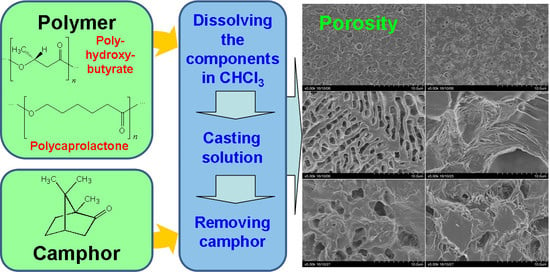
Production of Porous Films Based on Biodegradable Polyesters by the Casting Solution Technique Using a Co-Soluble Porogen (Camphor)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Films
2.3. Methods of Investigating the Films
2.4. Statistical Analysis
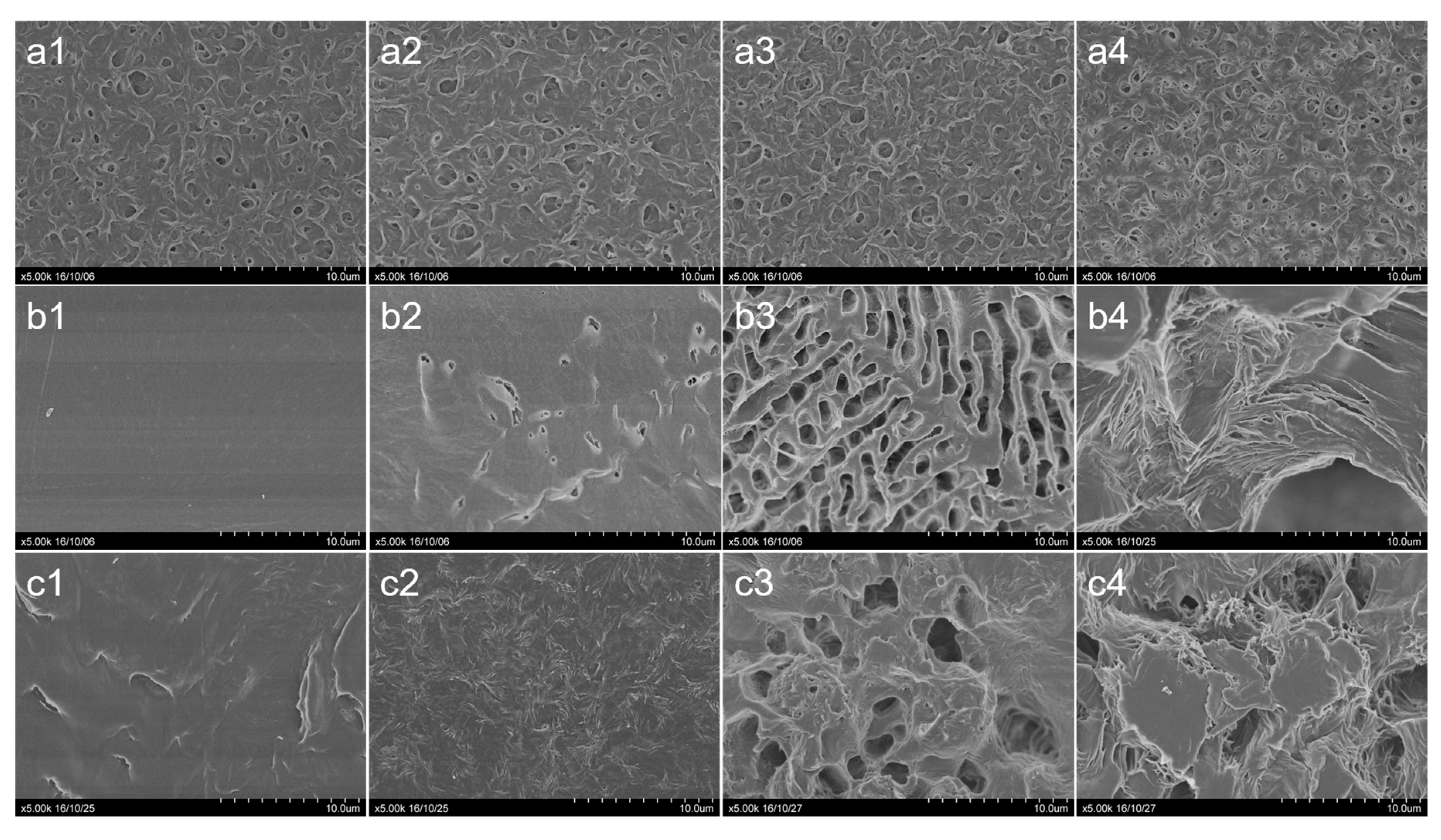
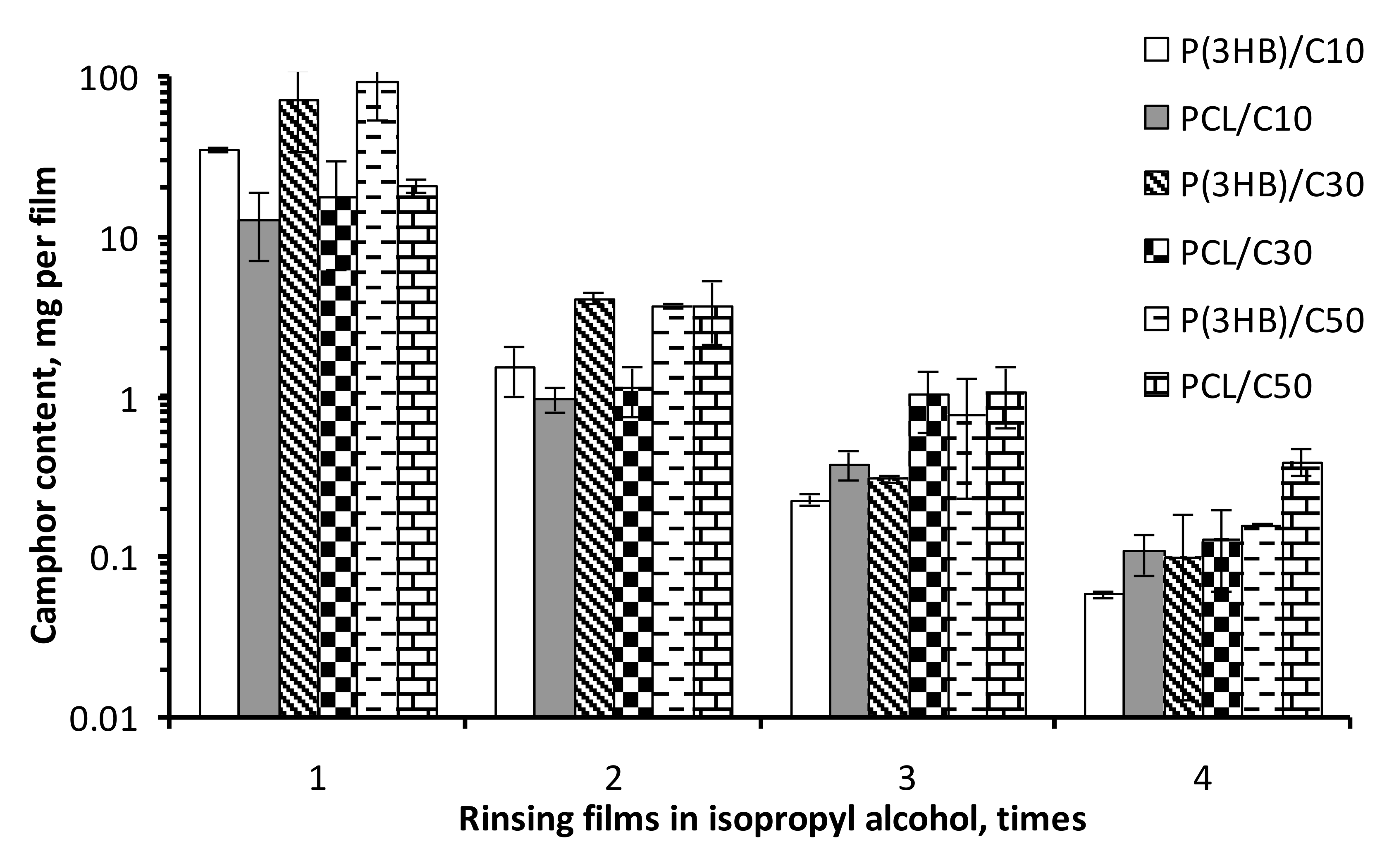
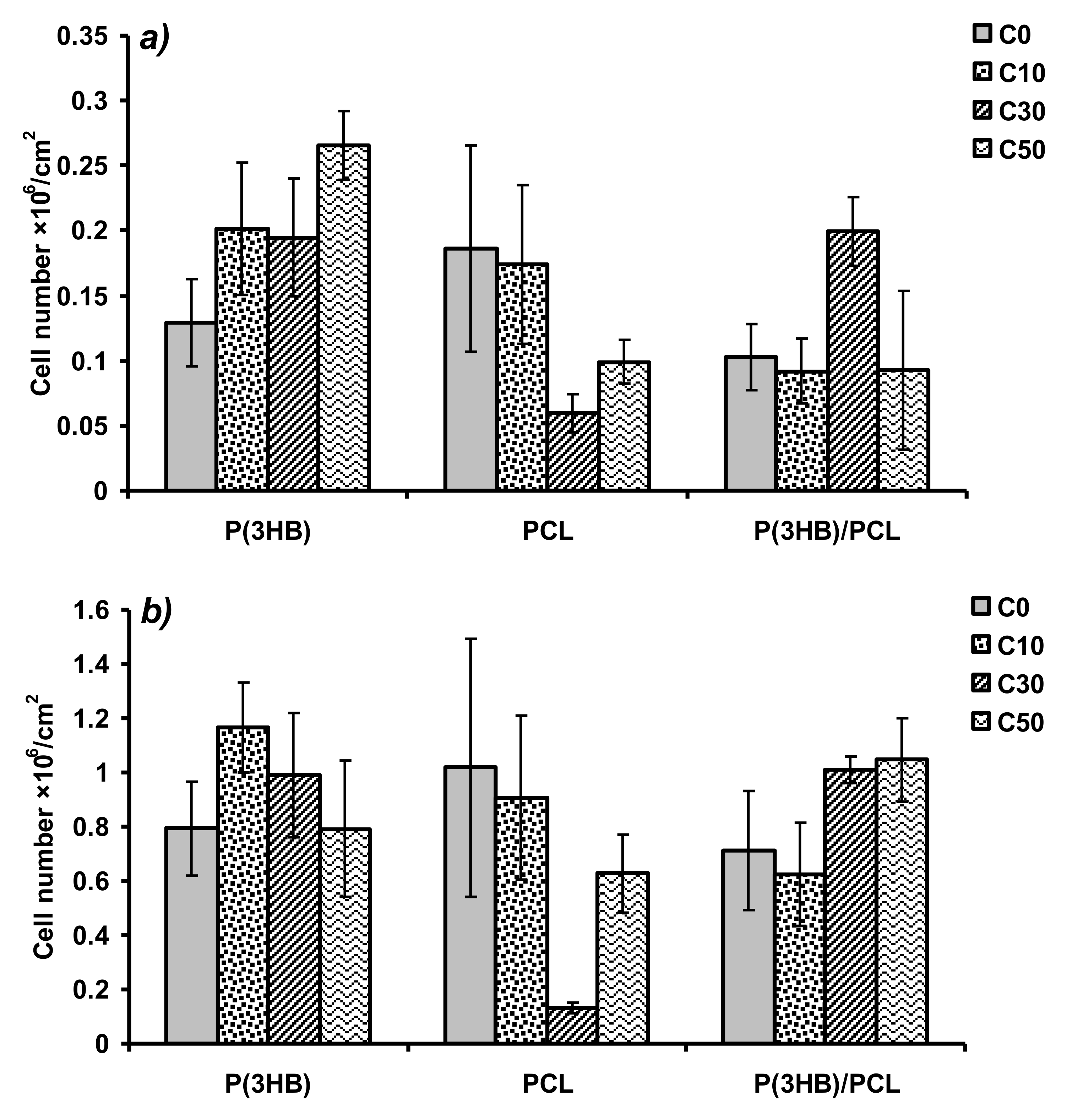
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Volova, T.G.; Vinnik, Y.S.; Shishatskaya, E.I.; Markelova, N.M.; Zaikov, G.E. Natural-Based Polymers for Biomedical Applications; Apple Academic Press: Toronto, ON, Canada, 2017. [Google Scholar]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical processing of polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of poly-hydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Steinbüchel, A. Production of rubber-like polymers by microorganisms. Curr. Opin. Microbiol. 2003, 6, 261–270. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G.W. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef]
- Volova, T.G. Microbial Polyhydroxyalkanoates—Plastic Materials of the 21st Century (Biosynthesis, Properties, Applications); Nova Science Publishing Inc.: New York, NY, USA, 2004. [Google Scholar]
- Hoque, M.E.; San, W.Y.; Wei, F.; Li, S.; Huang, M.H.; Vert, M.; Hutmacher, D.W. Processing of polycaprolactone and polycaprolactone-based copolymers into 3D scaffolds, and their cellular responses. Tissue Eng. Part A 2009, 15, 3013–3024. [Google Scholar] [CrossRef]
- Goh, B.T.; Lee, S.; Chanchareonsook, N.; Tideman, H. Modular endoprosthesis for mandibular reconstruction—A thematic research study at the National Dental Centre Singapore. Proc. Singap. Healthc. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Xue, R.; Qian, Y.; Li, L.; Yao, G.; Yang, L.; Sun, Y. