Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities
Abstract
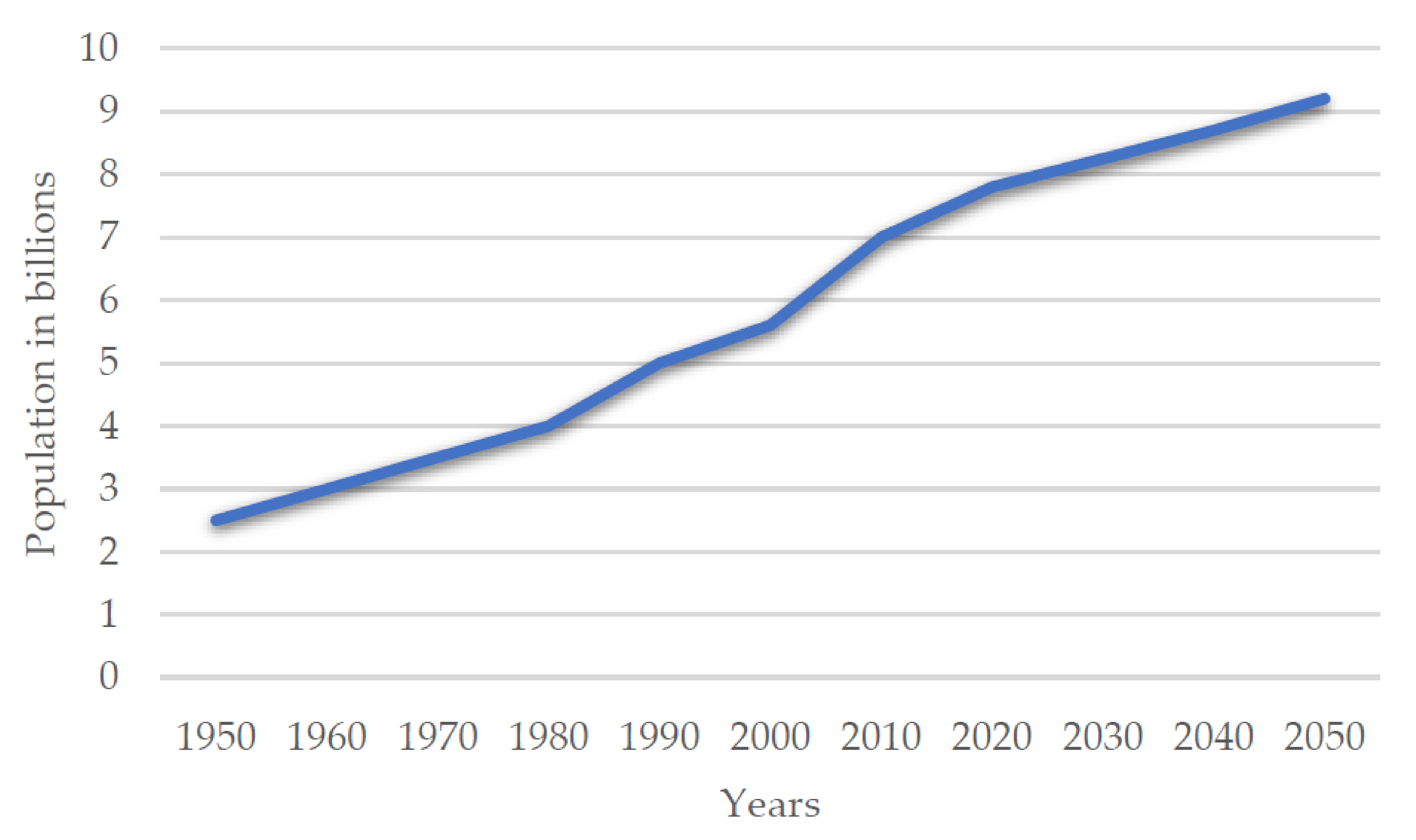
:1. Introduction
2. Functional Properties of Proteins
2.1. Functional Properties of Proteins
2.2. Surface Properties of Proteins
2.3. Foaming Capacity
2.4. Creating Emulsions
2.5. Protein-Based Bionanomaterials
3. The Most Common Sources of Plant and Animal Proteins
3.1. Soy
3.2. Wheat
3.3. Milk Proteins (Casein)
3.4. Whey Proteins
3.5. Egg White Proteins
3.6. Gelatin
3.7. Protein Hydrolysates—Food and Feed Additive and Use in Other Industrial Products
4. Unconventional and Alternative Sources of Proteins
4.1. Rice
4.2. Corn
4.3. Quinoa
4.4. Beans
4.5. Lupine
4.6. Sunflower
4.7. Insects
4.8. Algae
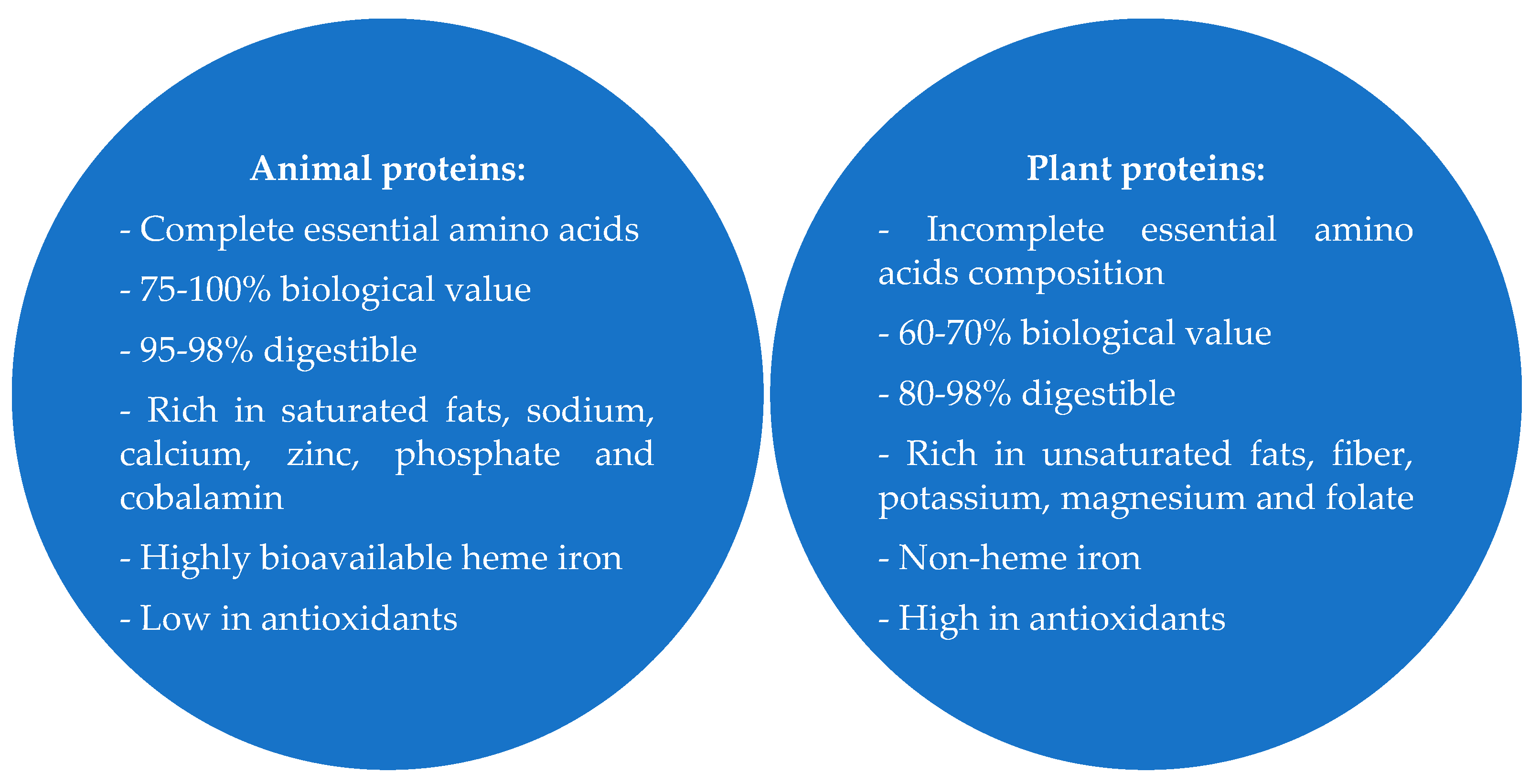

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, Y.; Isman, M.B. Insects as an Alternative Protein Source. In Proteins in Food Processing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 263–288. ISBN 9780081007297. [Google Scholar]
