Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
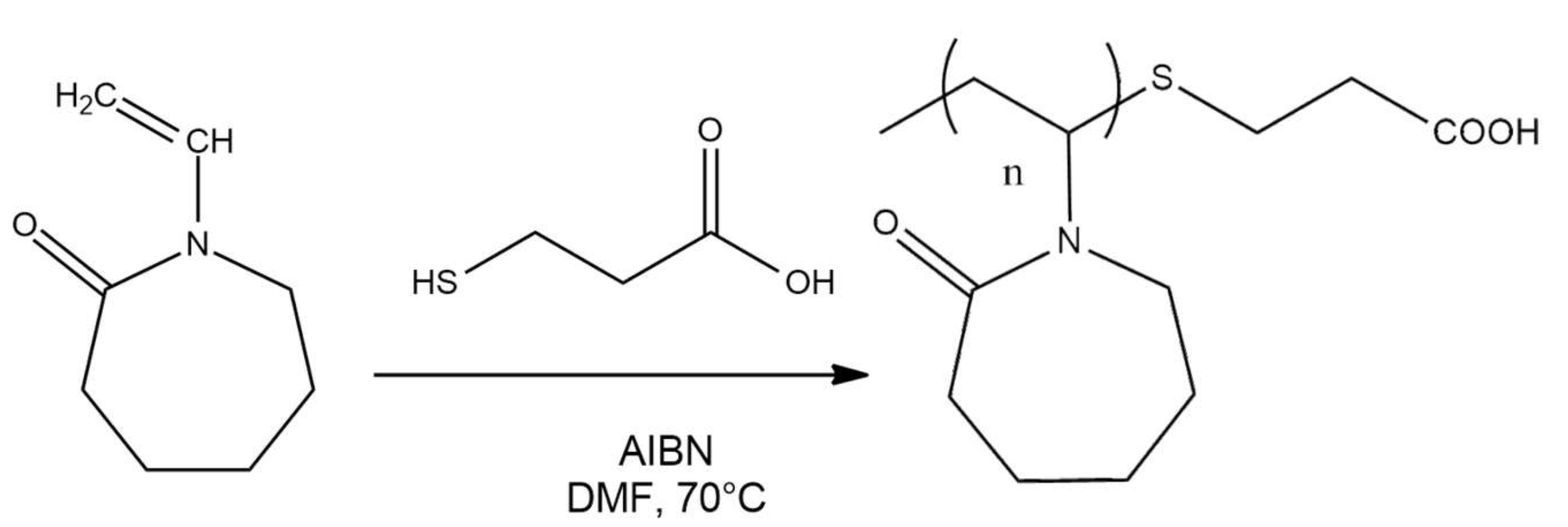
2.2. Synthesis of PNVCL-COOH Polymers
2.3. Structural Characterization of PNVCL-COOH Polymers
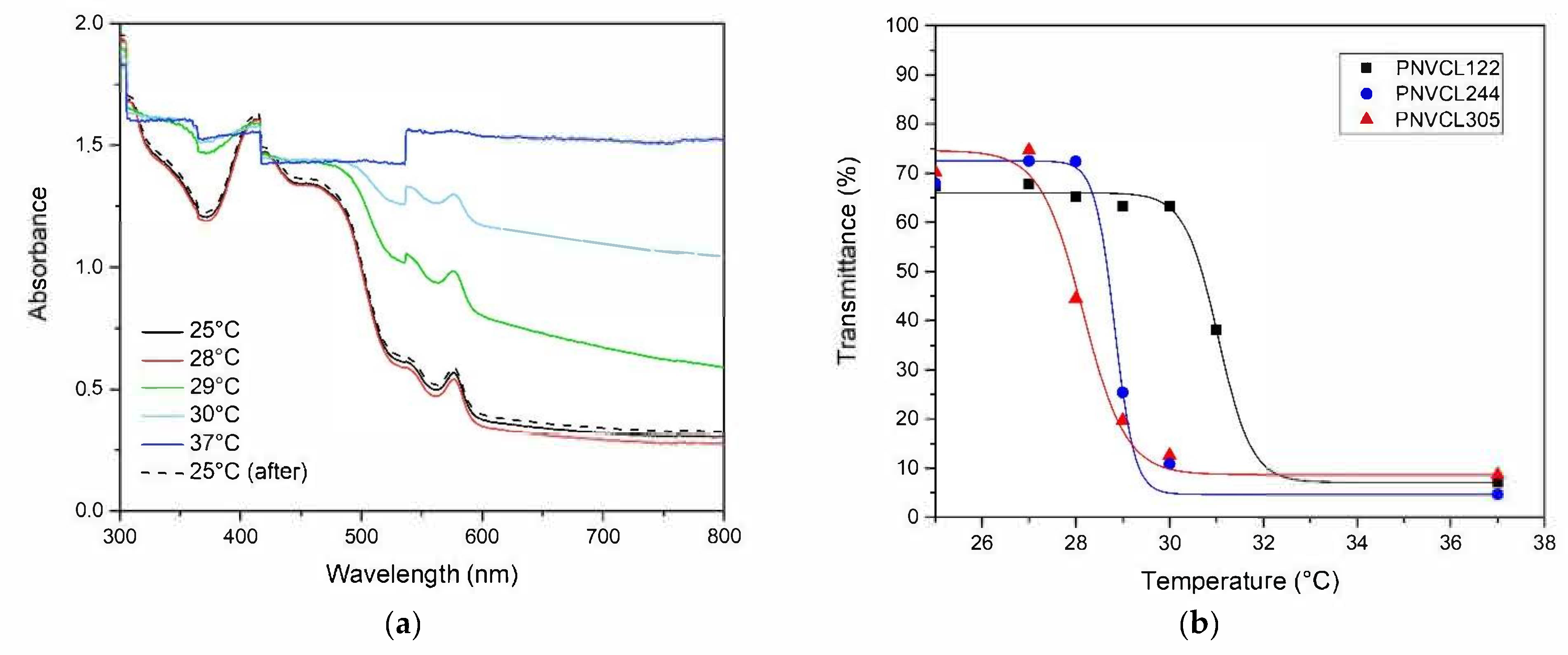
2.4. UV-VIS Determination of LCST
2.5. DLS Determination of LCST
2.6. Determination of Molecular Mass
2.6.1. Size Exclusion Chromatography
2.6.2. Dynamic Light Scattering
2.6.3. Intrinsic Viscosity Measurements
3. Results and Discussion
3.1. Structural Characterization of PNVCL-COOH Polymers
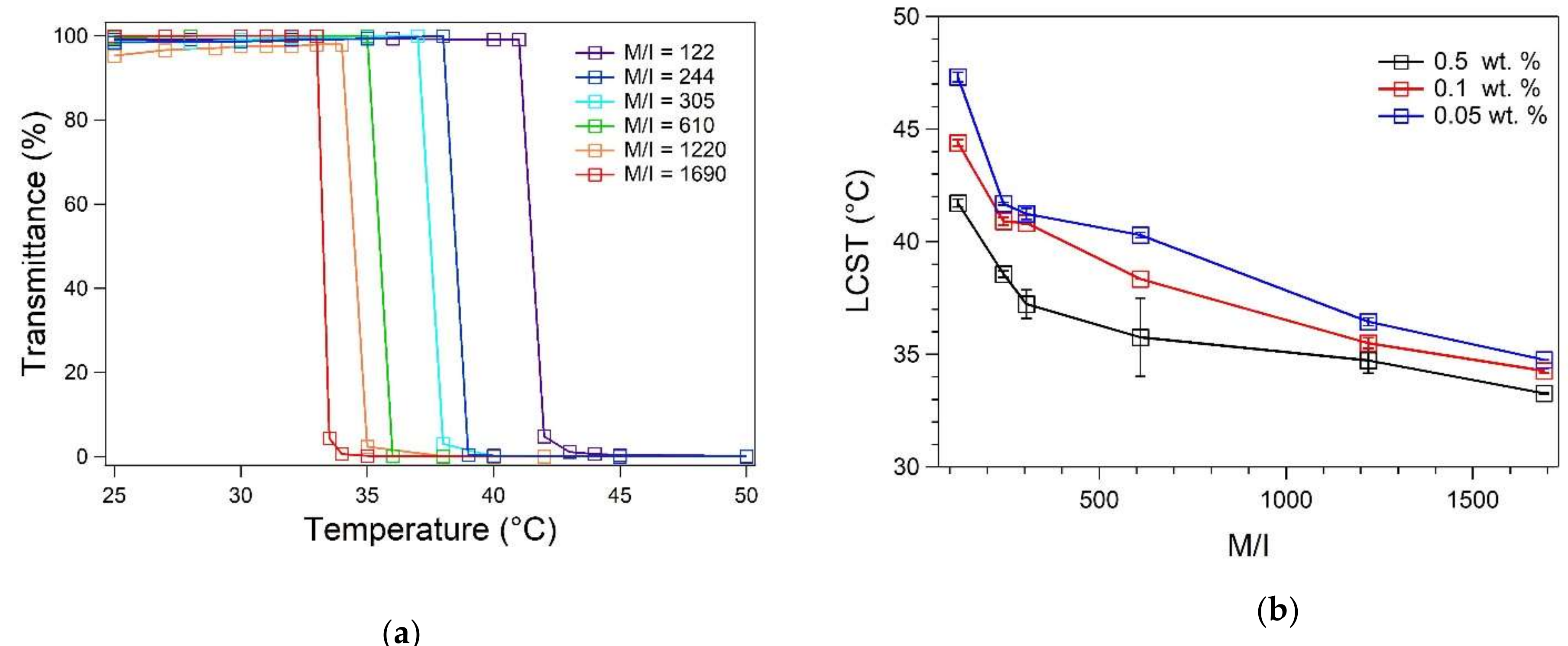
3.2. Spectroscopic Determination of LCST
3.3. Scattering Determination of LCST
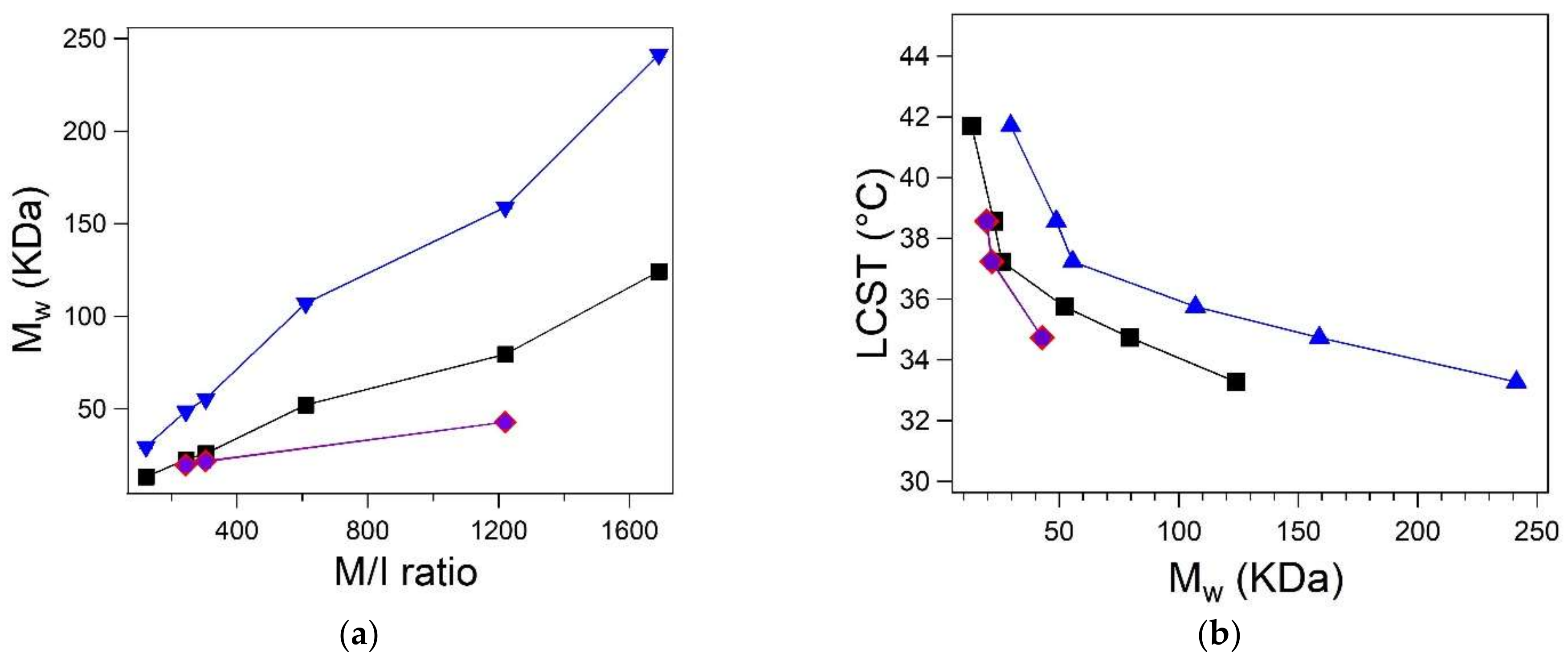
3.4. Determination of Molecular Mass
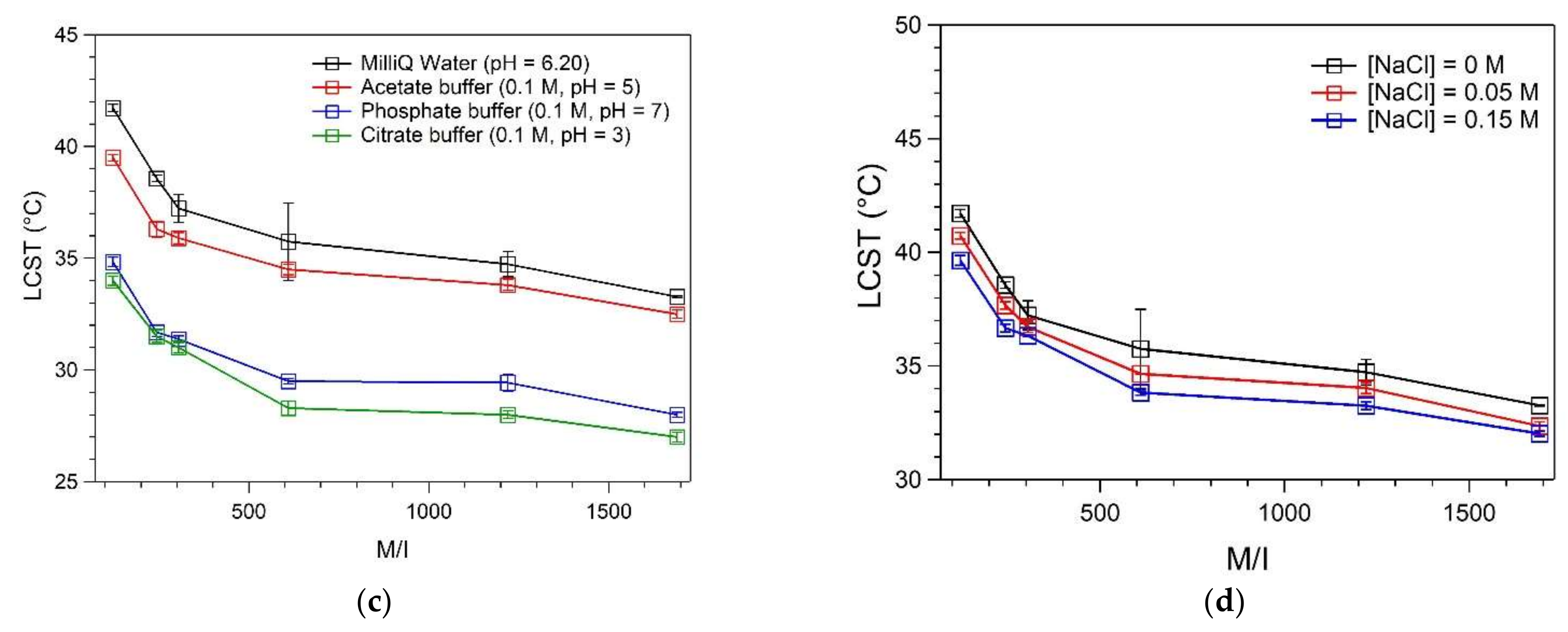
3.5. Determination of LCST in Human Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [η] | intrinsic viscosity |
| AIBN | 2,2′-Azobis(2-methylpropionitrile) |
| D2O | Deuterated water |
| Dh | Hydrodynamic diameter |
| DLS | Dynamic light scattering |
| DMF | N,N′-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| FRP | Free radical polymerization |
| FT-IR | Fourier-transform infrared |
| GPC-SEC | Gel Permeation Chromatography-Size exclusion chromatography |
| HSQC | heteronuclear single quantum coherence |
| HSQC-DEPT | heteronuclear single quantum coherence-distortionless enhanced polarization transfer |
| LCST | lower critical solution temperature |
| M/I | monomer to initiator ratio |
| Mn | Number average molecular weight |
| MPA | Mercaptopropionic acid |
| Mw | weight average molecular weight |
| MWCO | molecular weight cut-off |
| Mη | viscosimetric molecular weight |
| NHS | N-hydroxysuccinimide |
| NMR | nuclear magnetic resonance |
| NVCL | N-vinyl caprolactam |
| PBS | Phosphate-buffered saline |