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 148. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, G. A three-dimensional polycaprolactone scaffold combined with a drug delivery system consisting of electrospun nanofibers. J. Pharm. Sci. 2011, 100, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Pérez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.L.; Roy, T.D.; Parsons, J.R.; Rekow, E.D.; Thompson, V.P.; Kemnitzer, J.; Ricci, J.L. Engineered cellular response to scaffold architecture in a rabbit trephine defect. J. Biomed. Mater. Res. A 2003, 66, 275–282. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M.; Ahmad, S.R. Fabrication of polymeric biomaterials: A strategy for tissue engineering and medical devices. J. Mater. Chem. B 2015, 3, 8224–8249. [Google Scholar] [CrossRef]
- Henslee, A.M.; Spicer, P.P.; Yoon, D.M.; Nair, M.B.; Meretoja, V.V.; Witherel, K.E.; Jansen, J.A.; Mikos, A.G.; Kasper, F.K. Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 2011, 7, 3627–3637. [Google Scholar] [CrossRef]
- Liang, X.; Qi, Y.; Pan, Z.; He, Y.; Liu, X.; Cui, S.; Ding, J. Design and preparation of quasi-spherical salt particles as water-soluble porogens to fabricate hydrophobic porous scaffolds for tissue engineering and tissue regeneration. Mater. Chem. Front. 2018, 2, 1539–1553. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Hsu, S.H.; Whu, S.W.; Hsieh, S.C.; Tsai, C.L.; Chen, D.C.; Tan, T.S. Evaluation of chitosan-alginate-hyaluronate complexes modified by an RGD-containing protein as tissue-engineering scaffolds for cartilage regeneration. Artif. Organs 2004, 28, 693–703. [Google Scholar] [CrossRef]
