Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications
Abstract
1. Introduction
2. Cyclodextrin-Peptide Conjugates
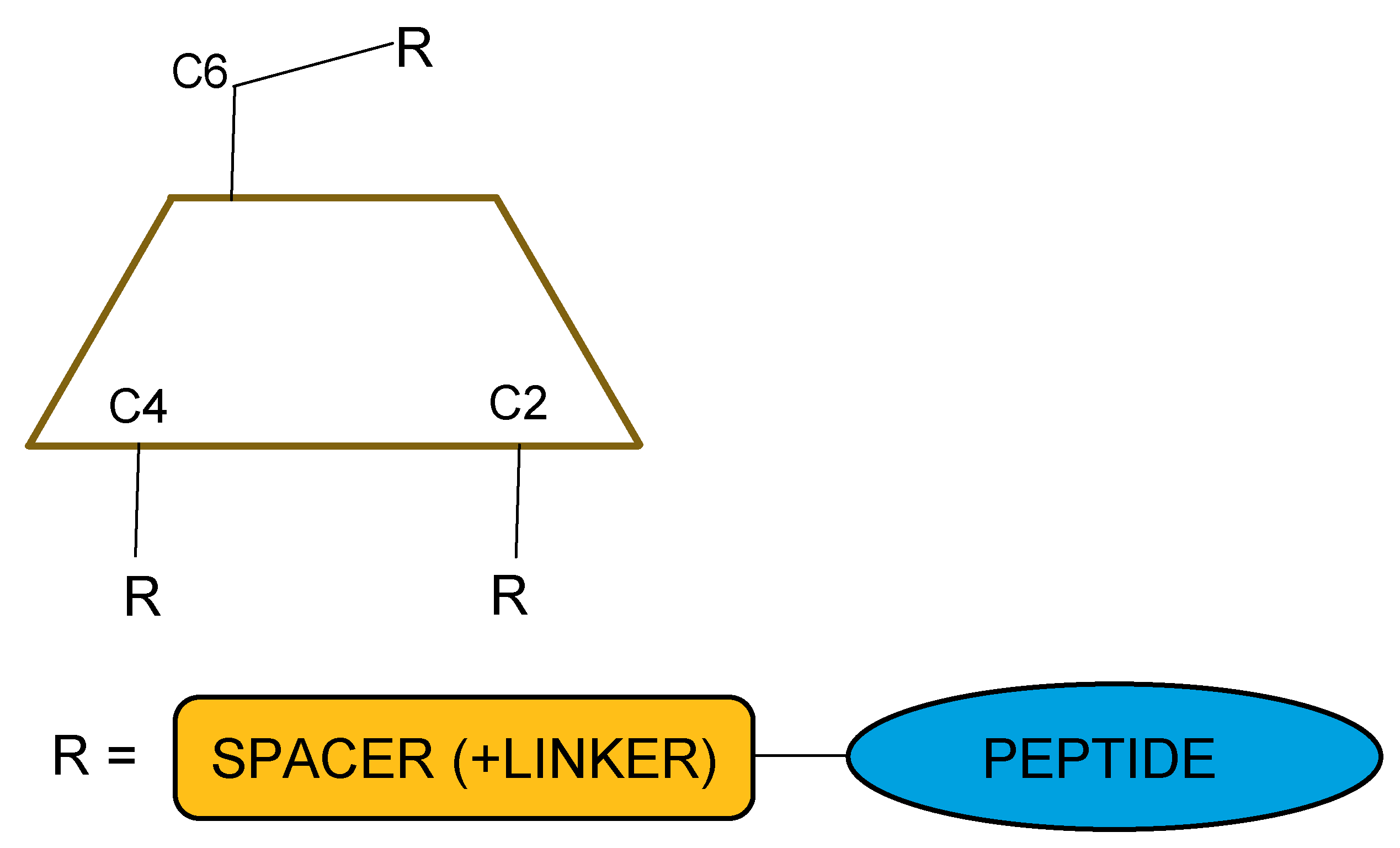
2.1. Purely Synthetic and Structural Aspects
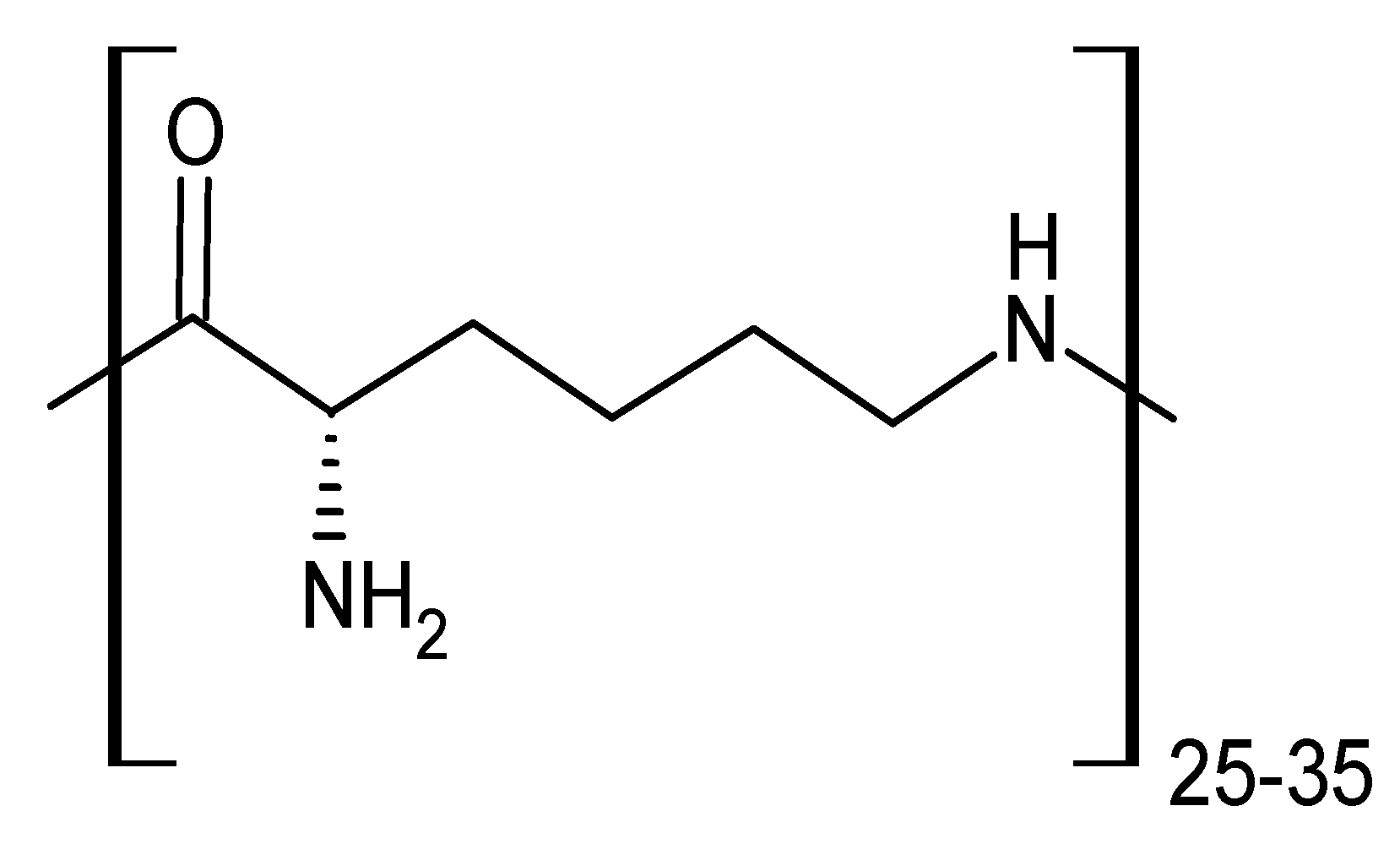
2.2. CD-Polylysine Conjugates
2.3. Cyclodextrin Polymers-Peptides Conjugated
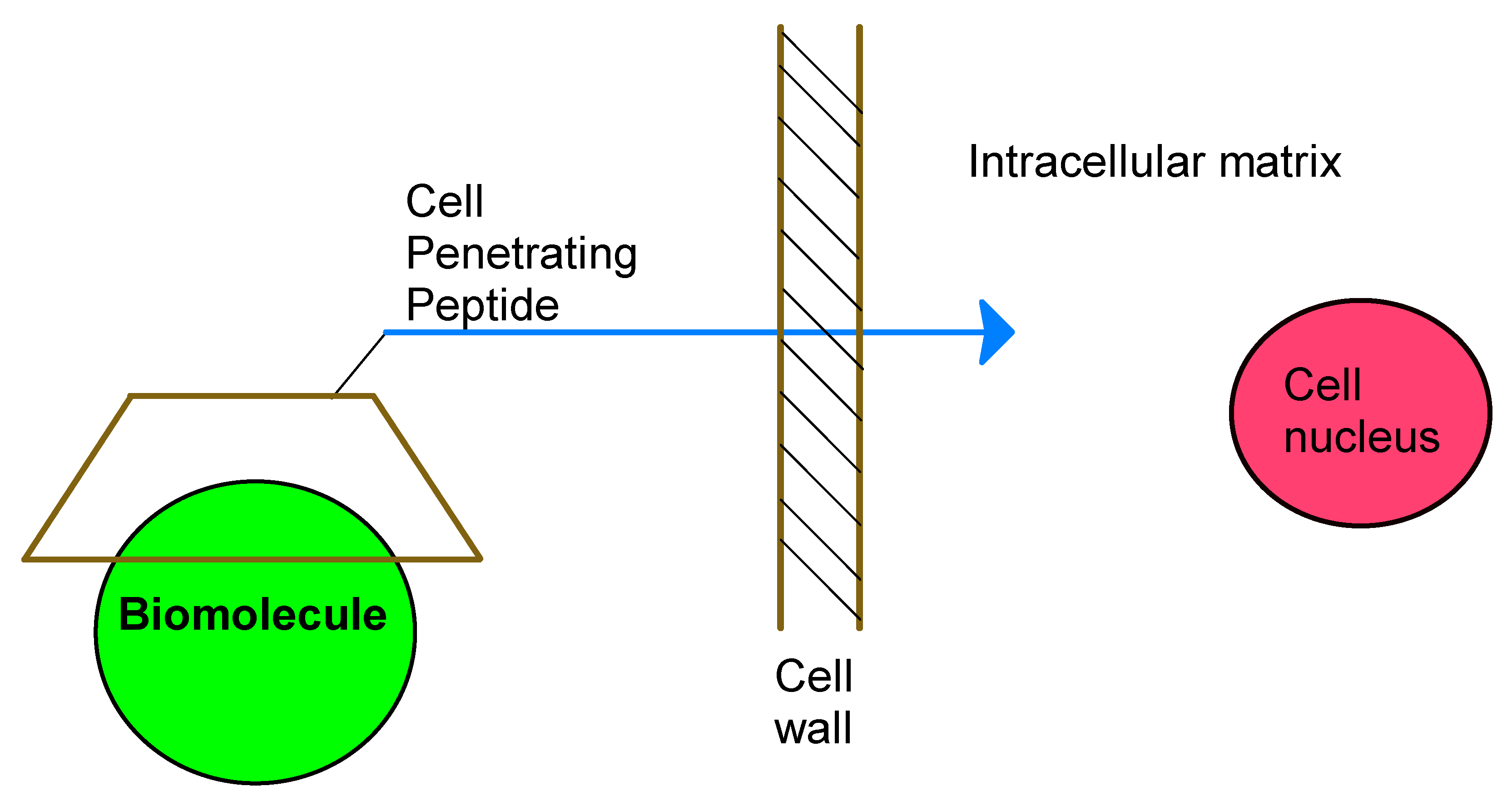
2.4. CD-Cell Penetrating Peptides Conjugates
2.5. CD-Peptide Conjugates in Fibrils Formation
2.6. CD-Peptide Conjugates as Tools in Chemical Biology
3. Cyclodextrin-Protein Conjugates
| Position at CD | Protein | Linker | Spacer | Ref. |
|---|---|---|---|---|
| C6 | Green fluorescent protein | Disulfide bridge -S-S- | - | [128] |
| Basic pancreatic trypsin inhibitor | Imine Bond -C=N- | - | [132] | |
| Lysozyme | - | [132,133] | ||
| Insulin | Amide Bond -CONH- | Glucuronylglucosyl- -Succinic Acid | [137] | |
| C2/C3/C6 | Bovine serum albumin | - | [140,141] | |
| C6 | Pneumococcal surface protein A | 1,2,3-Triazole moiety | Glycine-Glycine- -D-Propargylglycine | [144] |
| Pilus protein |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Śliwa, W.; Girek, T. Cyclodextrins Properties and Applications; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2017. [Google Scholar]
- Szejtli, J. Cyclodextrin Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988; pp. 1–170. [Google Scholar]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Shaarawy, S.; Shafie, A.E.; Hebeish, A. Development of antimicrobial medical cotton fabrics using synthesized nanoemulsion of reactive cyclodextrin hosted coconut oil inclusion complex. Fibers Polym. 2017, 18, 1486–1495. [Google Scholar] [CrossRef]
- Elsherbiny, D.A.; Abdelgawad, A.M.; El-Naggar, M.E.; El-Sherbiny, R.A.; El-Rafie, M.H.; El-Sayed, I.E.-T. Synthesis, antimicrobial activity, and sustainable release of novel α-aminophosphonate derivatives loaded carrageenan cryogel. Int. J. Biol. Macromol. 2020, 163, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sadaquat, H.; Akhtar, M. Comparative effects of β-cyclodextrin, HP-β-cyclodextrin and SBE7-β-cyclodextrin on the solubility and dissolution of docetaxel via inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 333–351. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Li, L.; Wang, G.; Su, X. β-Cyclodextrin modified silver nanoclusters for highly sensitive fluorescence sensing and bioimaging of intracellular alkaline phosphatase. Talanta 2020, 207, 120315. [Google Scholar] [CrossRef]
- Shuang, Y.; Zhang, T.; Li, L. Preparation of a stilbene diamido-bridged bis(β-cyclodextrin)-bonded chiral stationary phase for enantioseparations of drugs and pesticides by high performance liquid chromatography. J. Chromatogr. A 2020, 1614, 460702. [Google Scholar] [CrossRef]
- Czescik, J.; Lyu, Y.; Neuberg, S.; Scrimin, P.M.; Mancin, F. Host–Guest Allosteric Control of an Artificial Phosphatase. J. Am. Chem. Soc. 2020, 142, 6837–6841. [Google Scholar] [CrossRef]
- Irie, T. Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 1999, 36, 101–123. [Google Scholar] [CrossRef]
- Roy, M.N.; Ekka, D.; Saha, S.; Roy, M.C. Host–guest inclusion complexes of α and β-cyclodextrins with α-amino acids. RSC Adv. 2014, 4, 42383–42390. [Google Scholar] [CrossRef]
- Roy, M.N.; Roy, A.; Saha, S. Probing inclusion complexes of cyclodextrins with amino acids by physicochemical approach. Carbohydr. Polym. 2016, 151, 458–466. [Google Scholar] [CrossRef]
- Chu, H.M.; Zhang, R.X.; Huang, Q.; Bai, C.C.; Wang, Z.Z. Chemical conjugation with cyclodextrins as a versatile tool for drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 29–38. [Google Scholar] [CrossRef]