| PNIPAM | poly-N-isopropylamide |
| PNVCL | Poly-N-Vinylcaprolactam |
| Rg | Gyration radius |
| THF | Tetrahydrofuran |
| Tr | Transmittance |
| UCST | upper critical solution temperature |
| UV-VIS | UV-Visible |
| ηinh | inherent viscosity |
| ηred | reduced viscosity |
| ηsp | specific viscosity |
| degree of polymerization |
References
- Vihola, H. Studies on Thermosensitive Poly(N-vinylcaprolactam) Based Polymers for Pharmaceutical Applications. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 23 November 2007. [Google Scholar]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.N.; Bin Yusoh, K.; Shariffuddin, J.H.B.H. Poly(N-vinyl caprolactam) thermoresponsive polymer in novel drug delivery systems: A review. Mater. Express 2018, 8, 21–34. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Zaccarelli, E.; Chiessi, E. On the molecular origin of the cooperative coil-to-globule transition of poly(: N-isopropylacrylamide) in water. Phys. Chem. Chem. Phys. 2018, 20, 9997–10010. [Google Scholar] [CrossRef] [Green Version]
- Sund-Levander, M.; Forsberg, C.; Wahren, L.K. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: A systematic literature review. Scand. J. Caring Sci. 2002, 16, 122–128. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Indulekha, S.; Gottipalli, R.; Reddy, B.P.K.; Chikate, T.R.; Gupta, R.; Jahagirdar, D.N.; Prasad, R.; De, A.; Srivastava, R. NIR light-triggered shrinkable thermoresponsive PNVCL nanoshells for cancer theranostics. RSC Adv. 2017, 7, 44026–44034. [Google Scholar] [CrossRef] [Green Version]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Dual responsive magnetic composite nanogels for thermo-chemotherapy. Colloids Surfaces B Biointerfaces 2017, 155, 304–313. [Google Scholar] [CrossRef]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Thermoresponsive polymeric gel as an on-demand transdermal drug delivery system for pain management. Mater. Sci. Eng. C 2016, 62, 113–122. [Google Scholar] [CrossRef]