- Walia, A.; Gupta, A.K.; Sharma, V. Role of Bioactive Compounds in Human Health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Dietzen, D.J. Amino acids, peptides, and proteins. In Principles and Applications of Molecular Diagnostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–380. ISBN 9780128160619. [Google Scholar]
- Bigman, L.S.; Levy, Y. Proteins: Molecules defined by their trade-offs. Curr. Opin. Struct. Biol. 2020, 60, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, M.; Lin, S.; Jian, R.; Li, X.; Chan, J.; Dong, G.; Fang, H.; Robinson, A.E.; Aguet, F.; et al. A Quantitative Proteome Map of the Human Body. Cell 2020, 183, 269–283. [Google Scholar] [CrossRef]
- Chiesa, G.; Kiriakov, S.; Khalil, A.S. Protein assembly systems in natural and synthetic biology. BMC Biol. 2020, 18, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Scognamiglio, P.L.; Platella, C.; Napolitano, E.; Musumeci, D.; Roviello, G.N. From Prebiotic Chemistry to Supramolecular Biomedical Materials: Exploring the Properties of Self-Assembling Nucleobase-Containing Peptides. Molecules 2021, 26, 3558. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional colloids from proteins and polysaccharides for food applications. Trends Food Sci. Technol. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of processing on the chemistry and functionality of food proteins. In Proteins in Food Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–45. ISBN 9780081007297. [Google Scholar]
- Jensen, J.T.; Creinin, M.D. Family planning, population growth, and the environment. Contraception 2020, 101, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Henchion, M.; McCarthy, M.; Resconi, V.C.; Troy, D. Meat consumption: Trends and quality matters. Meat Sci. 2014, 98, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henchion, M.; De Backer, C.J.S.; Hudders, L. Ethical and Sustainable Aspects of Meat Production. New Asp. Meat Qual. 2017, 26, 649–666. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- World Plant-Based Protein Industry Report 2020–2025. Available online: https://www.globenewswire.com/news-release/2020/08/20/2081186/0/en/World-Plant-based-Protein-Industry-Report-2020-2025.html (accessed on 19 July 2021).
