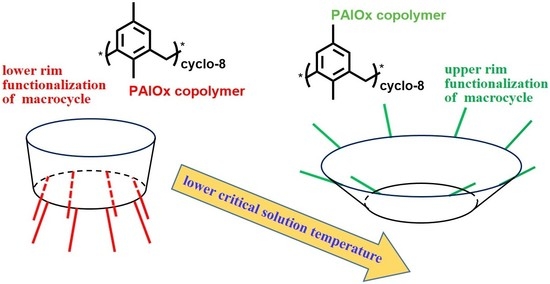
Features of Solution Behavior of Polymer Stars with Arms of Poly-2-alkyl-2-oxazolines Copolymers Grafted to the Upper Rim of Calix[8]arene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
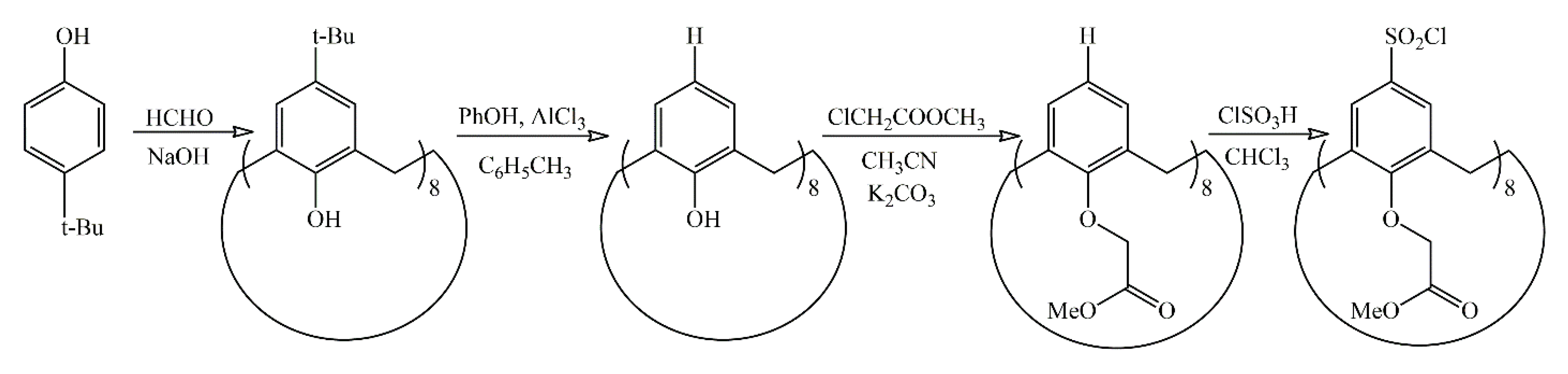
2.2. Synthesis of Multicenter Macroiniciater
2.2.1. 49,50,51,52,53,54,55,56-Octa(hydroxy)calix[8]arene
2.2.2. 49,50,51,52,53,54,55,56-Octa(methoxy(carbonylmethoxy)calix[8]arene
2.2.3. 5,11,17,23,29,35,41,47-Octachlorosulfonyl-49,50,51,52,53,54,55,56-octa(methoxy(carbonylmethoxy)calix[8]arene
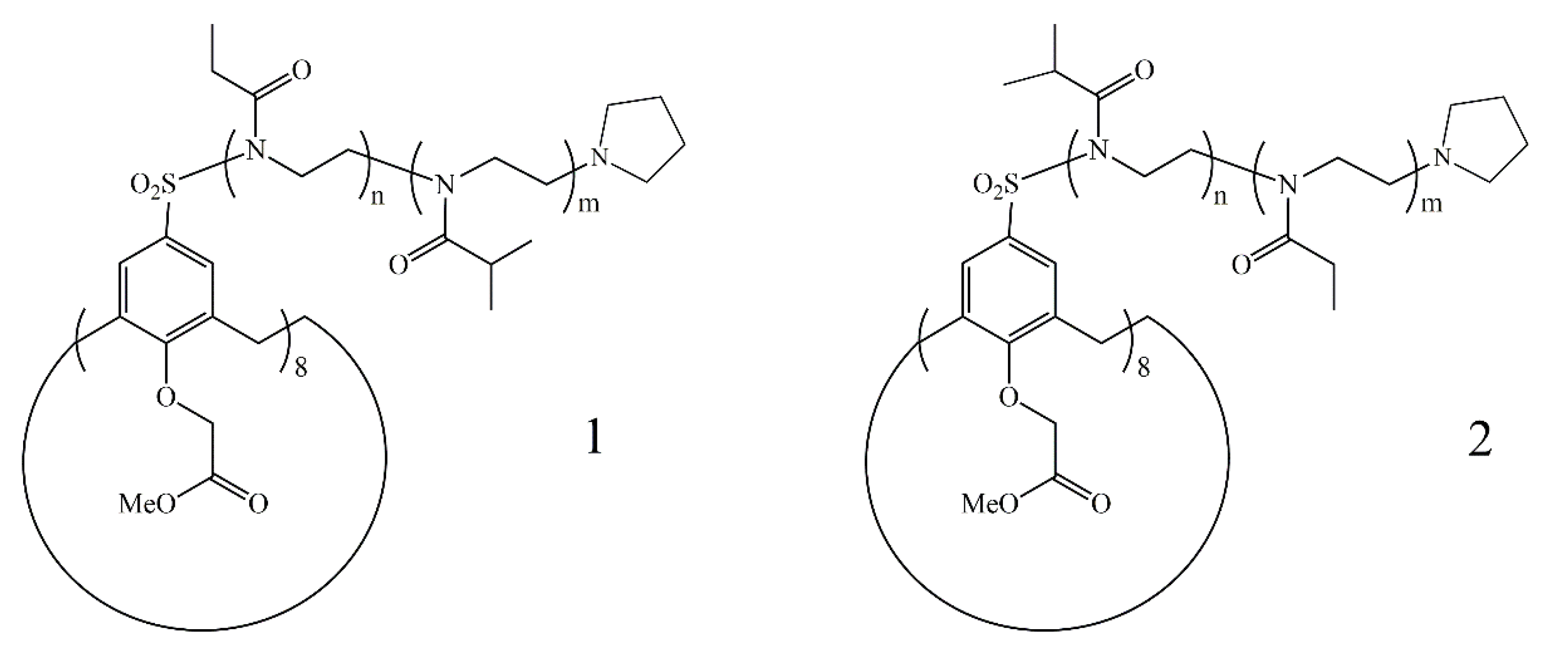
2.3. Eight-Arm Star Poly(2-Ethyl-2-Oxazoline-Block-2-Isopropyl-2-Oxazoline) Copolymer Synthesis
2.4. Eight-Arm Star Poly(2-Isopropyl-2-Oxazoline-Block-2-Ethyl-2-Oxazoline) Copolymer Synthesis
2.5. Eight-Arm Star Poly(2-Ethyl-2-Oxazoline-Grad-2-Isopropyl-2-Oxazoline) Gradient Copolymer Synthesis
2.6. Hydrolysis of Star-Shaped Polymers
2.7. Characterization of Prepared Star Samples
2.8. Investigation of Molecular-Dispersed Polymer Solutions
2.9. Investigation of Self-Organization in Aqueous Solutions
3. Results and Discussion
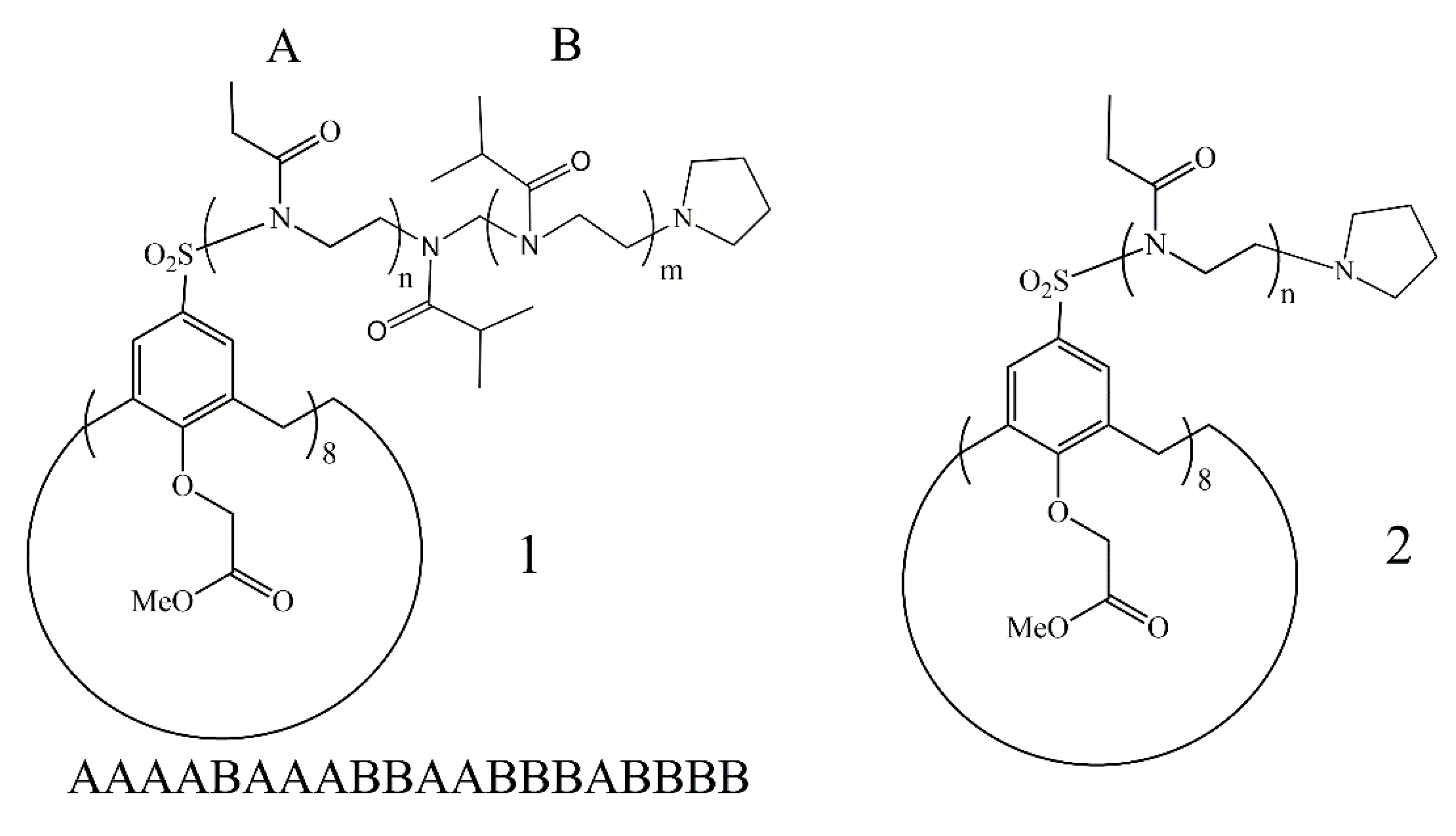
3.1. Synthetic Approach
3.2. Polymer Synthesis
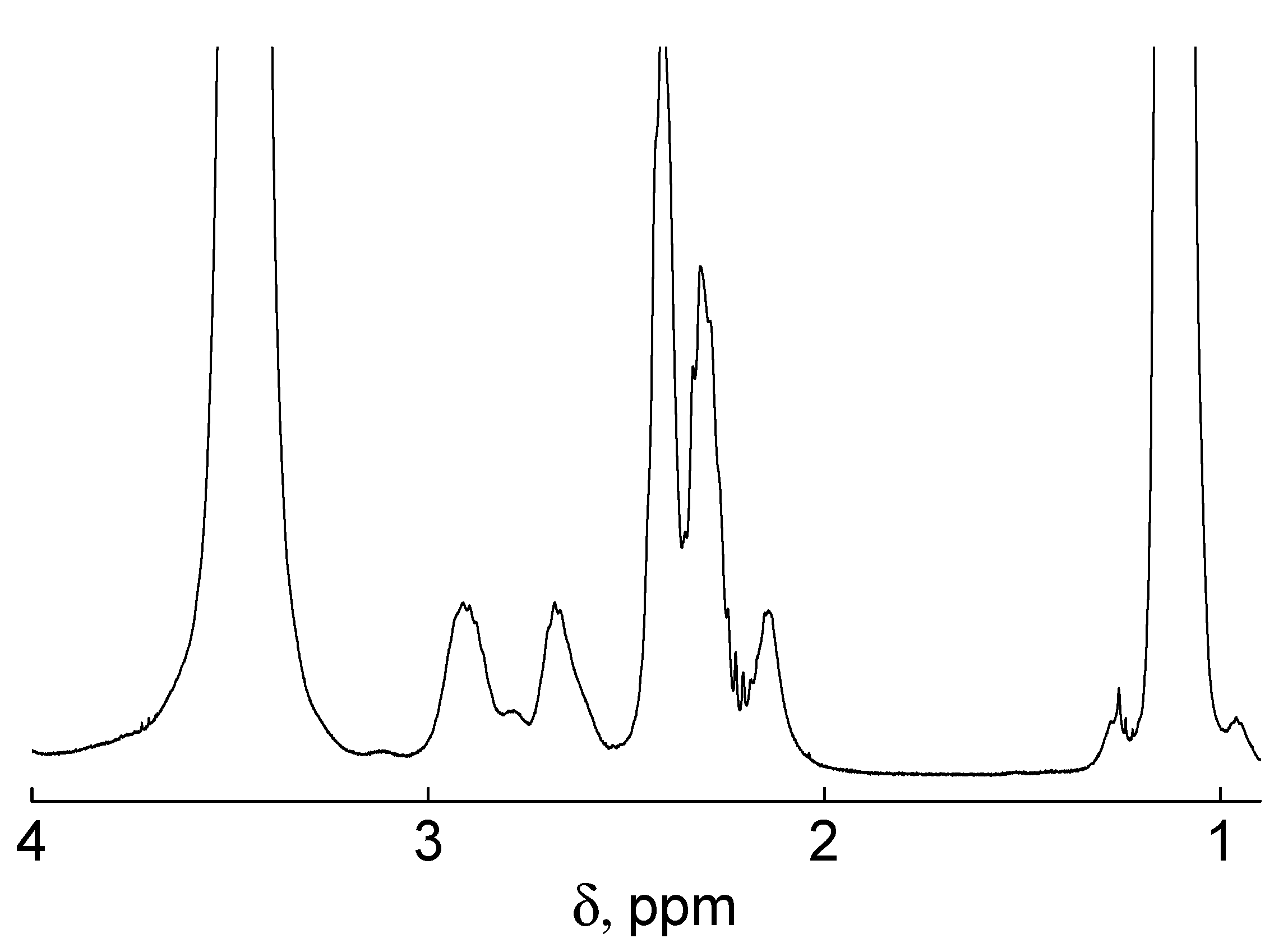
3.3. Characterization of Polymers
3.4. Hydrodynamic Characteristics and Conformation of Star-Shaped CA8-PAlOx-UR
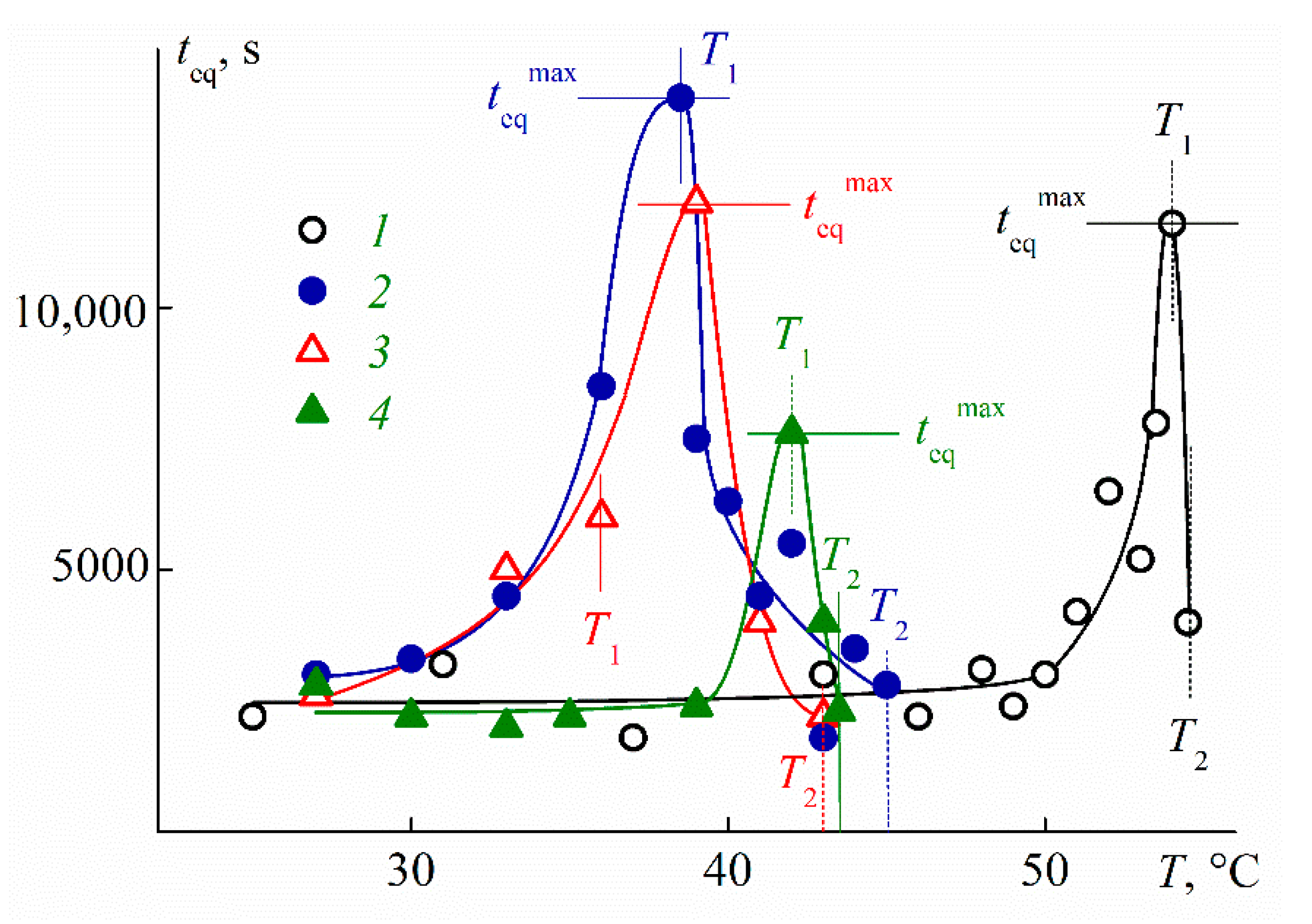
3.5. Self-Organization of C8A-PAlOx-UR Molecules in Aqueous Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Lee, H.; Pietrasik, J.; Sheiko, S.S.; Matyjaszewski, K. Stimuli-responsive molecular brushes. Prog. Polym. Sci. 2010, 35, 24–44. [Google Scholar] [CrossRef]
- Voit, B. Hyperbranched polymers—All problems solved after 15 years of research? J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2679–2699. [Google Scholar] [CrossRef] [Green Version]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Plamper, F.A.; Schmalz, A.; Penott-Chang, E.; Drechsler, M.; Jusufi, A.; Ballauff, M.A.; Muller, A.H.E. Synthesis and characterization of star-shaped poly(N,N-dimethylaminoethyl methacrylate) and its quaternized ammonium salts. Macromolecules 2007, 40, 5689–5697. [Google Scholar] [CrossRef]
- Rudolph, T.; Crotty, S.; von der Lühe, M.; Pretzel, D.; Schubert, U.S.; Schacher, F.H. Synthesis and solution properties of double hydrophilic poly(ethylene oxide)-block-poly(2-ethyl-2-oxazoline) (PEO-b-PEtOx) star block copolymers. Polymers 2013, 5, 1081–1101. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Kronek, J.; Bosowska, K.; Trzebicka, B.; Dworak, A. Star poly(2-ethyl-2-oxazoline)s—Synthesis and thermosensitivity. Polym. Int. 2011, 60, 1001–1009. [Google Scholar] [CrossRef]
- Kuckling, D.; Wycisk, A. Stimuli-responsive star polymers. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2980–2994. [Google Scholar] [CrossRef]
