Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
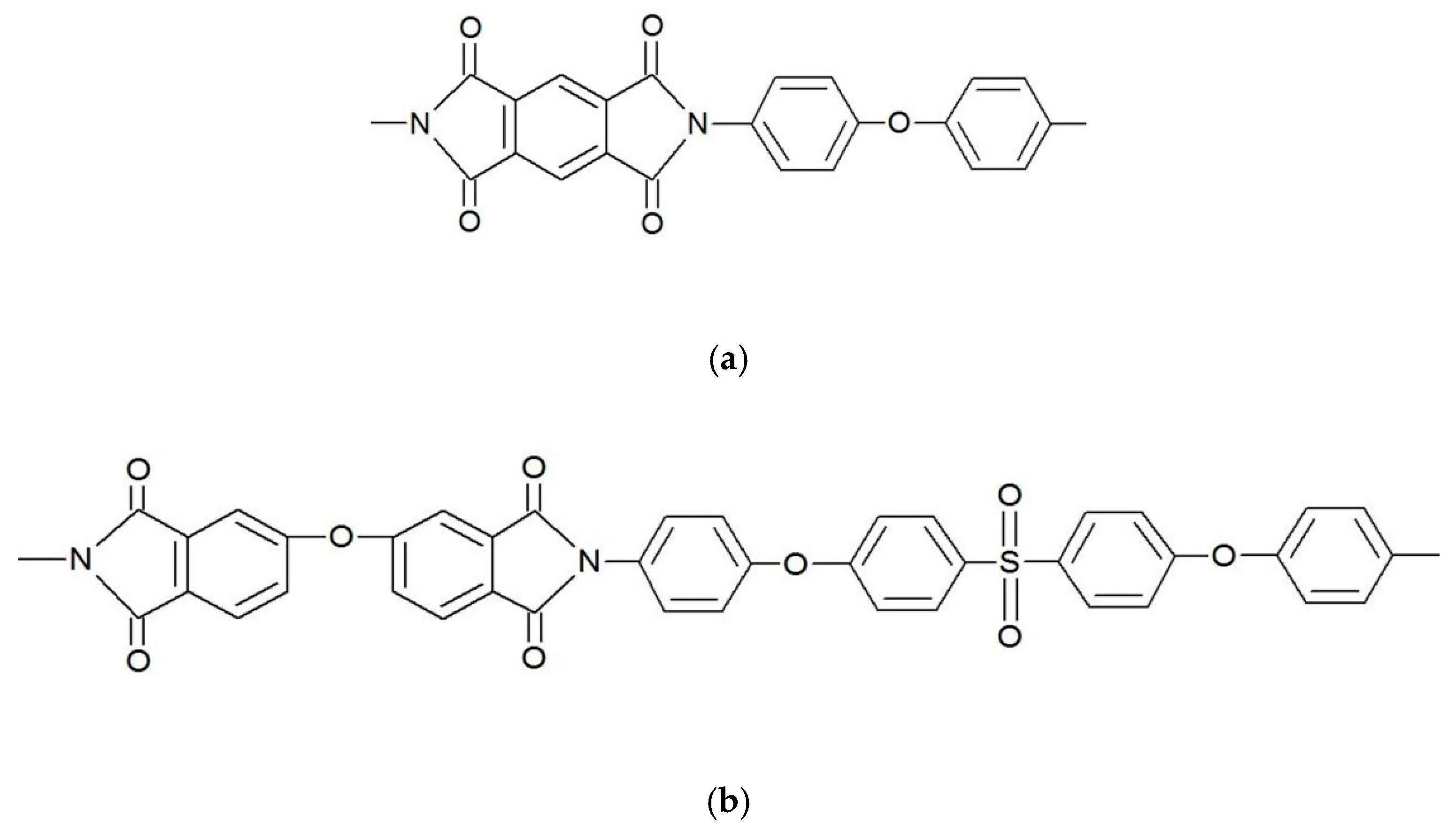
2.1. Materials
2.2. Films Preparation
2.3. Characterization Techniques
3. Results and Discussion
3.1. Optimization of Composition
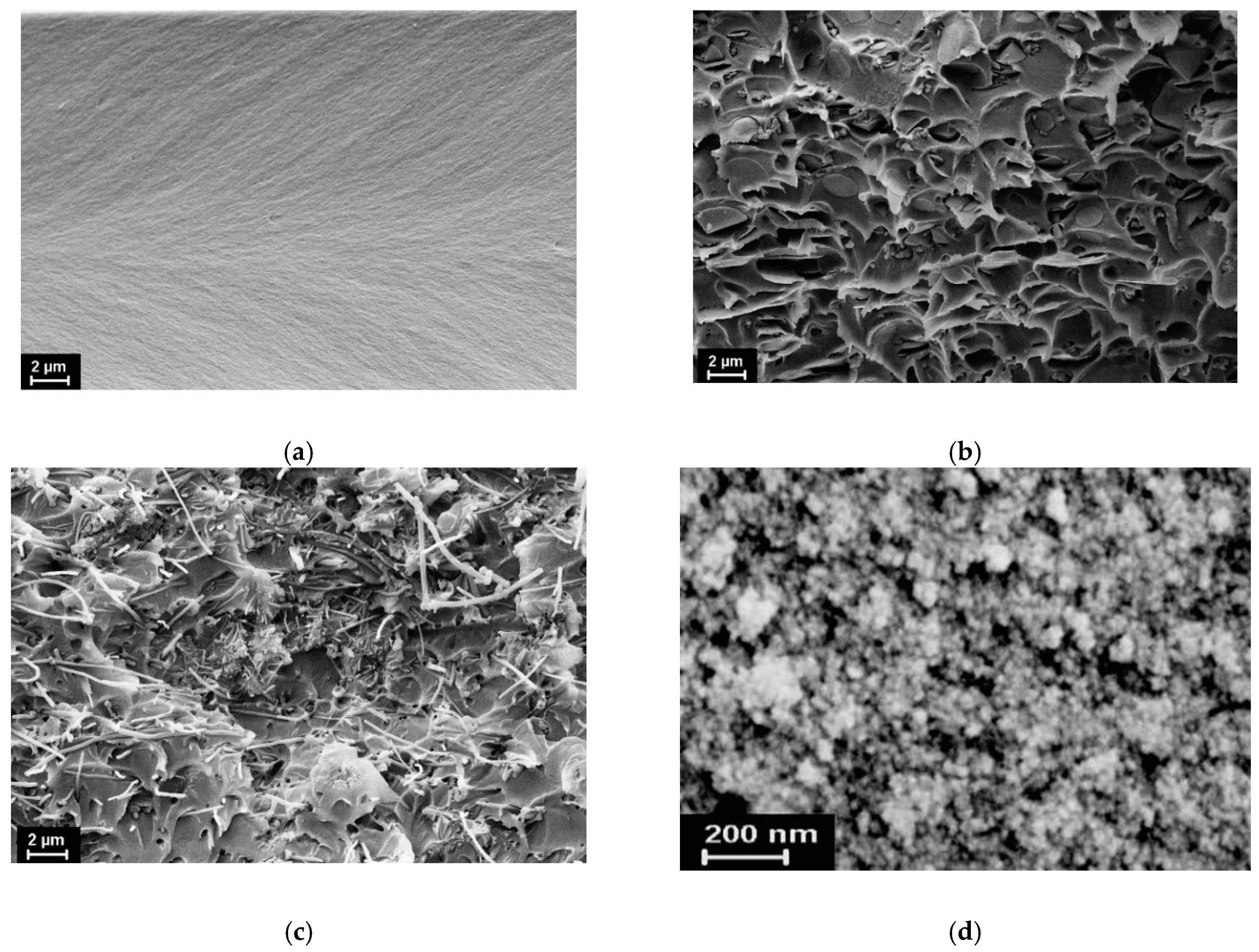
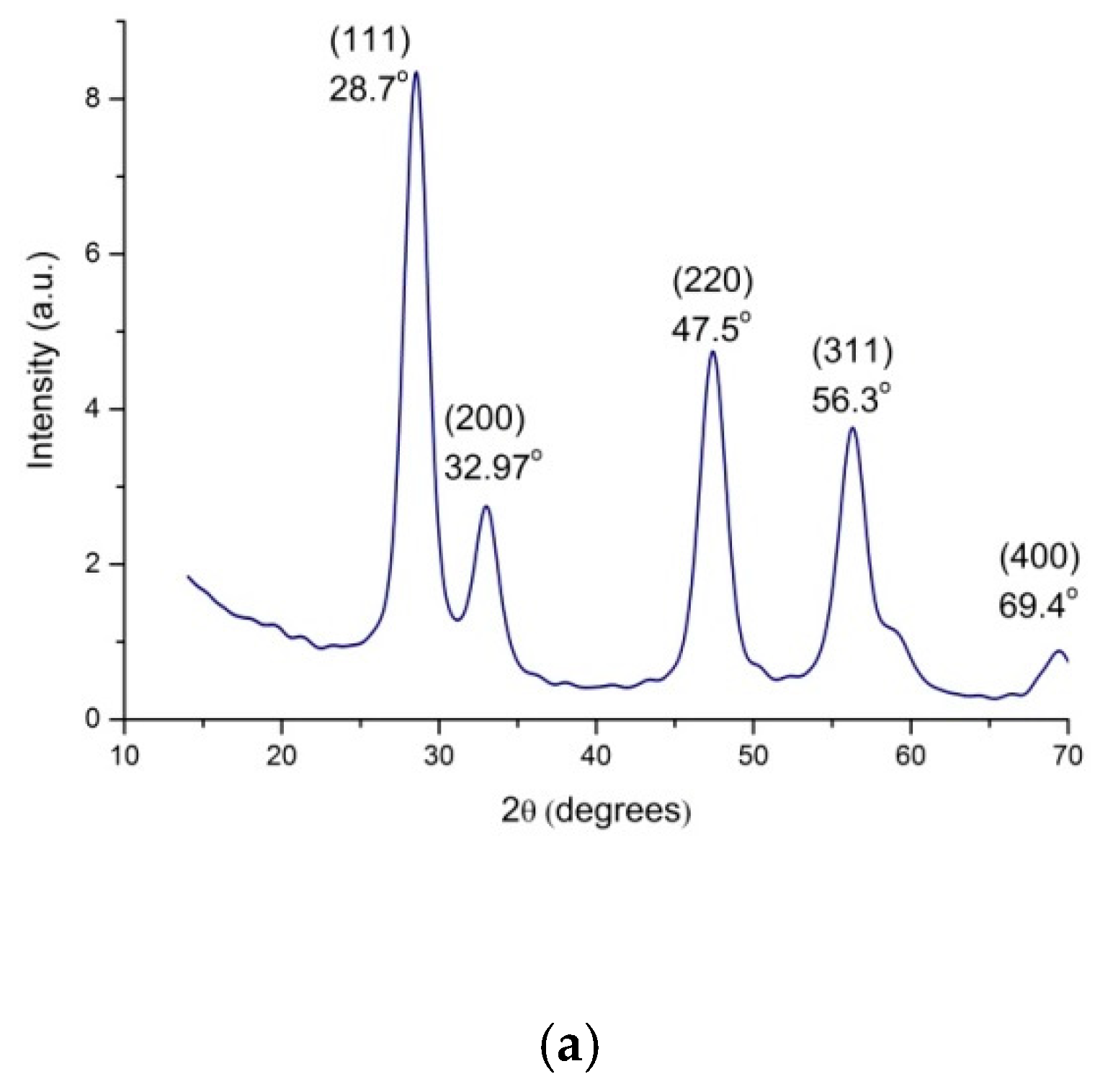
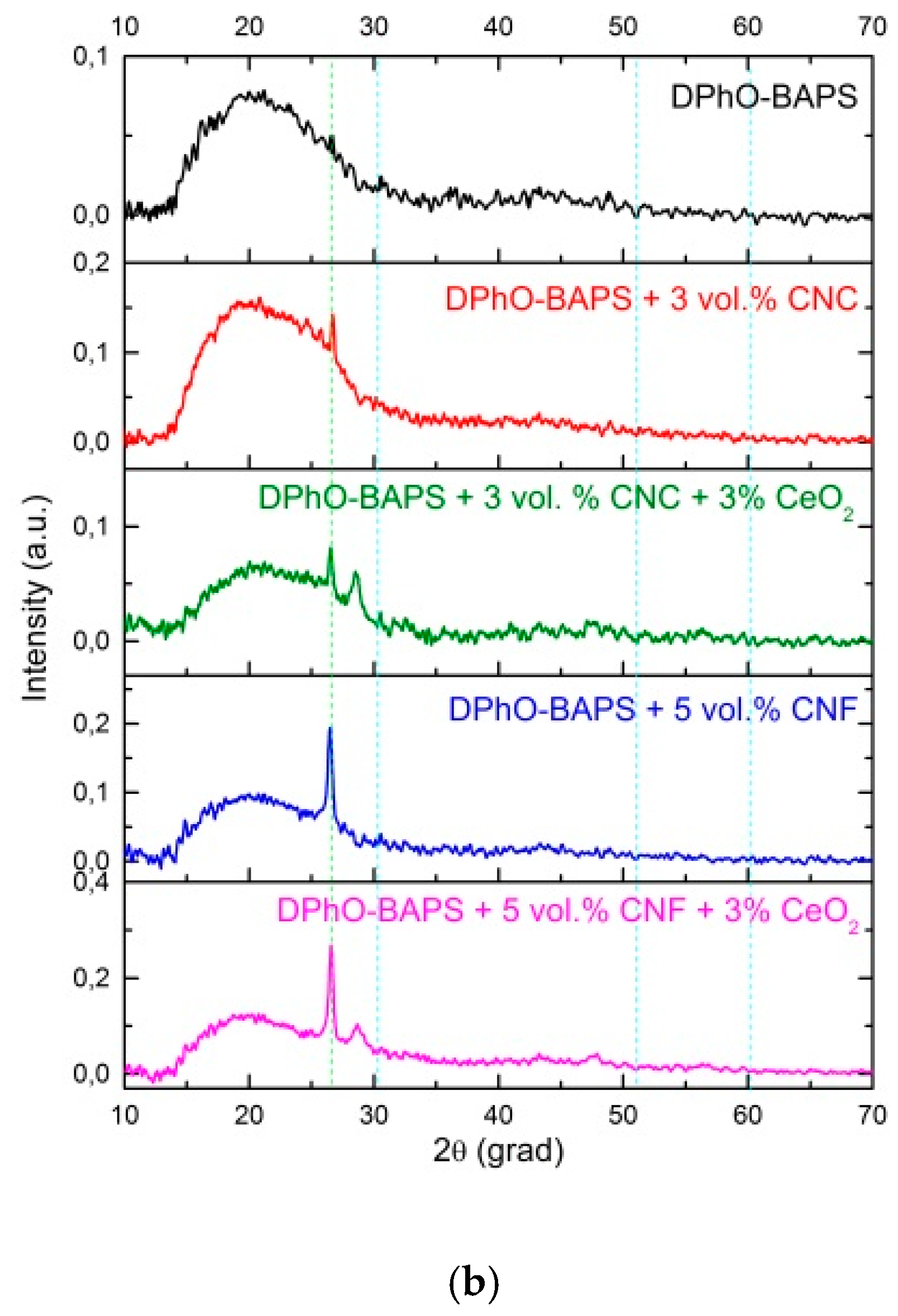
3.2. Investigation of Structure and Morphology
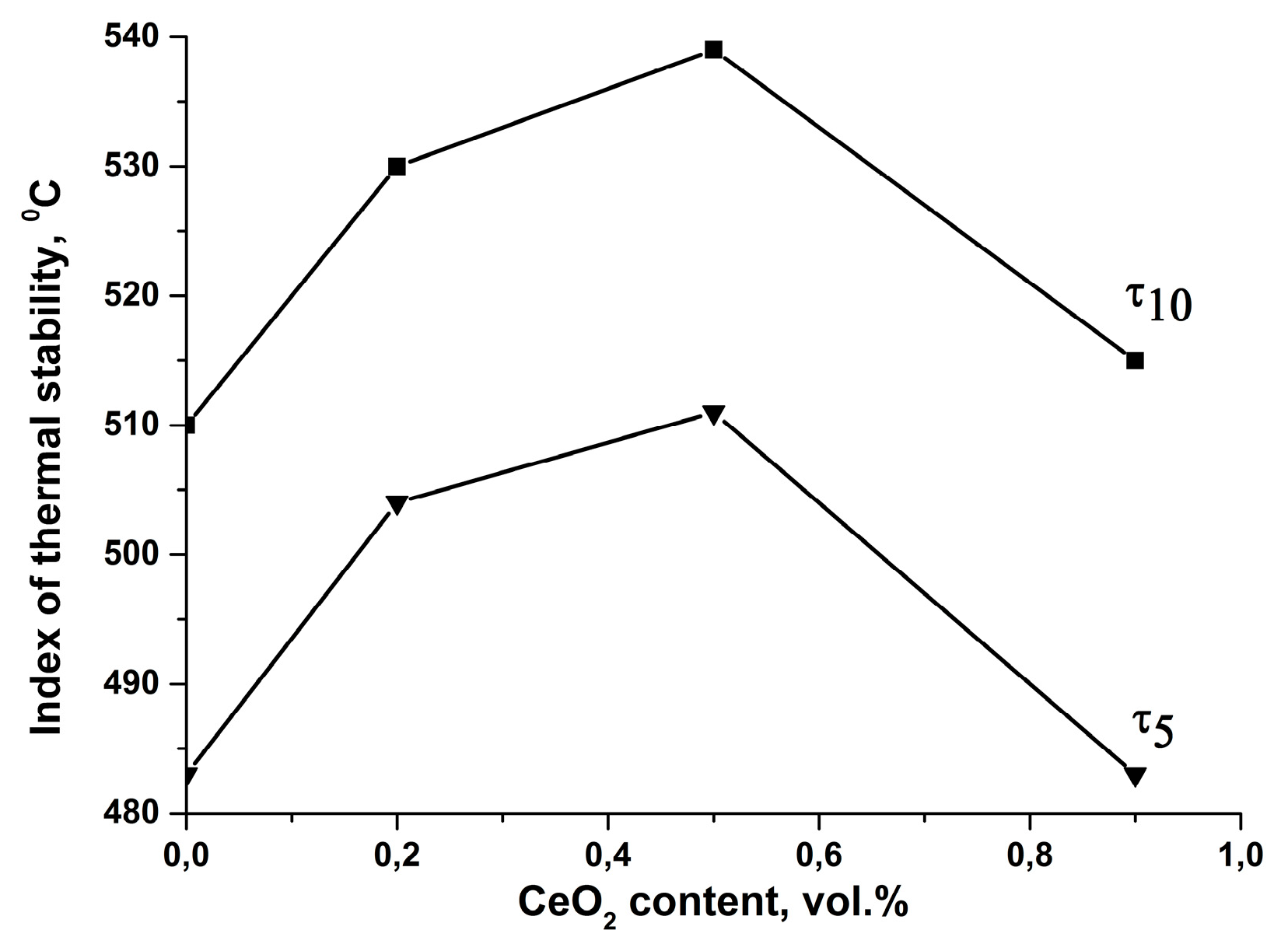
3.3. Thermal Properties
3.4. Thermomechanical Properties
3.4.1. PMDA-Based Compositions
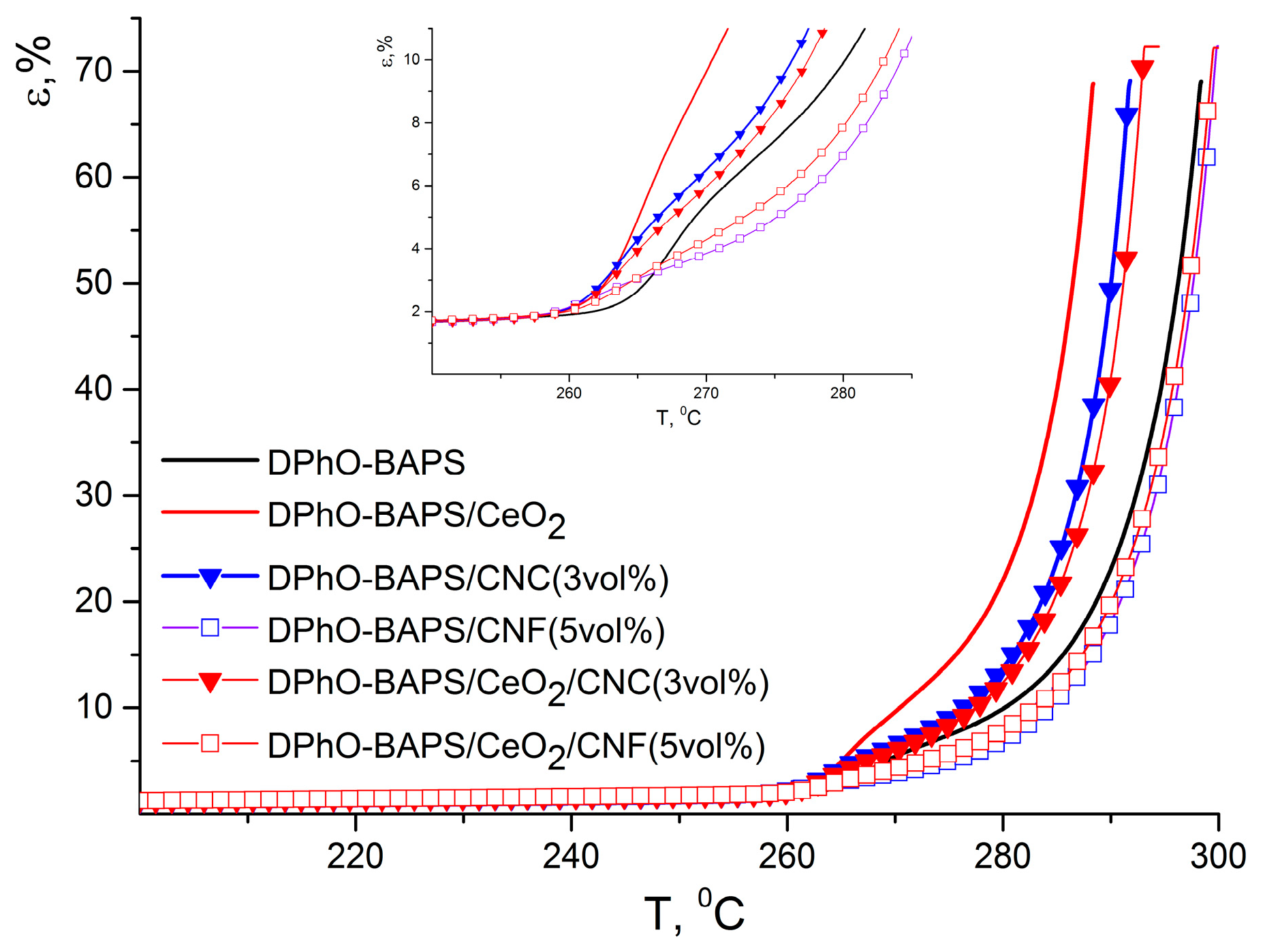
3.4.2. DPhO-BAPS-Based Compositions
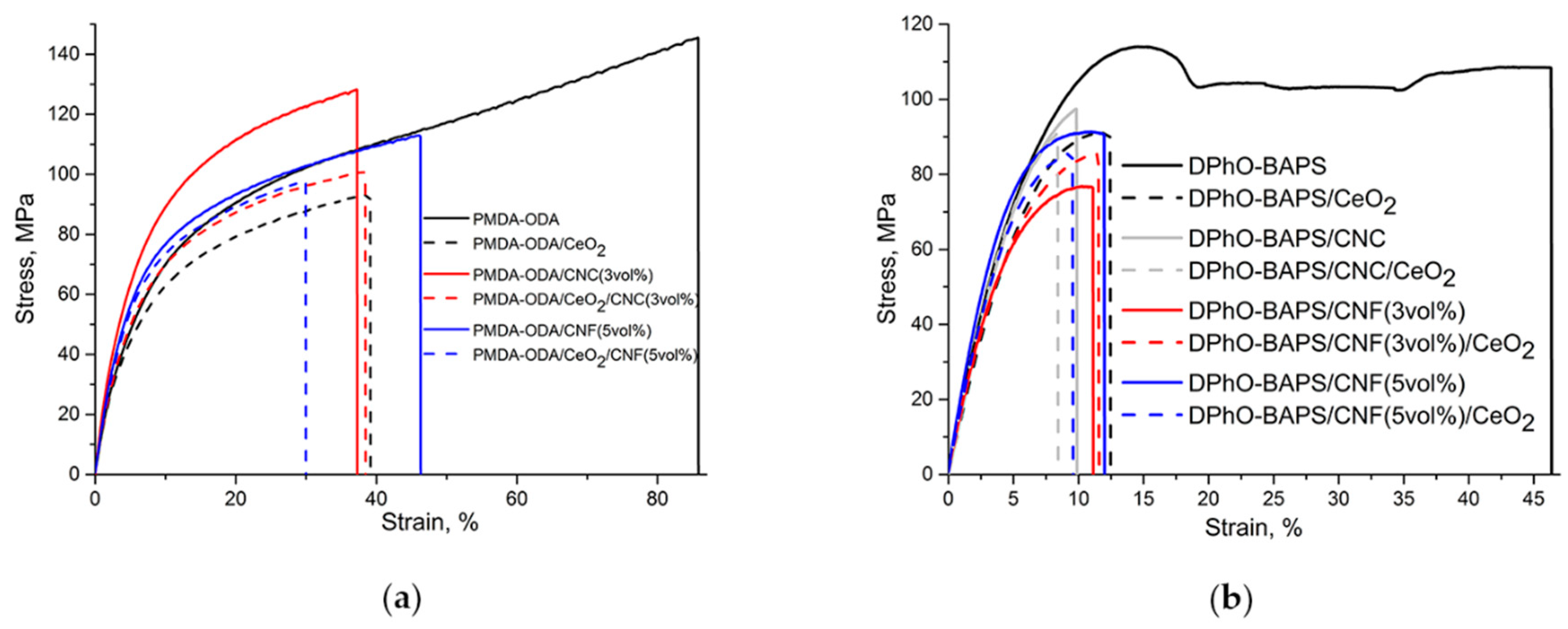
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, X.; Zhang, X.; Zhu, C.; Yu, Y.; Wei, J. Transparent, high glass-transition temperature, shape memory hybrid polyimides based on polyhedral oligomeric silsesquioxane. Polymers 2019, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Rasekh, M.; Rafiee, Z. Preparation and properties of polyimide-based nanocomposites containing functionalized Fe3O4 nanoparticles. High Perform. Polym. 2019, 32, 418–428. [Google Scholar] [CrossRef]
- Malav, J.K.; Rathod, R.; Umare, S.; Patil, A.; Ghugal, S. Production, measurements and anticorrosion properties of electroactive polyimide/tin oxide nanocomposites. Mater. Res. Express 2019, 6, 065306. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Bagheri, R.; Heidarian, P.; Davodi, S.M. Characterization and enhancement of the gas separation properties of mixed matrix membranes: Polyimide with nickel oxide nanoparticles. Chem. Eng. Res. Des. 2020, 153, 789–805. [Google Scholar] [CrossRef]