- Liñán, J.; Arroyo, P.; Carrete, L. Conceptualizing Healthy Food: How Consumer’s Values Influence the Perceived Healthiness of a Food Product. J. Food Nutr. Res. 2019, 7, 679–687. [Google Scholar] [CrossRef]
- Allison, T.M.; Bechara, C. Structural mass spectrometry comes of age: New insight into protein structure, function and interactions. Biochem. Soc. Trans. 2019, 47, 317–327. [Google Scholar] [CrossRef]
- Redfern, C.P.F. Vitamin A and its natural derivatives. Methods Enzymol. 2020, 637, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Van Vranken, D.; Weiss, G.A. Protein Function. In Introduction to Bioorganic Chemistry and Chemical Biology; Garland Science: New York, NY, USA, 2020; pp. 504–547. [Google Scholar]
- Meghwanshi, G.K.; Kaur, N.; Verma, S.; Dabi, N.K.; Vashishtha, A.; Charan, P.D.; Purohit, P.; Bhandari, H.S.; Bhojak, N.; Kumar, R. Enzymes for pharmaceutical and therapeutic applications. Biotechnol. Appl. Biochem. 2020, 67, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Lacroix, I.M.E. Properties of proteins in food systems: An introduction. In Proteins in Food Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–25. ISBN 9780081007297. [Google Scholar]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Gruner, P.; Riechers, B.; Semin, B.; Lim, J.; Johnston, A.; Short, K.; Baret, J.C. Controlling molecular transport in minimal emulsions. Nat. Commun. 2016, 10392, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udgaonkar, J.B. Polypeptide chain collapse and protein folding. Arch. Biochem. Biophys. 2013, 531, 24–33. [Google Scholar] [CrossRef]
- Wei, G.W. Multiscale, multiphysics and multidomain models I: Basic theory. J. Theor. Comput. Chem. 2013, 12, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Haque, E.; Bhandari, B.R. Effects of protein conformational modifications, enthalpy relaxation, and interaction with water on the solubility of milk protein concentrate powder. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; (Food Engineering Series); Springer: Berlin/Heidleberg, Germany, 2015; pp. 437–450. [Google Scholar]
- Haque, E.; Bhandari, B.R.; Gidley, M.J.; Deeth, H.C.; Whittaker, A.K. Ageing-induced solubility loss in milk protein concentrate powder: Effect of protein conformational modifications and interactions with water. J. Sci. Food Agric. 2011, 91, 2576–2581. [Google Scholar] [CrossRef]
- Nunes, L.; Tavares, G.M. Thermal treatments and emerging technologies: Impacts on the structure and techno-functional properties of milk proteins. Trends Food Sci. Technol. 2019, 99, 88–99. [Google Scholar] [CrossRef]
- Takeuchi, H. Biochemical significance of regulation of protein stability by Oglucose glycans. Seikagaku 2018, 90, 519–523. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Zapata, C.; Franco, D. Proteins and amino acids. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; pp. 347–357. ISBN 9780128141748. [Google Scholar]
- Martínez-Monteagudo, S.I.; Kamat, S.; Patel, N.; Konuklar, G.; Rangavajla, N.; Balasubramaniam, V.M. Improvements in emulsion stability of dairy beverages treated by high pressure homogenization: A pilot-scale feasibility study. J. Food Eng. 2017, 193, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Panagiotou, T.; Fisher, R. Improving Product Quality with Entrapped Stable Emulsions: From Theory to Industrial Application. Challenges 2012, 3, 84–114. [Google Scholar] [CrossRef] [Green Version]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Singh, H. Emulsions and foams stabilised by milk proteins. In Advanced Dairy Chemistry: Volume 1B: Proteins: Applied Aspects, 4th ed.; Springer: Berlin/Heidleberg, Germany, 2016; pp. 133–153. ISBN 9781493928002. [Google Scholar]
- Chung, C.; McClements, D.J. Structure-function relationships in food emulsions: Improving food quality and sensory perception. Food Struct. 2014, 1, 106–126. [Google Scholar] [CrossRef]
- Mleko, S.; Tomczyńska-Mleko, M.; Targoński, Z. Globular protein gels as carriers of active substances. Agro Food Ind. Hi-Tech 2010, 21, 26–28. [Google Scholar]
- Liu, C.-M.; Peng, Q.; Zhong, J.-Z.; Liu, W.; Zhong, Y.-J.; Wang, F. Molecular and Functional Properties of Protein Fractions and Isolate from Cashew Nut (Anacardium occidentale L.). Molecules 2018, 23, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppertz, T. Foaming properties of milk: A review of the influence of composition and processing. Int. J. Dairy Technol. 2010, 63, 477–488. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56. [Google Scholar] [CrossRef]