- Kozanoǧlu, S.; Özdemir, T.; Usanmaz, A. Polymerization of N-vinylcaprolactam and characterization of poly(N-vinylcaprolactam). J. Macromol. Sci. Part A Pure Appl. Chem. 2011, 48, 467–477. [Google Scholar] [CrossRef]
- Kozanoğlu, S. Polymerization and Characterization of N-Vinylcaprolactam. Master’s Thesis, Middle East Technical University, Ankara, Turkey, September 2008. [Google Scholar]
- Kirsh, Y.E.; Yanul, N.A.; Kalninsh, K.K. Structural transformations and water associate interactions in poly-N-vinylcaprolactam-water system. Eur. Polym. J. 1999, 25, 5697–5704. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Simenel, I.A.; Kurskaya, E.A.; Kulakova, V.K.; Galaev, I.Y.; Mattiasson, B.; Grinberg, V.Y.; Grinberg, N.V.; Khokhlov, A.R. Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer 2000, 41, 6507–6518. [Google Scholar] [CrossRef]
- Cheng, S.C.; Feng, W.; Pashikin, I.I.; Yuan, L.H.; Deng, H.C.; Zhou, Y. Radiation polymerization of thermo-sensitive poly (N-vinylcaprolactam). Radiat. Phys. Chem. 2002, 63, 517–519. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Kumari, K.; Maiti, T.K.; Mandal, M.; Chattopadhyay, S. Tailor-Made Temperature-Sensitive Micelle for Targeted and On-Demand Release of Anticancer Drugs. ACS Appl. Mater. Interfaces 2016, 8, 12063–12074. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Thomas, R.G.; Muthiah, M.; Lee, H.J.; Jeong, Y.Y.; Park, I.K.; Jayakumar, R. Breast tumor targetable Fe3O4 embedded thermo-responsive nanoparticles for radiofrequency assisted drug delivery. J. Biomed. Nanotechnol. 2016, 12, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sanoj Rejinold, N.