- Felberg, L.E.; Brookes, D.H.; Head-Gordon, T.; Rice, J.E.; Swope, W.C. Role of hydrophilicity and length of diblock arms for determining star polymer physical properties. J. Phys. Chem. B 2015, 119, 944–957. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kikuchi, S.; Sato, S.; Chen, Y.; Xu, L.; Song, B.; Duan, Q.; Wang, Y.; Kakuchi, T.; Shen, X. Core-first synthesis and thermoresponsive property of three-, four-, and six-arm star-shaped poly(N,N-diethylacrylamide)s and their block copolymers with poly(N,N-dimethylacrylamide). Macromolecules 2019, 52, 7207–7217. [Google Scholar] [CrossRef]
- Zhu, W.; Nese, A.; Matyjaszewsk, K. Thermoresponsive star triblock copolymers by combination of ROP and ATRP: From micelles to hydrogels. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1942–1952. [Google Scholar] [CrossRef]
- Sezonenko, T.; Qiu, X.-P.; Winnik, F.M.; Sato, T. Dehydration, micellization, and phase separation of thermosensitive polyoxazoline star block copolymers in aqueous solution. Macromolecules 2019, 52, 935–944. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, T.; Han, G.; Guo, T.; Zhang, W. Star block copolymer nanoassemblies: Block sequence is all-important. Macromolecules 2019, 52, 718–728. [Google Scholar] [CrossRef]
- Hirao, A.; Inushima, R.; Nakayama, T.; Watanabe, T.; Yoo, H.-S.; Ishizone, T.; Sugiyama, K.; Kakuchi, T.; Carlotti, S.; Deffieux, A. Precise synthesis of thermo-responsive and water-soluble star-branched polymers and star block copolymers by living anionic polymerization. Eur. Polym. J. 2011, 47, 713–722. [Google Scholar]
- Mori, H.; Ebina, Y.; Kambara, R.; Nakabayashi, K. Temperature-responsive self-assembly of star block copolymers with poly(ionic liquid) segments. Polym. J. 2012, 44, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Mendrek, B.; Fus, A.; Klarzyńska, K.; Sieroń, A.L.; Smet, M.; Kowalczuk, A.; Dworak, A. Synthesis, characterization and cytotoxicity of novel thermoresponsive star copolymers of N,N’-Dimethylaminoethyl methacrylate and hydroxyl-bearing oligo(ethylene glycol) methacrylate. Polymers 2018, 10, 1255. [Google Scholar] [CrossRef] [Green Version]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Preparation of a mini-library of thermo-responsive star (NVCL/NVP-VAc) polymers with tailored properties using a hexafunctional xanthate RAFT agent. Polymers 2018, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Gitsov, I.; Fréchet, J.M.J. Stimuli-responsive hybrid macromolecules: novel amphiphilic star copolymers with dendritic groups at the periphery. J. Am. Chem. Soc. 1996, 118, 3785–3786. [Google Scholar] [CrossRef]
- Cohen Stuart, M.A.; Huck, W.T.S.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsurkruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Borner, H.G.; Kuhnle, H.; Hentschel, J. Making “smart polymers” smarter: Modern concepts to regulate functions in polymer science. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1–14. [Google Scholar] [CrossRef]