- Karimi, M.; Ignasiak, M.T.; Chan, B.; Croft, A.K.; Radom, L.; Schiesser, C.H.; Pattison, D.I.; Davies, M.J. Reactivity of disulfide bonds is markedly affected by structure and environment: Implications for protein modification and stability. Sci. Rep. 2016, 38572, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Xi, Z.; Liu, W.; McClements, D.J.; Zou, L. Rheological, structural, and microstructural properties of ethanol induced cold-set whey protein emulsion gels: Effect of oil content. Food Chem. 2019, 291, 22–29. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; Charoux, C.M.G.; Cullen, P.J.; Tiwari, B.K. Chemical Modifications of Lipids and Proteins by Nonthermal Food Processing Technologies. J. Agric. Food Chem. 2018, 67, 2043–2051. [Google Scholar] [CrossRef]
- Walstra, P. Formation and Stability of Emulsions Made with Proteins and Peptides; Wageningen Universiteit: Wageningen, The Netherlands, 2000. [Google Scholar]
- Foegeding, E.A. Food Protein Functionality—A New Model. J. Food Sci. 2015, 80, 2670–2677. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 1–15. [Google Scholar] [CrossRef]
- Beloqui, A.; Cortajarena, A.L. Protein-based functional hybrid bionanomaterials by bottom-up approaches. Curr. Opin. Struct. Biol. 2020, 63, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Liu, Y.; Mai, S.; Li, N.; Yiu, C.K.Y.; Mao, J.; Pashley, D.H.; Tay, F.R. Differences between top-down and bottom-up approaches in mineralizing thick, partially-demineralized collagen scaffolds. Acta Biomater. 2011, 7, 1742–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Hirschi, K.D. Genetically Modified Plants: Nutritious, Sustainable, yet Underrated. J. Nutr. 2020, 150, 2628–2634. [Google Scholar] [CrossRef]
- Haslberger, A.G. Codex guidelines for GM foods include the analysis of unintended effects. Nat. Biotechnol. 2003, 21, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Bawa, A.S.; Anilakumar, K.R. Genetically modified foods: Safety, risks and public concerns—A review. J. Food Sci. Technol. 2012, 50, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- USDA. Grain: World Markets and Trade; USDA: Washington, DC, USA, 2019.
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Fernandez-Cornejo, J.; Wechsler, S. Soybeans. In Handbook on Agriculture, Biotechnology and Development; Edward Elgar: Cheltenham, UK, 2014; pp. 1–880. ISBN 9780857938350. [Google Scholar]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- USDA. World Agricultural Production Circular Series; USDA: Washington, DC, USA, 2019.
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crop. Res. 2017, 202, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Global Wheat Demand: Feeding the World by Milling and Feeding. Available online: https://research.rabobank.com/far/en/sectors/grains-oilseeds/global_wheat_demand_article_1.html (accessed on 19 July 2021).
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, F.M.; Ahmad, I.; Butt, M.S.; Sheikh, M.A.; Pasha, I. Amino acid composition of spring wheats and losses of lysine during chapati baking. J. Food Compos. Anal. 2005, 18, 523–532. [Google Scholar] [CrossRef]
- Day, L. Wheat gluten: Production, properties and application. In Handbook of Food Proteins; Woodhead Publishing: Sawston, UK, 2011; pp. 1–464. ISBN 9780857093639. [Google Scholar]
- Samard, S.; Gu, B.Y.; Ryu, G.H. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A.; American College of Gastroenterology. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non–Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Fernandez, M.A.; Fisberg, M.; Marette, A. Role of yogurt in the nutrition and health of children and adolescents. In Yogurt in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 491–505. ISBN 9780128052723. [Google Scholar]
- Badem, A.; Uçar, G. Production of caseins and their usages. Int. J. Food Sci. Nutr. 2017, 2, 4–9. [Google Scholar]
- O’Kennedy, B.T. Caseins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 13–29. [Google Scholar]
- Szpendowski, J.; Siemianowski, K. Właściwości odżywcze i funkcjonalne oraz zastosowanie kazeinianów w przetwórstwie spożywczym. Nauki Inż. Technol. Uniw. Ekon. Wrocławiu 2013, 3, 122–138. [Google Scholar]
- Singh, H. Milk Protein Products: Functional Properties of Milk Proteins. In Encyclopedia of Dairy Sciences: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 887–893. ISBN 9780123744029. [Google Scholar]
- Cirillo, G.; Spizzirri, U.G.; Iemma, F. Functional Polymers in Food Science. In Functional Polymers in Food Science: From Technology to Biology; Cirillo, G., Spizzirri, U.G., Iemma, F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 2, pp. 1–335. ISBN 9781119108580. [Google Scholar]
