; Burillo, G.; Ortega, A. Graft copolymerization of 2-hydroxyethyl methacrylate onto chitosan using radiation technique for release of diclofenac. Macromol. Res. 2018, 26, 690–695. [Google Scholar] [CrossRef]
- Ivanov, V.S. Radiation Chemistry of Polymers; VSP: Utrecht, The Netherlands, 1992; p. 319. [Google Scholar]
- Perez-Calixto, M.P.; Ortega, A.; García-Uriostegui, L.; Burillo, G. Synthesis and characterization of N-vinylcaprolactam/N-dimethylacrylamide grafted onto chitosan networks by gamma radiation. Radiat. Phys. Chem. 2016, 116, 228–235. [Google Scholar] [CrossRef]
- Patiño, Z.; Ortega, A.; Burillo, G. Removal of Cr (VI) ions using a binary grafting of N-vinylcaprolactam and N, N -dimethylacrylamide onto crosslinked chitosan, synthesized by gamma radiation. J. Mex. Chem. Soc. 2019, 63, 155–163. [Google Scholar] [CrossRef]
- Choi, W.S.; Ahn, K.J.; Lee, D.W.; Byun, M.W.; Park, H.J. Preparation of chitosan oligomers by irradiation. Polym. Degrad. Stab. 2002, 78, 533–538. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, F.; Ruiz, J.C.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Temperature- and pH-sensitive interpenetrating polymer networks grafted on PP: Cross-linking irradiation dose as a critical variable for the performance as vancomycin-eluting systems. Radiat. Phys. Chem. 2012, 81, 531–540. [Google Scholar] [CrossRef]
- Burillo, G.; Briones, M.; Adem, E. IPN’s of acrylic acid and N-isopropylacrylamide by gamma and electron beam irradiation. Nucl. Instrum. Methods B. 2007, 205, 104–108. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Hafte, M.; Sterubaut, W. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag. Sci. 2005, 61, 951–960. [Google Scholar] [CrossRef]
- Garcia-Vargas, M.; Gonzalez-Chomon, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2014, 461, 286–295. [Google Scholar] [CrossRef]
- Islas, L.; Alvarez-Lorenzo, C.; Margariños, B.; Concheiro, A.; Del-Castillo, L.F.; Burillo, G. Singly and binary grafted poly(vinylchloride) urinary catheters that elute ciprofloxacin and prevent bacteria adhesion. Int. J. Pharm. 2015, 488, 20–28. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hanifpour, F.; Taheri, R.; Javadian, H.; Ghasemi, M. Comparison of using formaldehyde and carboxy methyl chitosan in preparation of Fe3O4 superparamagnetic nanoparticles-chitosan hydrogel network: Sorption behavior toward bovine serum albumin. Process Saf. Environ. 2016, 102, 119–128. [Google Scholar] [CrossRef]
- Monteiro, O.A.; Airoldi, C. Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef]
- Iftime, M.M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a crosslinking method toward multifunctional hydrogels. Carbohyd. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Metzler, M.; Chylinska, M.; Kaczmarek, H. Preparation and characteristics of nanosilver composite based on chitosan-graft-acrylic acid copolymer. J. Polym. Res. 2015, 22, 146. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wang, C.; Zhang, Y.; Ye, L.; Qian, Y.; Shu, Y.; Wang, J.; Li, J.; Yao, F. A thermoresponsive poly(N-vinylcaprolactam-co-sulfobetaine methacrylate) zwitterionic hydrogel exhibiting switchable anti-biofouling and cytocompatibility. Polym. Chem. 2015, 6, 3431–3442. [Google Scholar] [CrossRef]
- González-Hernández, G.; Pino-Ramos, V.H.; Islas, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of N-vinylcaprolactam and 2-hydroxyethyl methacrylate onto polypropylene films to obtain a thermo-responsive drug delivery system. Radiat. Phys. Chem. 2019, 157, 6–14. [Google Scholar] [CrossRef]
- Ferraz, C.C.; Varca, G.H.; Ruiz, J.-C.; Lopes, P.S.; Mathor, M.B.; Lugao, A.B.; Bucio, E. Radiation-grafting of thermo- and pH-responsive poly(N-vinylcaprolactam-co-acrylic acid) onto silicone rubber and polypropylene films for biomedical purposes. Radiat. Phys. Chem. 2014, 97, 298–303. [Google Scholar] [CrossRef]
- Zhao, C.; Dolmans, L.; Zhu, X.X. Thermoresponsive behavior of Poly(acrylic acid-co-acrylonitrile) with UCST. Macromolecules 2019, 52, 4441–4446. [Google Scholar] [CrossRef]
- Whang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohyd. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Grafted cpolopymerized chitosan-present status and applications. Carbohyd. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Prabaharan, M.; Gralier, J.J.; Steeber, D.A.; Gong, S. Stimuli-Responsive Chitosan-graft-Poly(N-vinylcaprolactam) as a Promising Material for Controlled Hydrophobic Drug Delivery. Macomol. Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef]
- Banihashem, S.; Nezhati, M.N.; Panahia, H.A. Synthesis of chitosan-grafted-poly(N-vinylcaprolactam) coated on the thiolated gold nanoparticles surface for controlled release of cisplatin. Carbohyd. Polym. 2020, 227, 115333. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Singh, A.P.; Chauhan, G.S. Grafting of GMA and some comonomers onto chitosan for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2014, 64, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.A.; Matsuhiro, B.; Zapata, P.A.; Corrales, T.; Catalina, F. Preparation and characterization of maleoylagarose/PNIPAAm graft copolymers and formation of polyelectrolyte complexes with chitosan. Carbohyd. Polym. 2018, 182, 81–91. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, A.; Sánchez, A.; Burillo, G. Binary Graft of Poly(N-vinylcaprolactam) and Poly(acrylic acid) onto Chitosan Hydrogels Using Ionizing Radiation for the Retention and Controlled Release of Therapeutic Compounds. Polymers 2021, 13, 2641. https://doi.org/10.3390/polym13162641
Ortega A, Sánchez A, Burillo G. Binary Graft of Poly(N-vinylcaprolactam) and Poly(acrylic acid) onto Chitosan Hydrogels Using Ionizing Radiation for the Retention and Controlled Release of Therapeutic Compounds. Polymers. 2021; 13(16):2641. https://doi.org/10.3390/polym13162641
Chicago/Turabian StyleOrtega, Alejandra, Abigail Sánchez, and Guillermina Burillo. 2021. "Binary Graft of Poly(N-vinylcaprolactam) and Poly(acrylic acid) onto Chitosan Hydrogels Using Ionizing Radiation for the Retention and Controlled Release of Therapeutic Compounds" Polymers 13, no. 16: 2641. https://doi.org/10.3390/polym13162641
APA StyleOrtega, A., Sánchez, A., & Burillo, G. (2021). Binary Graft of Poly(N-vinylcaprolactam) and Poly(acrylic acid) onto Chitosan Hydrogels Using Ionizing Radiation for the Retention and Controlled Release of Therapeutic Compounds. Polymers, 13(16), 2641. https://doi.org/10.3390/polym13162641