- Bullet, J.R.; Korchagina, E.V.; Winnik, F.M. High-sensitivity microcalorimetry and gel permeation chromatography in tandem reveal the complexity of the synthesis of poly-(2-isopropyl-2-oxazoline) stars. Macromolecules 2021, 54, 6161–6170. [Google Scholar] [CrossRef]
- Trachsel, L.; Romio, M.; Zenobi-Wong, M.; Benetti, E.M. Hydrogels generated from cyclic poly(2-oxazoline)s display unique swelling and mechanical properties. Macromol. Rapid Commun. 2021, 42, 2000658. [Google Scholar] [CrossRef]
- Viegas, T.X.; Bentley, M.D.; Milton Harris, J.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Mero, A.; Pasut, G.; Veronese, F.M. Polyoxazoline: Chemistry, Properties, and Applications in Drug Delivery. Bioconjugate Chem. 2011, 22, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Kirila, T.Y.; Kurlykin, M.P.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of aqueous solutions of thermosensitive starlike polyalkyloxazolines with different arm structures. Polym. Sci. A 2017, 59, 826–838. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Self-organization in aqueous solutions of thermosensitive star-shaped and linear gradient copolymers of 2-ethyl-2-oxazoline and 2-isopropyl-2-oxazoline. Colloid. Polym. Sci. 2020, 298, 535–546. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Influence of a hydrophobic core on thermoresponsive behavior of dendrimer-based star-shaped poly(2-isopropyl-2-oxazoline) in aqueous solutions. J. Polym. Res. 2017, 24, 124. [Google Scholar] [CrossRef]
- Shinkai, S.; Mori, S.; Tsubaki, T.; Sone, T.; Manabe, O. New water-soluble host molecules derived from calix[6]arene. Tetrahedron Lett. 1984, 25, 5315–5318. [Google Scholar] [CrossRef]
- Schühle, D.T.; Peters, J.A.; Schatz, J. Metal binding calixarenes with potential biomimetic and biomedical applications. Coord. Chem. Rev. 2011, 255, 2727–2745. [Google Scholar] [CrossRef]
- Kiegiel, K.; Steczek, L.; Zakrzewska-Trznadel, G. Application of calixarenes as macrocyclic ligands for Uranium(VI): A Review. J. Chem. 2013, 2013, 762819. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-X.; Li, T.; Peng, S.; Tao, D. Supramolecular nanocapsules from the self-assembly of amphiphilic calixarene as a carrier for paclitaxel. New J. Chem. 2016, 40, 9923–9929. [Google Scholar] [CrossRef]
- Di Bari, I.; Picciotto, R.; Granata, G.; Blanco, A.R.; Consoli, G.M.L.; Sortino, S. A bactericidal calix[4]arene-based nanoconstruct with amplified NO photorelease. Org. Biomol. Chem. 2016, 14, 8047–8052. [Google Scholar] [CrossRef]