- Liu, X.; Yin, J.; Kong, Y.; Chen, M.; Feng, Y.; Wu, Z.; Su, B.; Lei, Q. The property and microstructure study of polyimide/nano-TiO2 hybrid films with sandwich structures. Thin Solid Films 2013, 544, 54–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, Y.; Yu, K.; Kang, Y.; Wang, H. Excellent thermal conductivity and dielectric properties of polyimide composites filled with silica coated self-passivated aluminum fibers and nanoparticles. Appl. Phys. Lett. 2013, 102, 1–6. [Google Scholar] [CrossRef]
- Shetty, K.; Jayadev; Raj, K.; Mohan, N. Synthesis, characterization and corrosion studies of polyanailine(PANI)/ceriem dioxide(CeO2) nano composite. In Proceedings of the International Conference on Materials and Manufacturing Methods, Tiruchirapalli, India, 5–7 July 2019. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Aboutalebi, K. Improving the anticorrosive performance of epoxy coatings by embedding various percentages of unmodified and imidazole modified CeO2 nanoparticles. Prog. Org. Coat. 2018, 122, 56–63. [Google Scholar] [CrossRef]
- Murali, A.; Lan, Y.P.; Sarswat, P.K.; Free, M.L. Synthesis of CeO2/reduced graphene oxide nanocomposite for electrochemical determination of ascorbic acid and dopamine and for photocatalytic applications. Mater. Today Chem. 2019, 12, 222–232. [Google Scholar] [CrossRef]
- Hadi, A.; Yaacob, I.I. Synthesis of PdO/CeO2 mixed oxides catalyst for automotive exhaust emissions control. Catal. Today 2004, 96, 165–170. [Google Scholar] [CrossRef]
- Sarıboğa, V.; Faruk Öksüzömer, M.A. Cu-CeO2 anodes for solid oxide fuel cells: Determination of infiltration characteristics. J. Alloys Compd. 2016, 688, 323–331. [Google Scholar] [CrossRef]
- Caputo, F.; De Nicola, M.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, Y.; Cheng, G.; Zhang, C.; Wang, S.; Zhang, J. Cerium Oxide Nanoparticles Protect Endothelial Cells from Apoptosis Induced by Oxidative Stress. Biol. Trace Elem. Res. 2013, 154, 156–166. [Google Scholar] [CrossRef]