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Bahna, S.L. Cow’s milk allergy versus cow milk intolerance. Ann. Allergy Asthma Immunol. 2002, 89, 56–60. [Google Scholar] [CrossRef]
- Kilara, A.; Vaghela, M.N. Whey proteins. In Proteins in Food Processing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–674. ISBN 9780081007297. [Google Scholar]
- Yang, J.; Liu, Y.; Mu, C.; Li, Y.; Tian, H. Influence of different nutrition supplement combinations on the training effect of athlete based on component analysis. Investig. Clin. 2020, 95, 4743–4747. [Google Scholar]
- Sharma, R. Whey proteins in functional foods. In Whey Proteins: From Milk to Medicine; Academic Press: London, UK, 2018; pp. 1–746. ISBN 9780128121245. [Google Scholar]
- De Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Jørgensen, C.E.; Abrahamsen, R.K.; Rukke, E.O.; Hoffmann, T.K.; Johansen, A.G.; Skeie, S.B. Processing of high-protein yoghurt—A review. Int. Dairy J. 2019, 88, 42–59. [Google Scholar] [CrossRef]
- Iqbal, S.; Ayyub, A.; Iqbal, H.; Chen, X.D. Protein microspheres as structuring agents in lipids: Potential for reduction of total and saturated fat in food products. J. Sci. Food Agric. 2020, 101, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Nys, Y.; Sauveur, B. Valeur nutritionnelle des œufs. Prod. Anim. 2004, 17, 385–393. [Google Scholar] [CrossRef]
- Drewnowski, A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am. J. Clin. Nutr. 2010, 91, 1095–1101. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, N.; Labbafi, M. Effect of processing on aggregation mechanism of egg white proteins. Food Chem. 2018, 252, 126–133. [Google Scholar] [CrossRef]
- Wu, J.; Acero-Lopez, A. Ovotransferrin: Structure, bioactivities, and preparation. Food Res. Int. 2012, 46, 480–487. [Google Scholar] [CrossRef]
- Chiang, W.D. Protein hydrolysate batch production with angiotensin I-converting enzyme inhibitory activity from egg whites. J. Food Drug Anal. 2006, 14, 385–390. [Google Scholar]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides from egg: A review. Nutr. Food Sci. 2015, 45, 190–212. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef]
- Zhu, Y.; Vanga, S.K.; Wang, J.; Raghavan, V. Impact of food processing on the structural and allergenic properties of egg white. Trends Food Sci. Technol. 2018, 78, 188–196. [Google Scholar] [CrossRef]
- Duconseille, A.; Wien, F.; Audonnet, F.; Traore, A.; Refregiers, M.; Astruc, T.; Santé-Lhoutellier, V. The effect of origin of the gelatine and ageing on the secondary structure and water dissolution. Food Hydrocoll. 2017, 66, 378–388. [Google Scholar] [CrossRef]
- Nuñez, S.M.; Guzmán, F.; Valencia, P.; Almonacid, S.; Cárdenas, C. Collagen as a source of bioactive peptides: A bioinformatics approach. Electron. J. Biotechnol. 2020, 48, 101–108. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Benjakul, S.; Kittiphattanabawon, P. Gelatin. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 2194–2220. ISBN 9780128140451. [Google Scholar]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Antihypertensive peptides of animal origin: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.; Petersen, K.; Kris-Etherton, P. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, 11–24. [Google Scholar] [CrossRef]
- Guruprasad, L. Protein Structure. Resonance 2019, 24, 327–338. [Google Scholar] [CrossRef]
- Chakraborty, H.J.; Gangopadhyay, A.; Ganguli, S.; Datta, A. Protein structure prediction. In Applying Big Data Analytics in Bioinformatics and Medicine; IGI Global: Hershey, PA, USA, 2017; pp. 1–465. ISBN 9781522526087. [Google Scholar]
- Lafarga, T.; Hayes, M. Bioactive protein hydrolysates in the functional food ingredient industry: Overcoming current challenges. Food Rev. Int. 2017, 33, 217–246. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess. Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Taghizadeh, M.S.; Niazi, A.; Moghadam, A.; Afsharifar, A.R. The potential application of the protein hydrolysates of three medicinal plants: Cytotoxicity and functional properties. J. Food Sci. 2020, 85, 3160–3167. [Google Scholar] [CrossRef]
- Liceaga, A.M.; Hall, F. Nutritional, functional and bioactive protein hydrolysates. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 456–465. ISBN 9780128140451. [Google Scholar]
- USDA. United States Department of Agriculture National Agricultural Statistics Service; USDA: Washington, DC, USA, 2018.