- Morozova, J.E.; Syakaev, V.V.; Kazakova, E.K.; Shalaeva, Y.V.; Nizameev, I.R.; Kadirov, M.K.; Voloshina, A.D.; Zobov, V.V.; Konovalov, A.I. Amphiphilic calixresorcinarene associates as effective solubilizing agents for hydrophobic organic acids: Construction of nano-aggregates. Soft Matter 2016, 12, 5590–5599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.; Badshah, L.S.; Ahmad, N.; Rashid, H.U.; Mabkhot, Y. Inclusion complexes of a new family of non-ionic amphiphilic dendrocalix[4]arene and poorly water-soluble drugs naproxen and ibuprofen. Molecules 2017, 22, 783. [Google Scholar] [CrossRef] [Green Version]
- Consoli, G.M.L.; Granata, G.; Picciotto, R.; Blanco, A.R.; Marino, A.; Nostro, A. Design, synthesis and antibacterial evaluation of a polycationic calix[4]arene derivative alone and in combination with antibiotics. Med. Chem. Comm. 2018, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Pisagatti, I.; Barbera, L.; Gattuso, G.; Patanè, S.; Parisi, M.F.; Notti, A. Novel PEGylated calix[5]arenes as carriers for Rose Bengal. Supramol. Chem. 2018, 30, 658–663. [Google Scholar] [CrossRef]
- Tu, C.; Zhu, L.; Li, P.; Chen, Y.; Su, Y.; Yan, D.; Zhu, X.; Zhou, G. Supramolecular polymeric micelles by the host–guest interaction of star-like calix[4]arene and chlorin e6 for photodynamic therapy. Chem. Commun. 2011, 47, 6063–6065. [Google Scholar] [CrossRef] [PubMed]
- Nimsea, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef]
- Amirova, A.; Tobolina, A.; Kirila, T.; Blokhin, A.N.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. Influence of core configuration and arm structure on solution properties of new thermosensitive star-shaped poly(2-alkyl-2-oxazolines). Int. J. Polym. Anal. Charact. 2018, 23, 278–285. [Google Scholar] [CrossRef]
- Percec, V.; Bera, T.K.; De, B.B.; Sanai, Y.; Smith, J.; Holerca, M.N.; Barboiu, B.; Grubbs, R.B.; Fréchet, J.M.J. Synthesis of functional aromatic multisulfonyl chlorides and their masked precursors. Org. Chem. 2001, 66, 2104–2117. [Google Scholar] [CrossRef]
- Shpyrkov, A.A.; Tarasenko, I.I.; Pankova, G.A.; Il’ina, I.E.; Tarasova, E.V.; Tarabukina, E.B.; Vlasov, G.P.; Filippov, A.P. Molecular mass characteristics and hydrodynamic and conformational properties of hyperbranched poly-l-lysines. Polym. Sci. A 2009, 51, 250–258. [Google Scholar] [CrossRef]
- Rueb, C.J.; Zukoski, C.F. Rheology of suspensions of weakly attractive particles: Approach to gelation. J. Rheol. 1998, 42, 1451–1476. [Google Scholar] [CrossRef]
- Amirova, A.I.; Rodchenko, S.V.; Filippov, A.P. Time dependence of the aggregation of star-shaped poly(2-isopropyl-2-oxazolines) in aqueous solutions. J. Polym. Res. 2016, 23, 221. [Google Scholar] [CrossRef]
- Kurlykin, M.P.; Razina, A.B.; Ten’kovtsev, A.V. The use of sulfonyl halides as initiators of cationic polymerization of oxazolines. Polym. Sci. B 2015, 57, 395–401. [Google Scholar] [CrossRef]