- Cai, G.; Xu, S.; Wang, Z.; Wilkie, C.A. Further studies on polystyrene/cerium (IV) oxide system: Melt blending and interaction with montmorillonite. Polym. Adv. Technol. 2014, 25, 217–222. [Google Scholar] [CrossRef]
- Huang, X.; Lin, S.; Shang, J.; He, W.; Lan, J. Mechanical, thermal, and ultraviolet resistance properties of poly(ether-ester)/cerium oxide (CeO2) composite fibers. J. Reinf. Plast. Compos. 2014, 33, 1207–1215. [Google Scholar] [CrossRef]
- Hemalatha, K.S.; Rukmani, K. Synthesis, characterization and optical properties of polyvinyl alcohol-cerium oxide nanocomposite films. RSC Adv. 2016, 6, 74354–74366. [Google Scholar] [CrossRef]
- Gofman, I.; Nikolaeva, A.; Yakimansky, A.; Ivanova, O.; Baranchikov, A.; Ivanov, V. Unexpected selective enhancement of the thermal stability of aromatic polyimide materials by cerium dioxide nanoparticles. Polym. Adv. Technol. 2019, 30, 1518–1524. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Gulii, N.S.; Teplonogova, M.A.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Interplay of polymer matrix and nanosized redox dopant with regard to thermo-oxidative and pyrolytic stability: CeO2 nanoparticles in a milieu of aromatic polyimides. Mater. Today Commun. 2020, 22, 100803. [Google Scholar] [CrossRef]
- Jia, R.P.; Wang, C.F.; Zheng, K.S.; He, X.Y.; Huang, M.S. Preparation, characterization, and properties of CeO2/thermoplastic polyurethane nanocomposites. J. Reinf. Plast. Compos. 2015, 34, 1090–1098. [Google Scholar] [CrossRef]
- Shang, Z.; Lü, C.; Lü, X.; Gao, L. Studies on syntheses and properties of novel CeO2/polyimide nanocomposite films from Ce(Phen)3 complex. Polymer 2007, 48, 4041–4046. [Google Scholar] [CrossRef]