- Malhotra, M.; Gooding, M.; Evans, J.C.; O’Driscoll, D.; Darcy, R.; O’Driscoll, C.M. Cyclodextrin-siRNA conjugates as versatile gene silencing agents. Eur. J. Pharm. Sci. 2018, 114, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, T.; Oda, Y.; Katsuraya, K.; Inazu, T.; Hattori, K. Synthesis, structure, and evaluation of a β-cyclodextrin-artificial carbohydrate conjugate for use as a doxorubicin-carrying molecule. Bioorg. Med. Chem. 2016, 24, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Hirayama, F.; Kamada, M.; Arima, H.; Uekama, K. Colon-specific delivery of prednisolone-appended α-cyclodextrin conjugate: Alleviation of systemic side effect after oral administration. J. Control. Release 2002, 79, 103–112. [Google Scholar] [CrossRef]
- Vieira, A.C.; Serra, A.C.; Carvalho, R.A.; Gonsalves, A.; Figueiras, A.; Veiga, F.; Basit, A.W.; Gonsalves, A.M.D.R. Microwave synthesis and in vitro stability of diclofenac-β-cyclodextrin conjugate for colon delivery. Carbohydr. Polym. 2013, 93, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjug. Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3, pp. 229–258. [Google Scholar]
- Boccardo, F.M.; Rubagotti, A.; Amoroso, D.; Agostara, B.; Amadori, D.; Gallo, L.; Iacobelli, S.; Massidda, B.; Mesiti, M.; Pacini, P.; et al. Endocrinological and clinical evaluation of two depot formulations of leuprolide acetate in pre- and perimenopausal breast cancer patients. Cancer Chemother. Pharmacol. 1999, 43, 461–466. [Google Scholar] [CrossRef]
- Schally, A.; Arimura, A.; Baba, Y.; Nair, R.; Matsuo, H.; Redding, T.; Debeljuk, L.; White, W. Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 1971, 43, 393–399. [Google Scholar] [CrossRef]
- Plosker, G.L.; Brogden, R.N. Leuprorelin: A Review of its Pharmacology and Therapeutic Use in Prostatic Cancer, Endometriosis and Other Sex Hormone-Related Disorders. Drugs 1994, 48, 930–967. [Google Scholar] [CrossRef]
- Wilson, A.C.; Meethal, S.V.; Bowen, R.L.; Atwood, C.S. Leuprolide acetate: A drug of diverse clinical applications. Expert Opin. Investig. Drugs 2007, 16, 1851–1863. [Google Scholar] [CrossRef]
- Kordopati, G.; Tselios, T.V.; Kellici, T.; Merzel, F.; Mavromoustakos, T.; Grdadolnik, S.G.; Tsivgoulis, G.M. A novel synthetic luteinizing hormone-releasing hormone (LHRH) analogue coupled with modified β-cyclodextrin: Insight into its intramolecular interactions. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 159–168. [Google Scholar] [CrossRef]
- Pickens, C.J.; Johnson, S.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide–Alkyne Cycloaddition. Bioconjug. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.; Presolski, S.; Ma, C.; Finn, M. Analysis and Optimization of Copper-Catalyzed Azideâ-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 2009, 48, 9879–9883. [Google Scholar] [CrossRef] [PubMed]
- Lartia, R.; Jankowski, C.K.; Arseneau, S. On the synthesis of cyclodextrin-peptide conjugates by the Huisgen reaction. J. Pept. Sci. 2016, 22, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Dommerholt, J.; Rutjes, F.P.J.T.; Van Delft, F.L. Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. Top. Curr. Chem. 2016, 374, 1–20. [Google Scholar] [CrossRef]