- Blokhin, A.N.; Razina, A.B.; Ten’kovtsev, A.V. Star poly(2-alkyl-2-oxazolines) based on octa-(chlorosulfonyl)-calix[8]arene. Polym. Sci. B 2018, 60, 307–316. [Google Scholar]
- Vergaelen, M.; Verbraeken, B.; Monnery, B.D.; Hoogenboom, R. Sulfolane as common rate accelerating solvent for the cationic ring-opening polymerization of 2-oxazolines. ACS Macro Lett. 2015, 4, 825–828. [Google Scholar] [CrossRef]
- Verbraeken, B.; Monnery, B.D.; Lava, K.; Hoogenboom, R. The chemistry of poly(2-oxazoline)s. Eur. Polym. J. 2017, 88, 451–469. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomers. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Razina, A.; Tenkovtsev, A.; Filippov, A. Synthesis and conformational characteristics of thermosensitive star-shaped six-arm polypeptoids. Polymers 2020, 12, 800. [Google Scholar] [CrossRef] [Green Version]
- Gubarev, A.S.; Monnery, B.D.; Lezov, A.A.; Sedlacek, O.; Tsvetkov, N.V.; Hoogenboom, R.; Filippov, S.K. Conformational properties of biocompatible poly(2-ethyl-2-oxazoline)s in phosphate buffered saline. Polym. Chem. 2018, 9, 2232–2237. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkov, V.N. Rigid-Chain Polymers; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Sung, J.H.; Lee, D.C. Molecular shape of poly(2-ethyl-2-oxazoline) chains in THF. Polymer 2001, 42, 5771–5779. [Google Scholar] [CrossRef]
- Grube, M.; Leiske, M.N.; Schubert, U.S.; Nischang, I. POx as an alternative to PEG? A hydrodynamic and light scattering study. Macromolecules 2018, 51, 1905–1916. [Google Scholar] [CrossRef]
- Smirnova, A.; Kirila, T.; Blokhin, A.; Kozina, N.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Linear and star-shaped poly(2-ethyl-2-oxazine)s. Synthesis, characterization and conformation in solution. Eur. Polym. J. 2021, 156, 110637. [Google Scholar] [CrossRef]
- Zimm, B.H.; Stockmayer, W.H. The dimensions of chain molecules containing branches and rings. J. Chem. Phys. 1949, 17, 1301–1314. [Google Scholar] [CrossRef]
- Burchard, W. Particle scattering factors of some branched polymers. Macromolecules 1977, 10, 919–927. [Google Scholar] [CrossRef]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. Adv. Polym. Sci. 1983, 48, 1–124. [Google Scholar]
- Daoud, M.; Cotton, J.P. Star shaped polymers: A model for the conformation and its concentration dependence. J. Phys. 1982, 43, 531–538. [Google Scholar] [CrossRef]
- Weissmuller, M.; Burchard, W. Molar mass distributions of end-linked polystyrene star macromolecules. Polym. Int. 1997, 44, 380–390. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer-molecules. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Tarabukina, E.B.; Amirova, A.I. Behavior of hyperbranched polymers in solutions. Polym. Sci. C 2011, 53, 107–117. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Roy, R.; Michailova, N.A.; Ortega, P.C.; Perez, M.A. Sedimentation, translational diffusion, and viscosity of lactosylated polyamidoamine dendrimers. Progr. Colloid Polym. Sci. 1999, 113, 150–157. [Google Scholar]