- Rahman, A.; Ali, I.; Al Zahrani, S.M.; Eleithy, R.H. A review of the applications of nanocarbon polymer composites. Nano 2011, 6, 185–203. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, N.; He, C. The superior mechanical and physical properties of nanocarbon reinforced bulk composites achieved by architecture design–A review. Prog. Mater. Sci. 2020, 13, 100672. [Google Scholar] [CrossRef]
- Smirnova, V.E.; Gofman, I.V.; Ivan’Kova, E.M.; Didenko, A.L.; Krestinin, A.V.; Zvereva, G.I.; Svetlichnyi, V.M.; Yudin, V.E. Effect of single-walled carbon nanotubes and carbon nanofibers on the structure and mechanical properties of thermoplastic polyimide matrix films. Polym. Sci.-Ser. A 2013, 55, 268–278. [Google Scholar] [CrossRef]
- Gofman, I.V.; Abalov, I.V.; Gladchenko, S.V.; Afanas’eva, N.V. Carbon nanocones/discs–A new type of filler to improve the thermal and mechanical properties of polymer films. Polym. Adv. Technol. 2012, 23, 408–413. [Google Scholar] [CrossRef]
- So, H.H.; Cho, J.W.; Sahoo, N.G. Effect of carbon nanotubes on mechanical and electrical properties of polyimide/carbon nanotubes nanocomposites. Eur. Polym. J. 2007, 43, 3750–3756. [Google Scholar] [CrossRef]
- Burgaz, E.; Kendirlioglu, C. Thermomechanical behavior and thermal stability of polyurethane rigid nanocomposite foams containing binary nanoparticle mixtures. Polym. Test. 2019, 77, 105930. [Google Scholar] [CrossRef]
- Iqbal, J.; Numan, A.; Ansari, M.O.; Jagadish, P.R.; Jafer, R.; Bashir, S.; Mohamad, S.; Ramesh, K.; Ramesh, S. Facile synthesis of ternary nanocomposite of polypyrrole incorporated with cobalt oxide and silver nanoparticles for high performance supercapattery. Electrochim. Acta 2020, 348, 136313. [Google Scholar] [CrossRef]
- Deepak, A.; Shankar, P. Exploring the properties of lead oxide and tungsten oxide based graphene mixed nanocomposite films. Nanosyst. Phys. Chem. Math. 2016, 7, 502–505. [Google Scholar] [CrossRef]