- Ni, J.; Singh, S.; Wang, L.-X. Synthesis of Maleimide-Activated Carbohydrates as Chemoselective Tags for Site-Specific Glycosylation of Peptides and Proteins. Bioconjug. Chem. 2003, 14, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Al Temimi, A.H.K.; Boltje, T.J.; Zollinger, D.; Rutjes, F.P.J.T.; Feiters, M.C. Peptide-Appended Permethylated β-Cyclodextrins with Hydrophilic and Hydrophobic Spacers. Bioconjug. Chem. 2017, 28, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Sitterley, G. Poly-l-lysine cell attachment protocol. BioFiles 2006, 3, 12. [Google Scholar]
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on production and medical applications of ɛ-polylysine. Biochem. Eng. J. 2012, 65, 70–81. [Google Scholar] [CrossRef]
- Eftekhari, R.B.; Maghsoudnia, N.; Samimi, S.; Zamzami, A.; Dorkoosh, F.A. Co-Delivery Nanosystems for Cancer Treatment: A Review. Pharm. Nanotechnol. 2019, 7, 90–112. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale Self-Assembly for Therapeutic Delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, H.-B.; Chen, Y.-Y.; Lin, J.-T.; Zhang, L.-M. New cyclodextrin derivative containing poly(L-lysine) dendrons for gene and drug co-delivery. J. Colloid Interface Sci. 2013, 405, 305–311. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Tao, W.; Zeng, X.; Liu, T.; Wang, Z.; Xiong, Q.; Ouyang, C.; Huang, L.; Mei, L. Docetaxel-loaded nanoparticles based on star-shaped mannitol-core PLGA-TPGS diblock copolymer for breast cancer therapy. Acta Biomater. 2013, 9, 8910–8920. [Google Scholar] [CrossRef]
- Liu, T.; Xue, W.; Ke, B.; Xie, M.-Q.; Ma, D. Star-shaped cyclodextrin-poly(l-lysine) derivative co-delivering docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials 2014, 35, 3865–3872. [Google Scholar] [CrossRef]
- Shima, S.; Sakai, H. Polylysine Produced by Streptomyces. Agric. Biol. Chem. 1977, 41, 1807–1809. [Google Scholar] [CrossRef]
- Shih, I.-L.; Shen, M.-H.; Van, Y.-T. Microbial synthesis of poly(ε-lysine) and its various applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef]
- Mandal, H.; Katiyar, S.S.; Swami, R.; Kushwah, V.; Katare, P.B.; Meka, A.K.; Banerjee, S.K.; Popat, A.; Jain, S. ε-Poly-l-Lysine/plasmid DNA nanoplexes for efficient gene delivery in vivo. Int. J. Pharm. 2018, 542, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Yamashita, A.; Ooya, T.; Yui, N.; Akita, H.; Kogure, K.; Ito, R.; Harashima, H. Sunflower-Shaped Cyclodextrin-Conjugated Poly(ε-Lysine) Polyplex as a Controlled Intracellular Trafficking Device. ChemBioChem 2005, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Hu, J.; Zhou, T.-Y.; Wang, P.; Xu, Y.-H. Synthesis, insecticidal evaluation of novel 1,3,4-thiadiazole chrysanthemamide derivatives formed by an EDCI/HOBt condensation. J. Chem. Res. 2011, 35, 703–706. [Google Scholar] [CrossRef]
- Yan, Q.; Zheng, H.-N.; Jiang, C.; Li, K.; Xiao, S.-J. EDC/NHS activation mechanism of polymethacrylic acid: Anhydride versus NHS-ester. RSC Adv. 2015, 5, 69939–69947. [Google Scholar] [CrossRef]
- Jiang, R.-J.; Yang, B.; Yi, D.; Wang, F.; Han, B.; Zhao, Y.-L.; Liao, X.-L.; Yang, J.; Gao, C.-Z. Synthesis and characterization of a series of novel amino β-cyclodextrin-conjugated poly(ε-lysine) derivatives. J. Polym. Eng. 2014, 34, 133–139. [Google Scholar] [CrossRef]
- Yi, S.; Yang, B.; Liao, R. Synthesis, characterization, and cytotoxicity studies of novel pendant polymers: Amino acid β-cyclodextrin-conjugated poly(ε-lysine) derivatives. Int. J. Polym. Anal. Charact. 2017, 22, 247–255. [Google Scholar] [CrossRef]
- Barshes, N.R.; Wyllie, S.; Goss, J.A. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J. Leukoc. Biol. 2005, 77, 587–597. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Jiang, X.; Kou, L.; Samuriwo, A.T.; Xu, H.-L.; Zhao, Y.-Z. Pharmacological actions and therapeutic potentials of bilirubin in islet transplantation for the treatment of diabetes. Pharmacol. Res. 2019, 145, 104256. [Google Scholar] [CrossRef]