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.P.; Manzoor, K.; Park, I.K.; Jeong, Y.Y.; Jayakumar, R. Anti-cancer, pharmacokinetics and tumor localization studies of pH-, RF- and thermo-responsive nanoparticles. Int. J. Biol. Macromol. 2015, 74, 249–262. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.P.; Park, I.K.; Jeong, Y.Y.; Manzoor, K.; Jayakumar, R. Radio frequency triggered curcumin delivery from thermo and pH responsive nanoparticles containing gold nanoparticles and its in vivo localization studies in an orthotopic breast tumor model. RSC Adv. 2014, 4, 39408–39427. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Sahebi, H.; Pourmortazavi, S.M.; Zandavar, H.; Mirsadeghi, S. Chitosan grafted onto Fe3O4@poly(: N -vinylcaprolactam) as a new sorbent for detecting Imatinib mesylate in biosamples using UPLC-MS/MS. Analyst 2019, 144, 7336–7350. [Google Scholar] [CrossRef]
- Banihashem, S.; Nezhati, M.N.; Panahia, H.A. Synthesis of chitosan-grafted-poly(N-vinylcaprolactam) coated on the thiolated gold nanoparticles surface for controlled release of cisplatin. Carbohydr. Polym. 2020, 227, 115333. [Google Scholar] [CrossRef]
- Meeussen, F.; Nies, E.; Berghmans, H.; Verbrugghe, S.; Goethals, E.; Du Prez, F. Phase behaviour of poly(N-vinyl caprolactam) in water. Polymer 2000, 41, 8597–8602. [Google Scholar] [CrossRef]
- Solomon, O.F.; Corciovei, M.; Ciută, I.; Boghină, C. Properties of solutions of poly-N-vinylcaprolactam. J. Appl. Polym. Sci. 1968, 12, 1835–1842. [Google Scholar] [CrossRef]
- Tager, A.A.; Safronov, A.P.; Sharina, S.V.; Galaev, I.Y. Thermodynamic study of poly(N-vinyl caprolactam) hydration at temperatures close to lower critical solution temperature. Colloid Polym. Sci. 1993, 271, 868–872. [Google Scholar] [CrossRef]