- Modi, S.H.; Dikovics, K.B.; Gevgilili, H.; Mago, G.; Bartolucci, S.F.; Fisher, F.T.; Kalyon, D.M. Nanocomposites of poly(ether ether ketone) with carbon nanofibers: Effects of dispersion and thermo-oxidative degradation on development of linear viscoelasticity and crystallinity. Polymer 2010, 51, 5236–5244. [Google Scholar] [CrossRef]
- Setua, D.K.; Mordina, B.; Srivastava, A.K.; Roy, D.; Eswara Prasad, N. Chapter 18—Carbon nanofibers-reinforced polymer nanocomposites as efficient microwave absorber. In Micro and Nano Technologies; Han, B., Sharma, S., Nguyen, T.A., Longbiao, L., Bhat, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-819904-6. [Google Scholar]
- Liu, X.; Yue, D.; Yang, C.; Li, N.; Gao, S.; Liu, Y.; Mo, G.; Wu, Z.; Yin, J.; Su, B.; et al. Fluorinated carbon nanofiber/polyimide composites: Electrical, mechanical, and hydrophobic properties. Surf. Coat. Technol. 2019, 361, 206–211. [Google Scholar] [CrossRef]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides–Thermally Stable Polymers; Springer: New York, NY, USA, 1987; ISBN 978-1-4615-7636-5. [Google Scholar]
- Ivanov, V.K.; Kopitsa, G.P.; Baranchikov, A.E.; Grigor’ev, S.V.; Runov, V.V.; Haramus, V.M. Hydrothermal growth of ceria nanoparticles. Russ. J. Inorg. Chem. 2009, 54, 1857–1861. [Google Scholar] [CrossRef]
- Balta-Calleja, F.J.; Vonk, C.G. X-ray Scattering of Synthetic Polymers; Elsevier: New York, NY, USA, 1989; ISBN 978-0444873859. [Google Scholar]
- Kim, B.S.; Bae, S.H.; Park, Y.-H.; Kim, J.-H. Preparation and characterization of polyimide/carbon-nanotube composites. Macromol. Res. 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Lin, C.-T.; Lee, C.-Y.; Chiu, H.-T.; Chin, T.-S. Graphene Structure in Carbon Nanocones and Nanodiscs. Langmuir 2007, 23, 12806–12810. [Google Scholar] [CrossRef] [PubMed]