- Yao, Q.; Jiang, X.; Huang, Z.-W.; Lan, Q.-H.; Wang, L.-F.; Chen, R.; Li, X.-Z.; Kou, L.; Xu, H.-L.; Zhao, Y.-Z. Bilirubin Improves the Quality and Function of Hypothermic Preserved Islets by Its Antioxidative and Anti-inflammatory Effect. Transplantation 2019, 103, 2486–2496. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, Z.-W.; Zhai, Y.-Y.; Yue, M.; Luo, L.-Z.; Xue, P.-P.; Han, Y.-H.; Xu, H.-L.; Kou, L.; Zhao, Y.-Z. Localized Controlled Release of Bilirubin from β-Cyclodextrin-Conjugated ε-Polylysine To Attenuate Oxidative Stress and Inflammation in Transplanted Islets. ACS Appl. Mater. Interfaces 2020, 12, 5462–5475. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(ethylene glycol) and Cyclodextrin-Grafted Chitosan: From Methodologies to Preparation and Potential Biotechnological Applications. Front. Chem. 2017, 5, 1–15. [Google Scholar] [CrossRef]
- Girek, T. Cyclodextrin-based polyrotaxanes. J. Incl. Phenom. Macrocycl. Chem. 2012, 76, 237–252. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Z.; Chen, Z.; Xu, G.; Chen, Y. Fibroblast Growth Factor Receptors (FGFRs): Structures and Small Molecule Inhibitors. Cells 2019, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Kallus, S.; Englinger, B.; Senkiv, J.; Laemmerer, A.; Heffeter, P.; Berger, W.; Kowol, C.R.; Keppler, B.K. Nanoformulations of anticancer FGFR inhibitors with improved therapeutic index. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, H.; Huang, H.; Shen, F.; Wu, X.; Li, J.; Wang, J.; Cao, X.; Wang, Q.; Tang, G. FGF Receptor-mediated Gene Delivery using Ligands Coupled to Polyethylenimine. J. Biomater. Appl. 2006, 22, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Maruta, F.; Parker, A.L.; Fisher, K.D.; Hallissey, M.T.; Ismail, T.; Rowlands, D.C.; Chandler, L.A.; Kerr, D.J.; Seymour, L.W. Identification of FGF receptor-binding peptides for cancer gene therapy. Cancer Gene Ther. 2002, 9, 543–552. [Google Scholar] [CrossRef]
- Li, D.; Ping, Y.; Xu, F.; Yu, H.; Pan, H.; Huang, H.; Wang, Q.; Tang, G.; Li, J. Construction of a Star-Shaped Copolymer as a Vector for FGF Receptor-Mediated Gene Delivery In Vitro and In Vivo. Biomacromolecules 2010, 11, 2221–2229. [Google Scholar] [CrossRef]
- Ping, Y.; Hu, Q.; Tang, G.; Li, J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials 2013, 34, 6482–6494. [Google Scholar] [CrossRef]
- Kelley, W.; Safari, H.; Lopez-Cazares, G.; Eniola-Adefeso, O. Vascular-targeted nanocarriers: Design considerations and strategies for successful treatment of atherosclerosis and other vascular diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 909–926. [Google Scholar] [CrossRef]
- Pawlowski, C.L.; Li, W.; Sun, M.; Ravichandran, K.; Hickman, D.; Kos, C.; Kaur, G.; Gupta, A.S. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials 2017, 128, 94–108. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Modery-Pawlowski, C.L.; Menegatti, S.; Kumar, S.; Vogus, D.R.; Tian, L.L.; Chen, M.; Squires, T.M.; Gupta, A.S.; Mitragotri, S. Platelet-like Nanoparticles (PLNs): Engineering Shape, Flexibility and Surface Chemistry of Nanocarriers to Target Vascular Injuries. ACS Nano 2014, 8, 11243–11253. [Google Scholar] [CrossRef]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Sun, X.; Ren, X.; Maharjan, A.; York, P.; Su, Y.; Li, H.; Zhang, J. Cuboidal tethered cyclodextrin frameworks tailored for hemostasis and injured vessel targeting. Theranostics 2019, 9, 2489–2504. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Aroui, S.; Kenani, A. Cheminformatics and its Applications, Cell-Penetrating Peptides: A Challenge for Drug Delivery. In Cheminformatics and Its Applications; IntechOpen: Rijeka, Croatia, 2020; Chapter 11. [Google Scholar] [CrossRef]