- Laukkanen, A.; Valtola, L.; Winnik, F.M.; Tenhu, H. Formation of colloidally stable phase separated poly(N-vinylcaprolactam) in water: A study by dynamic light scattering, microcalorimetry, and pressure perturbation calorimetry. Macromolecules 2004, 37, 2268–2274. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Barboza, J.C.S.; Ré, M.I.; Giudici, R.; Santos, A.M. Solution polymerization of N-vinylcaprolactam in 1,4-dioxane. Kinetic dependence on temperature, monomer, and initiator concentrations. J. Appl. Polym. Sci. 2010, 118, 229–240. [Google Scholar] [CrossRef]
- Shao, L.; Hu, M.; Chen, L.; Xu, L.; Bi, Y. RAFT polymerization of N-vinylcaprolactam and effects of the end group on the thermal response of poly(N-vinylcaprolactam). React. Funct. Polym. 2012, 72, 407–413. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Kamitakahara, H.; Takano, T.; Nakatsubo, F. Synthesis of diblock copolymers with cellulose derivatives. 3. Cellulose derivatives carrying a single pyrene group at the reducing-end and fluorescent studies of their self-assembly systems in aqueous NaOH solutions. Cellulose 2006, 13, 437–448. [Google Scholar] [CrossRef]
- Shah, S.; Pal, A.; Gude, R.; Devi, S. Synthesis and characterization of thermo-responsive copolymeric nanoparticles of poly(methyl methacrylate-co-N-vinylcaprolactam). Eur. Polym. J. 2010, 46, 958–967. [Google Scholar] [CrossRef]
- Ainara, I.; Jacqueline, F. N-vinylcaprolactam-based microgels for biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1173–1181. [Google Scholar] [CrossRef]
- Shakya, A.K.; Holmdahl, R.; Nandakumar, K.S.; Kumar, A. Polymeric cryogels are biocompatible, and their biodegradation is independent of oxidative radicals. J. Biomed. Mater. Res. Part A 2014, 102, 3409–3418. [Google Scholar] [CrossRef]