- Gofman, I.V.; Balik, K.; Cerny, M.; Zaloudkova, M.; Goikhman, M.J.; Yudin, V.E. Peculiarities of the initial stages of carbonization processes in polyimide-based nanocomposite films containing carbon nanoparticles. Cogent Chem. 2015, 1, 1–14. [Google Scholar] [CrossRef]
- Smirnova, V.E.; Gofman, I.V.; Maritcheva, T.A.; Yudin, V.E.; Eto, K.; Takeichi, T.; Kaburagi, Y.; Hishiyama, Y. The effect of different orientations in rigid rod polyimide films on the graphitized products. Carbon N. Y. 2007, 45, 839–846. [Google Scholar] [CrossRef]
- Sidorovich, A.V.; Kallistov, O.V.; Kydryavtsev, V.V.; Lavrent’ev, V.K.; Svetlichnyi, V.M. Nature of Fluidity of Some Polyimides. Vysok. Soedin. A 1985, 27, 1254–1261. [Google Scholar]
- Andrews, R.D. Transition phenomena and solid-state structure in glassy polymers. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 261–265. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Gofman, I.V.; Abalov, I.V.; Tiranov, V.G.; Yudin, V.E. Effect of carbon nanoparticles of different shapes on mechanical properties of aromatic polyimide-based composite films. Polym. Sci. Ser. A 2013, 55, 313–319. [Google Scholar] [CrossRef]
- Qi, Z.; Tan, Y.; Zhang, Z.; Gao, L.; Zhang, C.; Tian, J. Synergistic effect of functionalized graphene oxide and carbon nanotube hybrids on mechanical properties of epoxy composites. RSC Adv. 2018, 8, 38689–38700. [Google Scholar] [CrossRef]
- Tarasov, A.E.; Anokhin, D.V.; Propad, Y.V.; Bersenev, E.A.; Razorenov, S.V.; Garkushin, G.V.; Badamshina, E.R. Synergetic effect of fullerene and graphene oxide nanoparticles on mechanical characteristics of cross-linked polyurethanes under static and dynamic loading. J. Compos. Mater. 2019, 53, 3797–3805. [Google Scholar] [CrossRef]