- Martin, D.; Valdez, J.; Boren, J.; Mayersohn, M. Dermal absorption of camphor, menthol, and methyl salicylate in humans. J. Clin. Pharmacol. 2004, 44, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Kiselev, E.G.; Vinogradova, O.N.; Nikolaeva, E.D.; Chistyakov, A.A.; Sukovatyi, A.G.; Shishatskaya, E.I. A glucose-utilizing strain, Cupriavidus eutrophus B-10646: Growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS ONE 2014, 9, e87551. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Kavya, K.C.; Jayakumar, R.; Nair, S.; Chennazhi, K.P. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 59, 255–263. [Google Scholar] [CrossRef]
- Manoguerra, A.S.; Erdman, A.R.; Wax, P.M.; Nelson, L.S.; Caravati, E.M.; Cobaugh, D.J.; Chyka, P.A.; Olson, K.R.; Booze, L.L.; Woolf, A.D.; et al. Camphor poisoning: An evidence-based practice guideline for out-of-hospital management. Clin. Toxicol. 2006, 44, 357–370. [Google Scholar] [CrossRef]
- Tsinontides, S.C.; Rajniak, P.; Pham, D.; Hunke, W.A.; Placek, J.; Reynolds, S.D. Freeze drying—Principles and practice for successful scale-up to manufacturing. Int. J. Pharm. 2004, 280, 1–16. [Google Scholar] [CrossRef]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Novel approach to fabricate porous gelatin-siloxane hybrids for bone tissue engineering. Biomaterials 2002, 23, 4765–4773. [Google Scholar] [CrossRef]
- Lee, K.-W.D.; Chan, P.K.; Feng, X. Morphology development and characterization of the phase-separated structure resulting from the thermal-induced phase separation phenomenon in polymer solutions under a temperature gradient. Chem. Eng. Sci. 2004, 59, 1491–1504. [Google Scholar] [CrossRef]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Preparation, characterization, and in vitro degradation of bioresorbable and bioactive composites based on Bioglass-filled polylactide foams. J. Biomed. Mater. Res. A 2003, 66, 335–346. [Google Scholar] [CrossRef]
- Gassner, F.; Owen, A.J. Physical properties of poly(β-hydroxybutyrate)-poly(ε-caprolactone) blends. Polymer 1994, 35, 2233–2236. [Google Scholar] [CrossRef]