- BASF Luviskol ® Plus Technical Information. Available online: http://https://docplayer.net/207667766-Luviskol-plus-technical-information-nonionic-film-forming-agent-for-hair-setting-products-registered-trademark-of-basf-in-many-countries.html (accessed on 4 August 2011).
- Madhusudana Rao, K.; Krishna Rao, K.S.V.; Sudhakar, P.; Chowdoji Rao, K.; Subha, M.C.S. Synthesis and characterization of biodegradable poly (vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013, 3, 61–69. [Google Scholar] [CrossRef]
- Prabaharan, M.; Grailer, J.J.; Steeber, D.A.; Gong, S. Stimuli-responsive chitosan-graft-Poly(N-vinylcaprolactam) as a promising material for controlled hydrophobic drug delivery. Macromol. Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef]
- Feng, S.; Zheng, E.; Liu, H.; Wang, C.; Liu, F. Synthesis and characterization of poly (N-vinyl caprolactam) and its graft copolymers of dextran and dextrose. Polymer 2008, 49, 1081–1082. [Google Scholar]
- Shi, H.Y.; Zhang, L.M. Phase-transition and aggregation characteristics of a thermoresponsive dextran derivative in aqueous solutions. Carbohydr. Res. 2006, 341, 2414–2419. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Lau, A.C.W.; Wu, C. Thermally Sensitive and Biocompatible Poly(N-vinylcaprolactam): Synthesis and Characterization of High Molar Mass Linear Chains. Macromolecules 1999, 32, 581–584. [Google Scholar] [CrossRef]
- Eisele, M.; Burchard, W. Hydrophobic water-soluble polymers, 1. Dilute solution properties of poly(1-vinyl-2-piperidone) and poly(N-vinylcaprolactam). Die Makromol. Chem. 1990, 191, 169–184. [Google Scholar] [CrossRef]
- Kirsh, Y.E. Water Soluble Poly-N-Vinylamides: Synthesis and Physicochemical Properties; John Wiley & Sons: Chicester, UK, 1998. [Google Scholar]