- Douglas, J.F.; Thorpe, F. Geometrical percolation. Phys. Rev. E 1995, 52, 819–828. [Google Scholar]
- Ardeshana, B.A.; Jani, U.B.; Patel, A.M.; Joshi, A.Y. Investigating the elastic behavior of carbon nanocone reinforced nanocomposites. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2020, 234, 2908–2922. [Google Scholar] [CrossRef]
- Mohamed, A. Chapter Eight—Synthesis, Characterization, and Applications Carbon Nanofibers; Yaragalla, S., Mishra, R., Thomas, S., Kalarikkal, N., Maria, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–257. ISBN 978-0-12-813248-7. [Google Scholar]


| Sample | τ5, °C | τ10, °C |
|---|---|---|
| DPhO-BAPS | 483 | 510 |
| DPhO-BAPS/CeO2 | 511 | 539 |
| DPhO-BAPS/CNF(3 vol%) | 491 | 520 |
| DPhO-BAPS/CNF(3 vol%)/CeO2 | 503 | 532 |
| DPhO-BAPS/CNF(5 vol%) | 478 | 504 |
| DPhO-BAPS/CNF(5 vol%)/CeO2 | 499 | 528 |
| DPhO-BAPS/CNC(3 vol%) | 494 | 524 |
| DPhO-BAPS/CNC(3 vol%)/CeO2 | 502 | 533 |
| PMDA-ODA | 519 | 542 |
| PMDA-ODA/CeO2 | 451 | 472 |
| PMDA-ODA/CNF(5 vol%) | 524 | 546 |
| PMDA-ODA/CNC(3 vol%) | 529 | 553 |
| PMDA-ODA/CNC(3 vol%)/CeO2 | 451 | 473 |
| PMDA-ODA/CNF(5 vol%)/CeO2 | 454 | 477 |
| Sample | Tg, °C | ε, % | ||
|---|---|---|---|---|
| εTg1 | εmax2 | Δε = εmax − εTg | ||
| PMDA-ODA | 397 | 2.9 | 15.6 | 12.7 |
| PMDA-ODA/CeO2 | 397 | 2.6 | 12.2 | 9.6 |
| PMDA-ODA/CNC(3 vol%) | 398 | 2.5 | 7.7 | 5.2 |
| PMDA-ODA/CNF(5 vol%) | 394 | 2.4 | 6.0 | 3.6 |
| PMDA-ODA/CNC(3 vol%)/CeO2 | 395 | 2.4 | 8.7 | 6.3 |
| PMDA-ODA/CNF(5 vol%)/CeO2 | 394 | 2.7 | 5.8 | 3.1 |
| Sample | Tg, °C (DTA) | Tg, °C (TMA) | Tfl, °C 1 |
|---|---|---|---|
| DPhO-BAPS | 257 | 264 | 288 |
| DPhO-BAPS/CeO2 | 255 | 262 | 284 |
| DPhO-BAPS/CNC(3 vol%) | 255 | 260 | 287 |
| DPhO-BAPS/CNC(3 vol%)/CeO2 | 255 | 260 | 288 |
| DPhO-BAPS/CNF(5 vol%) | 255 | 258 | 294 |