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Sotgiu, M.G.; Vinci, B.; Domenici, C.; Giusti, P. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(epsilon-caprolactone) blends for tissue engineering applications in the form of hollow fibers. J. Biomed. Mater. Res. A 2008, 85, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Boyandin, A.N.; Nikolaeva, E.D.; Sukovatiy, A.G. Properties and biocompatibility of poly-3-hydroxybutyrate-co-3-hydroxyvalerate/poly-ε-caprolactone blends. J. Sib. Fed. Univ. Biol. 2016, 9, 63–74. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Conde, C.M.; Demarco, F.F.; Casagrande, L.; Alcazar, J.C.; Nör, J.E.; Tarquinio, S.B.C. Influence of poly-L-lactic acid scaffold’s pore size on the proliferation and differentiation of dental pulp stem cells. Braz. Dent. J. 2015, 26, 93–98. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, Z.; Zhou, Q.; Li, J.; Gao, C.; Shen, J. Poly(lactic acid) scaffold fabricated by gelatin particle leaching has good biocompatibility for chondrogenesis. J. Biomater. Sci. Polym. Ed. 2008, 19, 207–221. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Kumar, V. A method for solvent-free fabrication of porous polymer using solid-state foaming and ultrasound for tissue engineering applications. Biomaterials 2006, 27, 1924–1929. [Google Scholar] [CrossRef]
- Singh, L.; Kumar, V.; Ratner, B.D. Generation of porous microcellular 85/15 poly(DL-lactide-co-glycolide) foams for biomedical applications. Biomaterials 2004, 25, 2611–2617. [Google Scholar] [CrossRef]
- Yin, H.-M.; Qian, J.; Zhang, J.; Lin, Z.-F.; Li, J.-S.; Xu, J.-Z.; Li, Z.-M. Engineering porous poly(lactic acid) scaffolds with high mechanical performance via a solid state extrusion/porogen leaching approach. Polymers 2016, 8, 213. [Google Scholar] [CrossRef]