- Colombo, P.; Sonvico, F.; Colombo, G.; Bettini, R. Novel Platforms for Oral Drug Delivery. Pharm. Res. 2009, 26, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Morishita, M.; Eda, Y.; Ida, N.; Nishio, R.; Takayama, K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J. Control. Release 2008, 132, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A. Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv. Drug Deliv. Rev. 2008, 60, 580–597. [Google Scholar] [CrossRef]
- Zhu, X.; Shan, W.; Zhang, P.; Jin, Y.; Guan, S.; Fan, T.; Yang, Y.; Zhou, Z.; Huang, Y. Penetratin Derivative-Based Nanocomplexes for Enhanced Intestinal Insulin Delivery. Mol. Pharm. 2014, 11, 317–328. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Kosuru, R.; Singh, S.K.; Prasad, C.B.; Narayan, G.; Muthu, M.S. Resveratrol loaded PLGA:d-α-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv. 2016, 6, 74254–74268. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Jia, X.; Lu, W.-L.; Lou, J.; Wei, Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef]
- Saha, S.; Yakati, V.; Shankar, G.; Jaggarapu, M.M.C.S.; Moku, G.; Madhusudana, K.; Banerjee, R.; Ramkrishna, S.; Srinivas, R.; Chaudhuri, A. Amphetamine decorated cationic lipid nanoparticles cross the blood–brain barrier: Therapeutic promise for combating glioblastoma. J. Mater. Chem. B 2020, 8, 4318–4330. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Gooding, M.; Malhotra, M.; McCarthy, D.J.; Godinho, B.M.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. Synthesis and characterization of rabies virus glycoprotein-tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: In vitro analysis. Eur. J. Pharm. Sci. 2015, 71, 80–92. [Google Scholar] [CrossRef]
- Lopes, M.; Abrahim, B.; Seiça, R.; Veiga, F.; Rodrigues, C.; Ribeiro, A. Intestinal Uptake of Insulin Nanoparticles: Facts or Myths? Curr. Pharm. Biotechnol. 2014, 15, 629–638. [Google Scholar] [CrossRef]
- Alai, M.S.; Lin, W.J.; Pingale, S.S. Application of polymeric nanoparticles and micelles in insulin oral delivery. J. Food Drug Anal. 2015, 23, 351–358. [Google Scholar] [CrossRef]
- Morishita, M.; Kamei, N.; Ehara, J.; Isowa, K.; Takayama, K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J. Control. Release 2007, 118, 177–184. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Sun, Y.; Zhang, L. A cell-penetrating peptide conjugated carboxymethyl-β-cyclodextrin to improve intestinal absorption of insulin. Int. J. Biol. Macromol. 2018, 111, 685–695. [Google Scholar] [CrossRef]
- Ray, M.; Lee, Y.-W.; Scaletti, F.; Yu, R.; Rotello, V.M. Intracellular delivery of proteins by nanocarriers. Nanomedicine 2017, 12, 941–952. [Google Scholar] [CrossRef]
- Seo, B.J.; Hong, Y.J.; Do, J.T. Cellular Reprogramming Using Protein and Cell-Penetrating Peptides. Int. J. Mol. Sci. 2017, 18, 552. [Google Scholar] [CrossRef]