- Pavlov, G.M.; Korneeva, E.V.; Nepogod’ev, S.A.; Jumel, K.; Harding, S.E. Translational and rotational of lactodendrimer molecules in solution. Polym. Sci. A 1998, 40, 12821289. [Google Scholar]
- Obeid, R.; Maltseva, E.; Thünemann, A.F.; Tanaka, F.; Winnik, F.M. Temperature response of self-assembled micelles of telechelic hydrophobically modified poly(2-alkyl-2-oxazoline)s in water. Macromolecules 2009, 42, 2204–2214. [Google Scholar] [CrossRef]
- Hruby, M.; Filippov, S.K.; Panek, J.; Novakova, M.; Mackova, H.; Kucka, J.; Ulbrich, K. Polyoxazoline thermoresponsive micelles as radionuclide delivery systems. Macromol. Biosci. 2010, 10, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Caponi, P.F.; Qiu, X.P.; Vilela, F.; Winnik, F.M.; Ulijn, R.V. Phosphatase/temperature responsive poly(2-isopropyl-2-oxazoline). Polym. Chem. 2011, 2, 306–308. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, T.; Terao, K.; Qiu, X.P.; Winnik, F.M. Self-association of a thermosensitive poly(alkyl-2-oxazoline) block copolymer in aqueous solution. Macromolecules 2012, 45, 6111–6119. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Cyclische imidsäureester aus nitrilen und aminoalkoholen. Just. Lieb. Annal. Chem. 1974, 6, 996–1009. [Google Scholar] [CrossRef]
- Dimitrov, I.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive watersoluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Trinh, L.T.T.; Lambermont-Thijs, H.M.L.; Schubert, U.S.; Hoogenboom, R.; Kjøniksen, A.L. Thermoresponsive poly(2-oxazoline) block copolymers exhibiting two cloud points: Complex multistep assembly behavior. Macromolecules 2012, 45, 4337–4345. [Google Scholar] [CrossRef] [Green Version]
- Steinschulte, A.A.; Schulte, B.; Rütten, S.; Eckert, T.; Okuda, J.; Möller, M.; Schneider, S.; Borisov, O.V.; Plamper, F.A. Effects of architecture on the stability of thermosensitive unimolecular micelles. Phys. Chem. Chem. Phys. 2014, 16, 4917–4932. [Google Scholar] [CrossRef] [Green Version]
- Steinschulte, A.A.; Schulte, B.; Erberich, M.; Borisov, O.V.; Plamper, F.A. Unimolecular Janus micelles by microenvironment-induced, internal complexation. ACS Macro Lett. 2012, 1, 504–507. [Google Scholar] [CrossRef]
- Kirila, T.U.; Smirnova, A.V.; Filippov, A.S.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. Thermosensitive star-shaped poly-2-ethyl-2-oxazine. Synthesis, structure characterization, conformation, and self-organization in aqueous solutions. Eur. Polym. J. 2019, 120, 109215. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solution; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin, Germany, 2007. [Google Scholar]