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Aseyev, V.; Hietala, S.; Laukkanen, A.; Nuopponen, M.; Confortini, O.; Du Prez, F.E.; Tenhu, H. Mesoglobules of thermoresponsive polymers in dilute aqueous solutions above the LCST. Polymer 2005, 46, 7118–7131. [Google Scholar] [CrossRef]
- Mikheeva, L.M.; Grinberg, N.V.; Mashkevich, A.Y.; Grinberg, V.Y.; Thanh, L.T.M.; Makhaeva, E.E.; Khokhlov, A.R. Microcalorimetric study of thermal cooperative transitions in poly(N-vinylcaprolactam) hydrogels. Macromolecules 1997, 30, 2693–2699. [Google Scholar] [CrossRef]
- Boyko, V.B. N-Vinylcaprolactam based Bulk and Microgels: Synthesis, Structural Formation and Characterization by Dynamic Light Scattering. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 29 October 2004. [Google Scholar]
- Gooch, J.W. Kraemer Equation. In Encyclopedic Dictionary of Polymers; Gooch, J.W., Ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-6247-8. [Google Scholar]
- Serra, A.C.; Góis, J.R.; Coelho, J.F.J.; Popov, A.V.; Costa, J.R.C. Synthesis of well-defined alkyne terminated poly(N-vinyl caprolactam) with stringent control over the LCST by RAFT. RSC Adv. 2016, 6, 16996–17007. [Google Scholar] [CrossRef] [Green Version]
- Morgner, F.; Stufler, S.; Geißler, D.; Medintz, I.L.; Algar, W.R.; Susumu, K.; Stewart, M.H.; Blanco-Canosa, J.B.; Dawson, P.E.; Hildebrandt, N. Terbium to quantum dot FRET bioconjugates for clinical diagnostics: Influence of human plasma on optical and assembly properties. Sensors 2011, 11, 9667–9684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zijlstra, W.G.; Buursma, A. Spectrophotometry of hemoglobin: Absorption spectra of bovine oxyhemoglobin, deoxyhemoglobin, carboxyhemoglobin, and methemoglobin. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 743–749. [Google Scholar] [CrossRef]