- Varoni, E.M.; Altomare, L.; Cochis, A.; GhalayaniEsfahani, A.; Cigada, A.; Rimondini, L.; De Nardo, L. Hierarchic micro-patterned porous scaffolds via electrochemical replica-deposition enhance neo-vascularization. Biomed. Mater. 2016, 11, 025018. [Google Scholar] [CrossRef]
| Polymer | Sample | CAW (°) | CADIM (°) | SFE (mN/m) | PSFE (mN/m) | DSFE (mN/m) |
|---|---|---|---|---|---|---|
| PHB | C0 | 78.5 ± 7.33 | 48.5 ± 1.86 | 40.5 ± 0.76 | 5.4 ± 0.38 | 35.1 ± 0.38 |
| PHB | C10 | 61.6 ± 17.32 | 38.3 ± 4.73 | 52.3 ± 2.38 | 11.8 ± 1.33 | 40.5 ± 1.05 |
| PHB | C30 | 74.6 ± 6.28 | 41.2 ± 1.4 | 45 ± 0.65 | 6 ± 0.34 | 39 ± 0.31 |
| PHB | C50 | 81.7 ± 5.31 | 43.2 ± 1.23 | 41.6 ± 0.49 | 3.6 ± 0.22 | 38 ± 0.26 |
| PCL | C0 | 54.7 ± 7.12 | 20.4 ± 3.42 | 60.7 ± 1.44 | 13 ± 0.61 | 47.7 ± 0.82 |
| PCL | C10 | 65.8 ± 5.56 | 23.6 ± 3.33 | 54.6 ± 1.19 | 7.9 ± 0.39 | 46.6 ± 0.79 |
| PCL | C30 | 95.3 ± 6.12 | 24.6 ± 5.79 | 46.4 ± 1.43 | 0.1 ± 0.06 | 46.3 ± 1.38 |
| PCL | C50 | 83.4 ± 2.62 | 26.5 ± 1.59 | 47.5 ± 0.47 | 1.9 ± 0.09 | 45.6 ± 0.37 |
| PHB/PCL | C0 | 85.4 ± 2.71 | 44.8 ± 2.37 | 39.8 ± 0.63 | 2.7 ± 0.13 | 37.1 ± 0.5 |
| PHB/PCL | C10 | 75.8 ± 1.56 | 38.1 ± 0.93 | 45.8 ± 0.3 | 5.2 ± 0.09 | 40.6 ± 0.21 |
| PHB/PCL | C30 | 83.4 ± 1.27 | 35.6 ± 0.94 | 44.2 ± 0.27 | 2.5 ± 0.05 | 41.7 ± 0.21 |
| PHB/PCL | C50 | 98.9 ± 4.2 | 35.1 ± 1.57 | 42 ± 0.38 | 0 ± 0.02 | 42 ± 0.36 |
| Polymer | Sample | Thickness (µm) | Porosity (%) | E (MPa) | P (MPa) | ε (%) | WVTR (g/m2/d) |
|---|---|---|---|---|---|---|---|
| PHB | C0 | 35 | 4.2 ± 3.6 | 3634.41 ± 284.20 | 40.11 ± 3.02 | 1.55 ± 0.09 | 197.37 ± 23.62 |
| PHB | C10 | 35 | 11.3 ± 3.1 | 3516.88 ± 217.34 | 32.79 ± 1.03 | 1.08 ± 0.05 | 226.62 ± 27.19 |
| PHB | C30 | 35 | 30.1 ± 10.3 | 2722.82 ± 88.23 | 24.18 ± 1.84 | 1.16 ± 0.30 | 232.00 ± 27.12 |
| PHB | C50 | 45 | 50.0 ± 12.8 | 1815.64 ± 17.84 | 13.54 ± 0.60 | 0.9 ± 0.03 | 934.03 ± 114.34 |
| PCL | C0 | 40 | 6.4 ± 5.5 | 302.15 ± 11.60 | 13.54 ± 0.42 | 55.37 ± 19.25 | 1027.99 ± 154.10 |