- Hu, Q.-D.; Tang, G.-P.; Chu, P.K. Cyclodextrin-Based Host–Guest Supramolecular Nanoparticles for Delivery: From Design to Applications. Accounts Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pan, X.; Chang, J.; Niu, W.; Hou, W.; Kuai, H.; Zhao, Z.; Liu, J.; Wang, M.; Tan, W. Supramolecularly Engineered Circular Bivalent Aptamer for Enhanced Functional Protein Delivery. J. Am. Chem. Soc. 2018, 140, 6780–6784. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, H.; Jiromaru, M.; Hasegawa, N. Intracellular Delivery of Adamantane-Tagged Small Molecule, Proteins, and Liposomes Using an Octaarginine-Conjugated β-Cyclodextrin. ACS Appl. Bio Mater. 2020, 3, 4902–4911. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Peptide Fibrillization. Angew. Chem. Int. Ed. 2007, 46, 8128–8147. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, V.; Kar, R.K.; Datta, A.; Parthasarathi, K.; Chatterjee, S.; Das, K.P.; Bhunia, A. Use of a Small Peptide Fragment as an Inhibitor of Insulin Fibrillation Process: A Study by High and Low Resolution Spectroscopy. PLoS ONE 2013, 8, e72318. [Google Scholar] [CrossRef]
- Smith, J.F.; Knowles, T.; Dobson, C.M.; MacPhee, C.E.; Welland, M.E. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef]
- Jeppesen, M.D.; Westh, P.; Otzen, D.E. The role of protonation in protein fibrillation. FEBS Lett. 2010, 584, 780–784. [Google Scholar] [CrossRef]
- Aggeli, A.; Nyrkova, I.A.; Bell, M.; Harding, R.; Carrick, L.; McLeish, T.C.B.; Semenov, A.N.; Boden, N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide -sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. USA 2001, 98, 11857–11862. [Google Scholar] [CrossRef]
- Reches, M. Casting Metal Nanowires Within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Christoffersen, H.F.; Andreasen, M.; Zhang, S.; Nielsen, E.H.; Christiansen, G.; Dong, M.; Skrydstrup, T.; Otzen, D.E. Scaffolded multimers of hIAPP20–29 peptide fragments fibrillate faster and lead to different fibrils compared to the free hIAPP20–29 peptide fragment. Biochim. Biophys. Acta BBA Proteins Proteom. 2015, 1854, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A Century of Alzheimer’s Disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Salawu, F.K.; Umar, J.T.; Olokoba, A.B. Alzheimer′s disease: A review of recent developments. Ann. Afr. Med. 2011, 10, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997, 20, 154–159. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bett, C.K.; Ngunjiri, J.N.; Serem, W.K.; Fontenot, K.R.; Hammer, R.P.; McCarley, R.L.; Garno, J.C. Structure−Activity Relationships in Peptide Modulators of β-Amyloid Protein Aggregation: Variation in α,α-Disubstitution Results in Altered Aggregate Size and Morphology. ACS Chem. Neurosci. 2010, 1, 608–626. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Liu, F.; Zheng, J.; Sun, Y. Ac-LVFFARK-NH 2 conjugation to β-cyclodextrin exhibits significantly enhanced performance on inhibiting amyloid β-protein fibrillogenesis and cytotoxicity. Biophys. Chem. 2018, 235, 40–47. [Google Scholar] [CrossRef]
- Oprea, T.I.; Tropsha, A.; Faulon, J.-L.; Rintoul, M.D. Systems chemical biology. Nat. Chem. Biol. 2007, 3, 447–450. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Y.; Ding, S. A chemical approach to stem-cell biology and regenerative medicine. Nat. Cell Biol. 2008, 453, 338–344. [Google Scholar] [CrossRef]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef]
- Joddar, B.; Ito, Y. Biological modifications of materials surfaces with proteins for regenerative medicine. J. Mater. Chem. 2011, 21, 13737–13755. [Google Scholar] [CrossRef]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Frith, J.; Cooper-White, J.J. Modulation of Stem Cell Adhesion and Morphology via Facile Control over Surface Presentation of Cell Adhesion Molecules. Biomacromolecules 2013, 15, 43–52. [Google Scholar] [CrossRef]