- Al-Doury, M.K.W.; Hettiarachchy, N.S.; Horax, R. Rice-Endosperm and Rice-Bran Proteins: A Review. J. Am. Oil Chem. Soc. 2018, 95, 943–956. [Google Scholar] [CrossRef]
- Khir, R.; Pan, Z. Rice. In Integrated Processing Technologies for Food and Agricultural By-Products; Academic Press: London, UK, 2019; pp. 1–452. ISBN 9780128141397. [Google Scholar]
- Ferreira, S.M.R.; De Mello, A.P.; De Caldas Rosa Dos Anjos, M.; Krüger, C.C.H.; Azoubel, P.M.; De Oliveira Alves, M.A. Utilization of sorghum, rice, corn flours with potato starch for the preparation of gluten-free pasta. Food Chem. 2016, 191, 147–151. [Google Scholar] [CrossRef]
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Małecki, J.; Tomasevic, I.; Djekic, I.; Sołowiej, B.G. The Effect of Protein Source on the Physicochemical, Nutritional Properties and Microstructure of High-Protein Bars Intended for Physically Active People. Foods 2020, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.A.; Drago, S.R.; Bassett, M.N.; Lobo, M.O.; Sammán, N.C. Nutritional improvement of corn pasta-like product with broad bean (Vicia faba) and quinoa (Chenopodium quinoa). Food Chem. 2016, 199, 150–156. [Google Scholar] [CrossRef] [PubMed]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Loy, D.D.; Lundy, E.L. Nutritional properties and feeding value of corn and its coproducts. In Corn: Chemistry and Technology, 3rd ed.; Woodhead Publishing: Sawston, UK; AACC International Press: Washington, DC, USA, 2018; pp. 1–690. ISBN 9780128119716. [Google Scholar]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Li, H.M.; Wen, L.K.; Li, S.J.; Zhang, D.L.; Lin, B.S. In vitro and in vivo effects and safety assessment of corn peptides on alcohol dehydrogenase activities. Chem. Res. Chin. Univ. 2011, 11, 1813–1835. [Google Scholar]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant. Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Craine, E.B.; Murphy, K.M. Corrigendum: Seed Composition and Amino Acid Profiles for Quinoa Grown in Washington State. Front. Nutr. 2020, 7, 22–43. [Google Scholar] [CrossRef]
- Sobral, J.; Amaral, P.; Menegalli, F. Comparison of starch pasting and retrogradation properties of quinoa (Chenopodium quinoa), rice, potato, cassava, wheat and corn starches. In Proceedings of the 2nd Mercosur Congress on Process Systems Engineering, Costa Verde, Brasil, January 2005; pp. 1–7. [Google Scholar]
- Lonnie, M.; Laurie, I.; Myers, M.; Horgan, G.; Russell, W.R.; Johnstone, A.M. Exploring health-promoting attributes of plant proteins as a functional ingredient for the food sector: A systematic review of human interventional studies. Nutrients 2020, 12, 2291. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.A.; Mojica, L.; González de Mejía, E.; Mendoza, S.; Loarca-Piña, G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): A review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.M.; Hellström, J.; Pihlanto, A. Nutritional Value of Commercial Protein-Rich Plant Products. Plant. Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Scheelbeek, P.F.D.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allen, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, N.; Curtain, F.; Beck, E.; Grafenauer, S. Consumer Understanding and Culinary Use of Legumes in Australia. Nutrients 2019, 11, 1575. [Google Scholar] [CrossRef] [Green Version]