| PCL | C10 | 60 | 11.0 ± 3.4 | 219.20 ± 4.28 | 10.70 ± 0.02 | 20.14 ± 4.65 | 1410.94 ± 134.36 |
| PCL | C30 | 75 | 30.7 ± 6.4 | 114.24 ± 6.67 | 5.58 ± 0.15 | 26.93 ± 3.21 | 4674.51 ± 118.42 |
| PCL | C50 | 80 | 54.5 ± 6.0 | 121.99 ± 6.29 | 5.62 ± 0.20 | 68.4 ± 2.53 | 7014.62 ± 280.81 |
| PHB/PCL | C0 | 20 | 4.9 ± 4.8 | 502.07 ± 62.42 | 14.12 ± 1.55 | 45.69 ± 18.16 | 715.47 ± 50.08 |
| PHB/PCL | C10 | 40 | 14.3 ± 4.8 | 562.32 ± 46.23 | 13.81 ± 1.29 | 33.94 ± 12.35 | 712.92 ± 47.10 |
| PHB/PCL | C30 | 50 | 33.1 ± 4.4 | 359.57 ± 46.73 | 8.97 ± 1.36 | 48.00 ± 15.72 | 1608.00 ± 126.20 |
| PHB/PCL | C50 | 65 | 51.5 ± 5.8 | 199.38 ± 15.59 | 7.37 ± 0.35 | 103.80 ± 12.99 | 4239.09 ± 275.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyandin, A.N.; Dvoinina, L.M.; Sukovatyi, A.G.; Sukhanova, A.A. Production of Porous Films Based on Biodegradable Polyesters by the Casting Solution Technique Using a Co-Soluble Porogen (Camphor). Polymers 2020, 12, 1950. https://doi.org/10.3390/polym12091950
Boyandin AN, Dvoinina LM, Sukovatyi AG, Sukhanova AA. Production of Porous Films Based on Biodegradable Polyesters by the Casting Solution Technique Using a Co-Soluble Porogen (Camphor). Polymers. 2020; 12(9):1950. https://doi.org/10.3390/polym12091950
Chicago/Turabian StyleBoyandin, Anatoly Nikolayevich, Ljublyana Mikhailovna Dvoinina, Aleksey Grigorievich Sukovatyi, and Anna Alekseevna Sukhanova. 2020. "Production of Porous Films Based on Biodegradable Polyesters by the Casting Solution Technique Using a Co-Soluble Porogen (Camphor)" Polymers 12, no. 9: 1950. https://doi.org/10.3390/polym12091950
APA StyleBoyandin, A. N., Dvoinina, L. M., Sukovatyi, A. G., & Sukhanova, A. A. (2020). Production of Porous Films Based on Biodegradable Polyesters by the Casting Solution Technique Using a Co-Soluble Porogen (Camphor). Polymers, 12(9), 1950. https://doi.org/10.3390/polym12091950