| M/I Ratio | LCST (°C) | Average Molecular Weight (kDa) | ||||
| UV-VIS | DLS | Equation (2) | Equation (3) | Equation (6) | Equation (7) | |
| 122 | 41.71 ± 0.17 | 41 | 13.29 ± 0.36 | 29.60 ± 0.99 | - | - |
| 244 | 38.56 ± 0.14 | 37 | 22.60 ± 0.06 | 48.74 ± 0.19 | 19.74 ± 1.12 | 19.56 ± 1.18 |
| 305 | 37.23 ± 0.63 | 37 | 25.96 ± 0.06 | 55.53 ± 0.17 | 21.87 ± 0.88 | 21.65 ± 0.89 |
| 610 | 35.75 ± 1.74 | 34 | 52.20 ± 0.36 | 107.0 ± 1.00 | - | - |
| 1220 | 34.73 ± 0.56 | 33.5 | 79.54 ± 0.25 | 158.9 ± 0.70 | 42.87 ± 1.90 | 42.94 ± 1.93 |
| 1690 | 33.27 ± 0.03 | 32.5 | 124.1 ± 0.60 | 241.4 ± 1.64 | - | - |
| M/I Ratio | LCST (°C) | ||
| MilliQ Water | PBS (0.1 M, pH = 7) | Human Plasma | |
| 122 | 41.71 ± 0.17 | 34.83 ± 0.21 | 31.04 ± 0.05 |
| 244 | 38.56 ± 0.14 | 31.68 ± 0.17 | 28.83 ± 0.11 |
| 305 | 37.23 ± 0.63 | 31.38 ± 0.14 | 28.15 ± 6.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsili, L.; Dal Bo, M.; Eisele, G.; Donati, I.; Berti, F.; Toffoli, G. Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications. Polymers 2021, 13, 2639. https://doi.org/10.3390/polym13162639
Marsili L, Dal Bo M, Eisele G, Donati I, Berti F, Toffoli G. Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications. Polymers. 2021; 13(16):2639. https://doi.org/10.3390/polym13162639
Chicago/Turabian StyleMarsili, Lorenzo, Michele Dal Bo, Giorgio Eisele, Ivan Donati, Federico Berti, and Giuseppe Toffoli. 2021. "Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications" Polymers 13, no. 16: 2639. https://doi.org/10.3390/polym13162639
APA StyleMarsili, L., Dal Bo, M., Eisele, G., Donati, I., Berti, F., & Toffoli, G. (2021). Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications. Polymers, 13(16), 2639. https://doi.org/10.3390/polym13162639