- Amirova, A.I.; Golub, O.V.; Kirila, T.U.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. Influence of arm length and number on star-shaped poly(2-isopropyl-2-oxazoline) aggregation in aqueous solutions near cloud point. Soft Mater. 2016, 14, 15–26. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarasova, E.V.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix[8]arene core. Int. J. Polym. Anal. Charact. 2019, 24, 87–95. [Google Scholar] [CrossRef]
- Smirnova, A.V.; Kirila, T.U.; Kurlykin, M.P.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of aqueous solutions of polymer star with block copolymer poly(2-ethyl-2-oxazoline) and poly(2-isopropyl-2-oxazoline) arms. Int. J. Polym. Anal. Charact. 2017, 22, 677–684. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Milenin, S.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Self-organization of thermosensitive star-shaped poly(2-isopropyl-2-oxazolines) influenced by arm number and generation of carbosilane dendrimer core in aqueous solutions. Colloid Polym. Sci. 2020, 298, 355–363. [Google Scholar] [CrossRef]
- Kirile, T.Y.; Tobolina, A.I.; Elkina, A.A.; Kurlykin, M.P.; Tenkovtsev, A.V.; Filippov, A.P. Self-assembly processes in aqueous solutions of heat-sensitive star-shaped poly-2-ethyl-2-oxazoline. Fiber. Chem. 2018, 50, 248–251. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, V.R.; Nau, W.M.; Hoogenboom, R. Tuning temperature responsive poly(2-alkyl-2-oxazoline)s by supramolecular host–guest interactions. Org. Biomol. Chem. 2015, 13, 3048–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccone, A.; Crassous, J.J.; Béri, B.; Ballauff, M. Quantifying the reversible association of thermosensitive nanoparticles. Phys. Rev. Lett. 2011, 107, 168303. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Xu, J.; Hu, J.; Wang, X.; Zhang, G.; Liu, S.; Wu, C. Comparative study of temperature-induced association of cyclic and linear poly(N-isopropylacrylamide) chains in dilute solutions by laser light scattering and stopped-flow temperature jump. Macromolecules 2008, 41, 4416–4422. [Google Scholar] [CrossRef]
- Zhao, J.; Hoogenboom, R.; Van Assche, G.; Van Mele, B. Demixing and remixing kinetics of poly(2-isopropyl-2-oxazoline) (PIPOZ) aqueous solutions studied by modulated temperature differential scanning calorimetry. Macromolecules 2010, 43, 6853–6860. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.L.; Hu, Y. Effect of composition of PDMAEMA-b-PAA block copolymers on their pH and temperature-responsive behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef]
- Adelsberger, J.; Grillo, I.; Kulkarni, A.; Sharp, M.; Bivigou-Koumba, A.M.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Kinetics of aggregation in micellar solutions of thermoresponsive triblock copolymers—Influence of concentration, start and target temperatures. Soft Matter 2013, 9, 1685–1699. [Google Scholar] [CrossRef]