- Goverdhana, S.; Puntel, M.; Xiong, W.; Zirger, J.; Barcia, C.; Curtin, J.; Soffer, E.; Mondkar, S.; King, G.; Hu, J.; et al. Regulatable gene expression systems for gene therapy applications: Progress and future challenges. Mol. Ther. 2005, 12, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Klug, A. The Discovery of Zinc Fingers and Their Applications in Gene Regulation and Genome Manipulation. Annu. Rev. Biochem. 2010, 79, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, T.E.; Brandl, C.J.; Struhl, K.; Harrison, S.C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted α Helices: Crystal structure of the protein-DNA complex. Cell 1992, 71, 1223–1237. [Google Scholar] [CrossRef]
- García, Y.R.; Zelenka, J.; Pabon, Y.V.; Iyer, A.; Buděšínský, M.; Kraus, T.; Smith, C.I.E.; Madder, A. Cyclodextrin–peptide conjugates for sequence specific DNA binding. Org. Biomol. Chem. 2015, 13, 5273–5278. [Google Scholar] [CrossRef]
- Kiessling, L. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000, 4, 696–703. [Google Scholar] [CrossRef]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.-W.; Haag, R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.A.; Mellet, C.O.; Fernández, J.M.G. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate–protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. [Google Scholar] [CrossRef] [PubMed]
- Sebestik, J.; Niederhafner, P.; Jezek, J. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids 2010, 40, 301–370. [Google Scholar] [CrossRef]
- Gaidzik, N.; Westerlind, U.; Kunz, H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 2013, 42, 4421–4442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-M.; Chen, P.-G.; Huang, Z.-H.; Sun, Z.-Y.; Li, Q.-Q.; Chen, Y.-X.; Zhao, Y.-F. Synthesis of an MUC1 Glycopeptide Dendrimer Based on β-Cyclodextrin by Click Chemistry. Synlett 2017, 28, 1961–1965. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Li, W.; Johnson, N.S. Bile Salts as Semiochemicals in Fish. Chem. Senses 2014, 39, 647–654. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef]
- Holm, R.; Müllertz, A.; Mu, H. Bile salts and their importance for drug absorption. Int. J. Pharm. 2013, 453, 44–55. [Google Scholar] [CrossRef]
- Wenz, G.; Strassnig, C.; Thiele, C.; Engelke, A.; Morgenstern, B.; Hegetschweiler, K. Recognition of Ionic Guests by Ionic β-Cyclodextrin Derivatives. Chem. Eur. J. 2008, 14, 7202–7211. [Google Scholar] [CrossRef]
- Vurgun, N.; Nitz, M. Highly Functionalized β-Cyclodextrins by Solid-Supported Synthesis. Chem. Eur. J. 2018, 24, 4459–4467. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M. Green Fluorescent Protein (GFP): Applications, Structure, and Related Photophysical Behavior. Chem. Rev. 2002, 102, 759–782. [Google Scholar] [CrossRef]
- Remington, S.J. Green fluorescent protein: A perspective. Protein Sci. 2011, 20, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ishimaru, Y.; Saito, A.; Nishigaki, K. Simple Preparation of Green Fluorescent Protein Conjugated with β-Cyclodextrin in a Site Specific Manner. Anal. Sci. 2013, 29, 811–814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parkin, S.; Rupp, B.; Hope, H. Structure of bovine pancreatic trypsin inhibitor at 125 K definition of carboxyl-terminal residues Gly57 and Ala58. Acta Crystallogr. Sect. D Biol. Crystallogr. 1996, 52, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Bocedi, A.; Bolognesi, M.; Spallarossa, A.; Coletta, M.; Cristofaro, R.; Menegatti, E. The Bovine Basic Pancreatic Trypsin Inhibitor (Kunitz Inhibitor): A Milestone Protein. Curr. Protein Pept. Sci. 2003, 4, 231–251. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Girek, T.; Goszczyński, T.M.; Girek, B.; Ciesielski, W.; Boratynski, J.; Rychter, P. β-Cyclodextrin/protein conjugates as a innovative drug systems: Synthesis and MS investigation. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 293–296. [Google Scholar] [CrossRef]