| DPhO-BAPS/CNF(5 vol%)/CeO2 | 254 | 260 | 293 |
| Sample | E, GPa | σy, MPa | σb, MPa | εb, % |
|---|---|---|---|---|
| PMDA-ODA | 2.6 | 103 | 145 | 90 |
| PMDA-ODA/CeO2 | 2.1 | 77 | 95 | 39 |
| PMDA-ODA/CNC(3 vol%) | 3.0 | 102 | 113 | 36 |
| PMDA-ODA/CNC(3 vol%)/CeO2 | 2.3 | 80 | 95 | 38 |
| PMDA-ODA/CNF(5 vol%) | 2.9 | 90 | 114 | 44 |
| PMDA-ODA/CNF(5 vol%)/CeO2 | 2.5 | 78 | 98 | 30 |
| Sample | E, GPa | σy, MPa | σb, MPa | εb, % |
|---|---|---|---|---|
| DPhO-BAPS | 2.6 | 104 | 104 | 47 |
| DPhO-BAPS/CeO2 | 2.4 | - | 90 | 12 |
| DPhO-BAPS/CNC(3 vol%) | 2.9 | - | 97 | 10 |
| DPhO-BAPS/CNC(3 vol%)/CeO2 | 2.9 | - | 90 | 8.5 |
| DPhO-BAPS/CNF(3 vol%) | 2.6 | 80 | 76 | 12 |
| DPhO-BAPS/CNF(3 vol%)/CeO2 | 2.8 | - | 89 | 11 |
| DPhO-BAPS/CNF(5 vol%) | 3.1 | 91 | 95 | 11 |
| DPhO-BAPS/CNF(5 vol%)/CeO2 | 3.0 | - | 84 | 9.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Abalov, I.V.; Baranchikov, A.E.; Ivanov, V.K. Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties. Polymers 2020, 12, 1952. https://doi.org/10.3390/polym12091952
Nikolaeva AL, Gofman IV, Yakimansky AV, Ivan’kova EM, Abalov IV, Baranchikov AE, Ivanov VK. Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties. Polymers. 2020; 12(9):1952. https://doi.org/10.3390/polym12091952
Chicago/Turabian StyleNikolaeva, Alexandra L., Iosif V. Gofman, Alexander V. Yakimansky, Elena M. Ivan’kova, Ivan V. Abalov, Alexander E. Baranchikov, and Vladimir K. Ivanov. 2020. "Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties" Polymers 12, no. 9: 1952. https://doi.org/10.3390/polym12091952
APA StyleNikolaeva, A. L., Gofman, I. V., Yakimansky, A. V., Ivan’kova, E. M., Abalov, I. V., Baranchikov, A. E., & Ivanov, V. K. (2020). Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties. Polymers, 12(9), 1952. https://doi.org/10.3390/polym12091952