- Meyer, M.; Antonietti, M.; Schlaad, H. Unexpected thermal characteristics of aqueous solutions of poly(2-isopropyl-2-oxazoline). Soft Matter 2007, 3, 430–431. [Google Scholar] [CrossRef]
- Bühler, J.; Muth, S.; Fischer, K.; Schmidt, M. Collapse of cylindrical brushes with 2-isopropyloxazoline side chains close to the phase boundary. Macromol. Rapid Commun. 2013, 34, 588–594. [Google Scholar] [CrossRef]
- Kudryavtseva, A.A.; Kurlykin, M.P.; Tarabukina, E.B.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of thermosensitive graft copolymer with aromatic polyester backbone and poly-2-ethyl-2-oxazoline side chains in aqueous solutions. Int. J. Polym. Anal. Charact. 2017, 22, 526–533. [Google Scholar] [CrossRef]

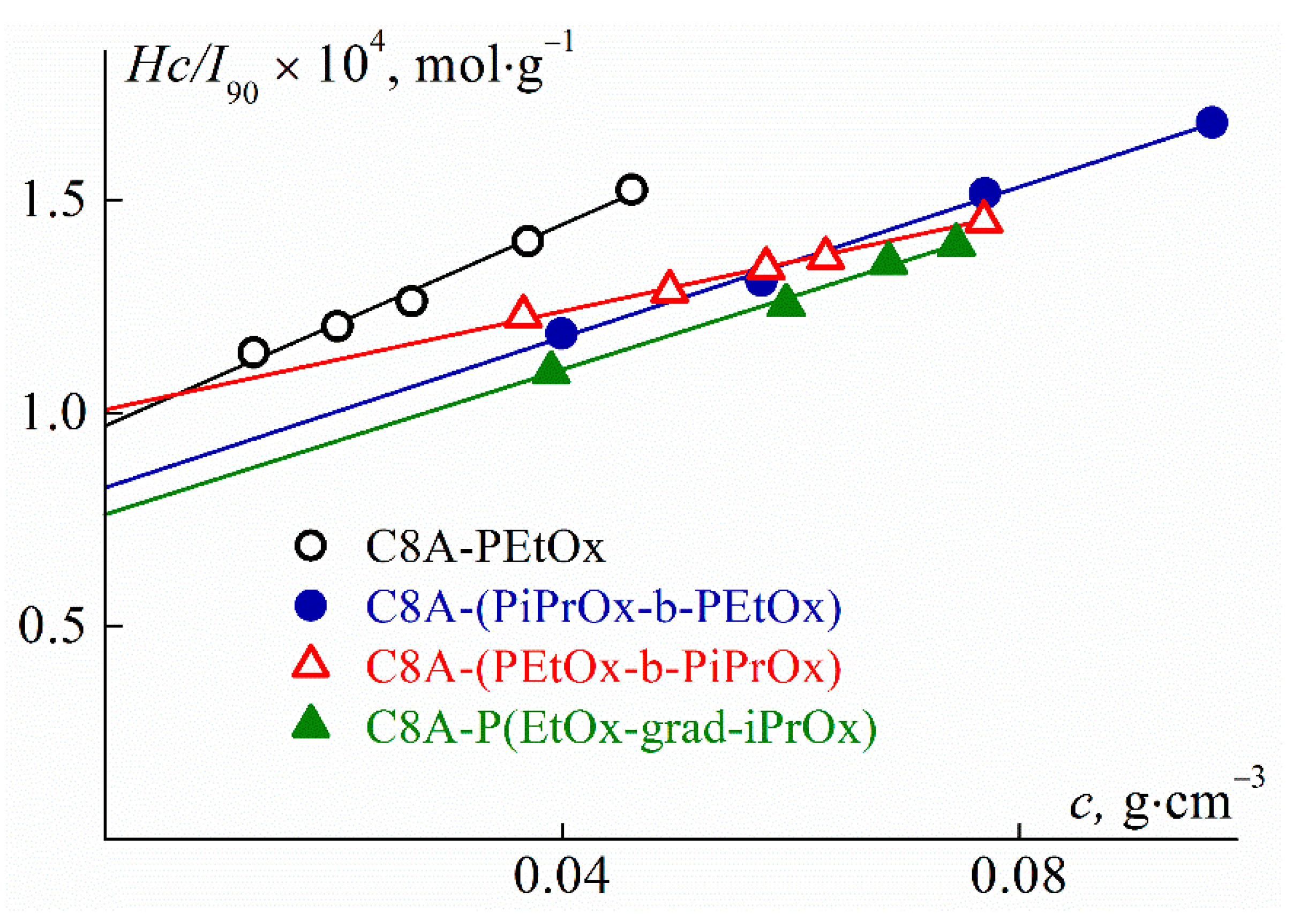

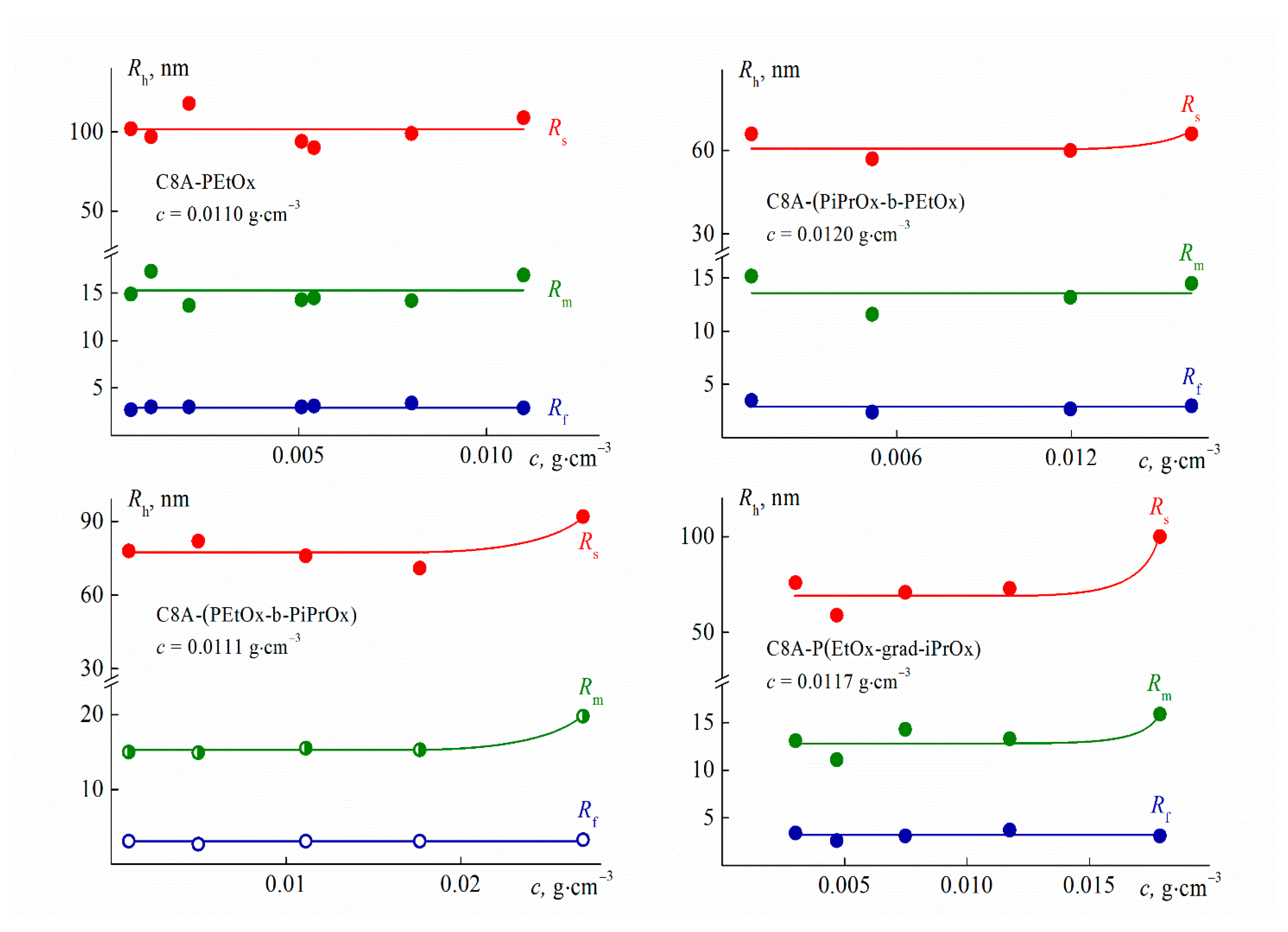



| Sample | Mw, g·mol−1 | Đ | Rh-D, nm | [η], cm3·g−1 | Rh-[η], nm | dn/dc, cm3·g−1 | A2·104, cm3·mol·g−2 |
|---|---|---|---|---|---|---|---|
| C8A-PEtOx | 10,300 | 1.38 | 2.6 | 8.2 | 2.4 | 0.1246 | 5.9 |
| C8A-(PiPrOx-b-PEtOx) | 12,100 | 1.21 | 2.4 | 5.9 | 2.2 | 0.1166 | 4.4 |
| C8A-(PEtOx-b-PiPrOx) | 10,000 | 1.35 | 2.0 | 4.9 | 2.0 | 0.1156 | 2.8 |
| C8A-P(EtOx-grad-iPrOx) | 13,200 | 1.41 | 2.9 | 7.7 | 2.5 | 0.1160 | 4.3 |
| Sample | Mw, g·mol−1 | Mw (arm), g·mol−1 | fa | La, nm | N* | g′ | g | A0 × 1010, erg·K−1mol−1/3 |
|---|---|---|---|---|---|---|---|---|
| C8A-PEtOx | 10,300 | 1100 | 8.0 | 4.0 | 2.4 | 0.45 | 0.37 | 2.7 |
| C8A-(PiPrOx-b-PEtOx) | 12,100 | 1300 | 8.0 | 4.5 | 2.8 | 0.29 | 0.23 | 2.7 |
| C8A-(PEtOx-b-PiPrOx) | 10,000 | 1050 | 8.1 | 3.6 | 2.3 | 0.27 | 0.21 | 2.9 |
| C8A-P(EtOx-grad-iPrOx) | 13,200 | 1400 | 7.6 | 4.7 | 2.9 | 0.34 | 0.27 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirila, T.; Amirova, A.; Blokhin, A.; Tenkovtsev, A.; Filippov, A. Features of Solution Behavior of Polymer Stars with Arms of Poly-2-alkyl-2-oxazolines Copolymers Grafted to the Upper Rim of Calix[8]arene. Polymers 2021, 13, 2507. https://doi.org/10.3390/polym13152507
Kirila T, Amirova A, Blokhin A, Tenkovtsev A, Filippov A. Features of Solution Behavior of Polymer Stars with Arms of Poly-2-alkyl-2-oxazolines Copolymers Grafted to the Upper Rim of Calix[8]arene. Polymers. 2021; 13(15):2507. https://doi.org/10.3390/polym13152507
Chicago/Turabian StyleKirila, Tatyana, Alina Amirova, Alexey Blokhin, Andrey Tenkovtsev, and Alexander Filippov. 2021. "Features of Solution Behavior of Polymer Stars with Arms of Poly-2-alkyl-2-oxazolines Copolymers Grafted to the Upper Rim of Calix[8]arene" Polymers 13, no. 15: 2507. https://doi.org/10.3390/polym13152507
APA StyleKirila, T., Amirova, A., Blokhin, A., Tenkovtsev, A., & Filippov, A. (2021). Features of Solution Behavior of Polymer Stars with Arms of Poly-2-alkyl-2-oxazolines Copolymers Grafted to the Upper Rim of Calix[8]arene. Polymers, 13(15), 2507. https://doi.org/10.3390/polym13152507