- Goszczyński, T.M.; Gawłowski, M.; Girek, B.; Kowalski, K.; Boratynski, J.; Girek, T. Synthesis of β-cyclodextrin-lysozyme conjugates and their physicochemical and biochemical properties. J. Incl. Phenom. Macrocycl. Chem. 2017, 87, 341–348. [Google Scholar] [CrossRef]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
- Zaykov, A.; Mayer, J.P.; DiMarchi, R.D. Pursuit of a perfect insulin. Nat. Rev. Drug Discov. 2016, 15, 425–439. [Google Scholar] [CrossRef]
- Jono, H.; Anno, T.; Motoyama, K.; Misumi, Y.; Tasaki, M.; Oshima, T.; Mori, Y.; Mizuguchi, M.; Ueda, M.; Shono, M.; et al. Cyclodextrin, a novel therapeutic tool for suppressing amyloidogenic transthyretin misfolding in transthyretin-related amyloidosis. Biochem. J. 2011, 437, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, T.; Higashi, T.; Motoyama, K.; Hirayama, F.; Uekama, K.; Arima, H. Improvement of pharmaceutical properties of insulin through conjugation with glucuronylglucosyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 107–112. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Lee, P. Review: Modifications of Human Serum Albumin and their Binding Effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef]
- Shi, Y.; Su, C.; Cui, W.; Li, H.; Liu, L.; Feng, B.; Liu, M.; Su, R.; Zhao, L. Gefitinib loaded folate decorated bovine serum albumin conjugated carboxymethyl-beta-cyclodextrin nanoparticles enhance drug delivery and attenuate autophagy in folate receptor-positive cancer cells. J. Nanobiotechnol. 2014, 12, 43. [Google Scholar] [CrossRef]
- Su, C.; Li, H.; Shi, Y.; Wang, G.; Liu, L.; Zhao, L.; Su, R. Carboxymethyl-β-cyclodextrin conjugated nanoparticles facilitate therapy for folate receptor-positive tumor with the mediation of folic acid. Int. J. Pharm. 2014, 474, 202–211. [Google Scholar] [CrossRef]
- Cossart, P.; Jonquières, R. Sortase, a universal target for therapeutic agents against Gram-positive bacteria? Proc. Natl. Acad. Sci. USA 2000, 97, 5013–5015. [Google Scholar] [CrossRef]
- Hendrickx, A.P.A.; Budzik, J.M.; Oh, S.-Y.; Schneewind, O. Architects at the bacterial surface—Sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Genet. 2011, 9, 166–176. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, K.; Shukla, S.; Sampathkumar, S.-G.; Roy, R.P. Sortase-click strategy for defined protein conjugation on a heptavalent cyclodextrin scaffold. PLoS ONE 2019, 14, e0217369. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łagiewka, J.; Girek, T.; Ciesielski, W. Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers 2021, 13, 1759. https://doi.org/10.3390/polym13111759
Łagiewka J, Girek T, Ciesielski W. Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers. 2021; 13(11):1759. https://doi.org/10.3390/polym13111759
Chicago/Turabian StyleŁagiewka, Jakub, Tomasz Girek, and Wojciech Ciesielski. 2021. "Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications" Polymers 13, no. 11: 1759. https://doi.org/10.3390/polym13111759
APA StyleŁagiewka, J., Girek, T., & Ciesielski, W. (2021). Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers, 13(11), 1759. https://doi.org/10.3390/polym13111759