- Van de Noort, M. Lupin: An Important Protein and Nutrient Source. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–456. ISBN 9780128027769. [Google Scholar]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. Nutritional, Health, and Technological Functionality of Lupin Flour Addition to Bread and Other Baked Products: Benefits and Challenges. Crit. Rev. Food Sci. Nutr. 2016, 56, 835–857. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Czubinski, J.; Feder, S. Lupin seeds storage protein composition and their interactions with native flavonoids. J. Sci. Food Agric. 2019, 99, 4011–4018. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S. Sunflower Proteins. In Sunflower: Chemistry, Production, Processing, and Utilization; Academic Press: London, UK; AOCS Press: Urbana, IL, USA, 2015; pp. 1–728. ISBN 9781630670627. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 25 July 2021).
- González-Pérez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Wahlstrom, R.C. Sunflower seeds. In Non-Traditional Feeds for Use in Swine Production (1992); Butterworths: Stoneham, MA, USA, 2017; pp. 1–528. ISBN 9781351359795. [Google Scholar]
- WHO. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Collins, C.M.; Vaskou, P.; Kountouris, Y. Insect Food Products in the Western World: Assessing the Potential of a New “Green” Market. Ann. Entomol. Soc. Am. 2019, 112, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, A.K.; Jayasimha, G.T.; Rachana, R.R.; Rohini, G. Insects as human food. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems: Sustaining Regulatory Mechanisms; Springer: Singapore, 2016; pp. 133–146. ISBN 9789811015243. [Google Scholar]
- Rumpold, B.A.; Schlüter, O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015, 5, 20–24. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6343–6372. [Google Scholar] [CrossRef]
- Huang, C.; Feng, W.; Xiong, J.; Wang, T.; Wang, W.; Wang, C.; Yang, F. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: Amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 2019, 245, 11–21. [Google Scholar] [CrossRef]
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. A systematic review of nutrient composition data available for twelve commercially available edible insects, and comparison with reference values. Trends Food Sci. Technol. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Lamsal, B.; Wang, H.; Pinsirodom, P.; Dossey, A.T. Applications of Insect-Derived Protein Ingredients in Food and Feed Industry. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 1–17. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Ohara, A.; dos Santos Aguilar, J.G.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida ) and nori (Porphyra purpurea ) as food supplements. J. Appl. Phycol. 2012, 25, 1271–1276. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Madsen, M. Algal proteins. In Handbook of Food Proteins; Woodhead Publishing: Sawston, UK, 2011; pp. 353–394. [Google Scholar]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małecki, J.; Muszyński, S.; Sołowiej, B.G. Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities. Polymers 2021, 13, 2506. https://doi.org/10.3390/polym13152506
Małecki J, Muszyński S, Sołowiej BG. Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities. Polymers. 2021; 13(15):2506. https://doi.org/10.3390/polym13152506
Chicago/Turabian StyleMałecki, Jan, Siemowit Muszyński, and Bartosz G. Sołowiej. 2021. "Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities" Polymers 13, no. 15: 2506. https://doi.org/10.3390/polym13152506
APA StyleMałecki, J., Muszyński, S., & Sołowiej, B. G. (2021). Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities. Polymers, 13(15), 2506. https://doi.org/10.3390/polym13152506






