Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure of the Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate
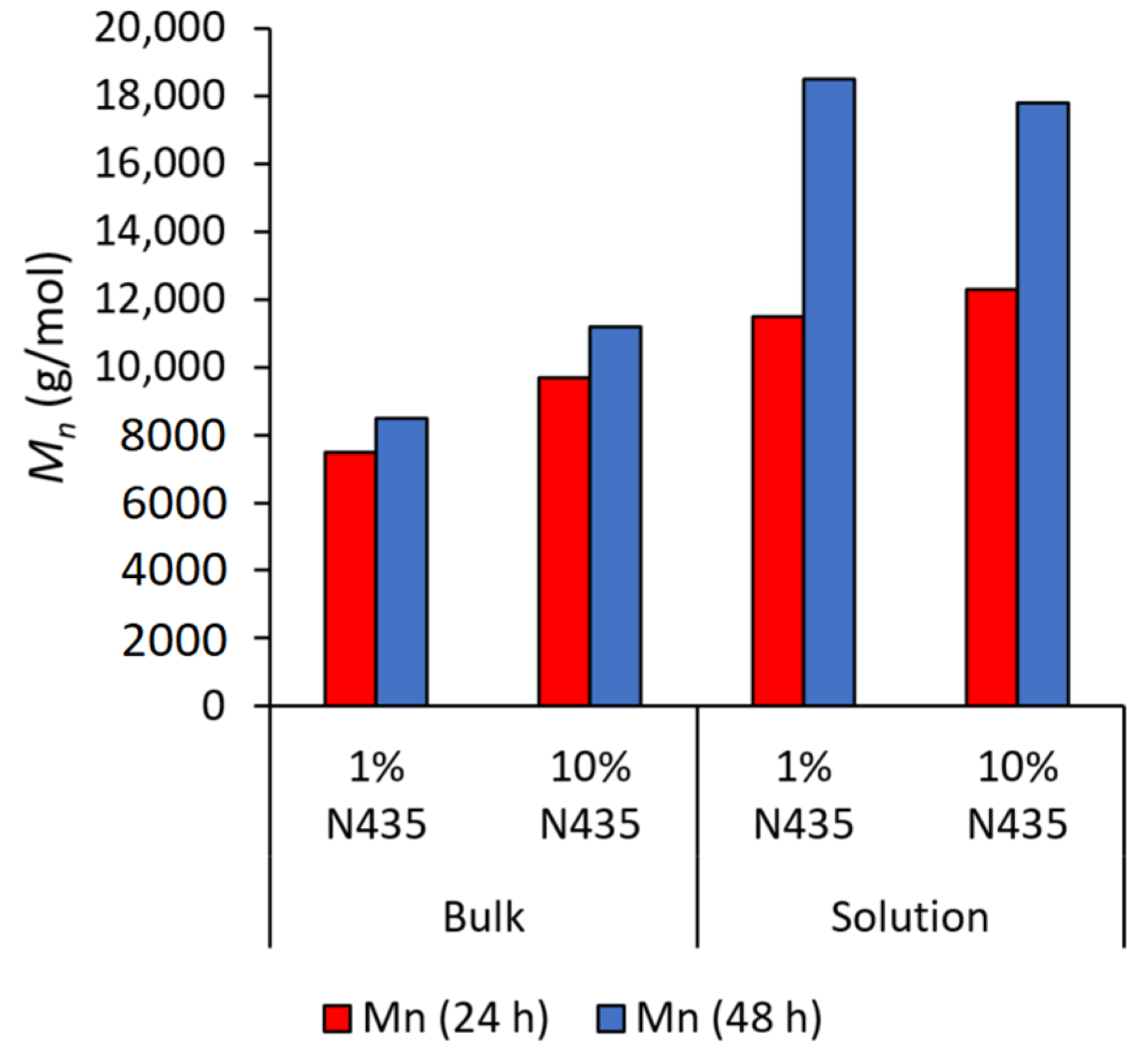
2.3. Effect of Solvent Volume on the Achieved Number-Average Molecular Weight (Mn)
2.4. Effect of the Oligomerization Step Time on Conversion in Solution and Bulk
2.5. Effect of the Oligomerization Step Time on the Achieved Number-Average Molecular Weight (Mn) after 24 h of Post Vacuum Application in Bulk
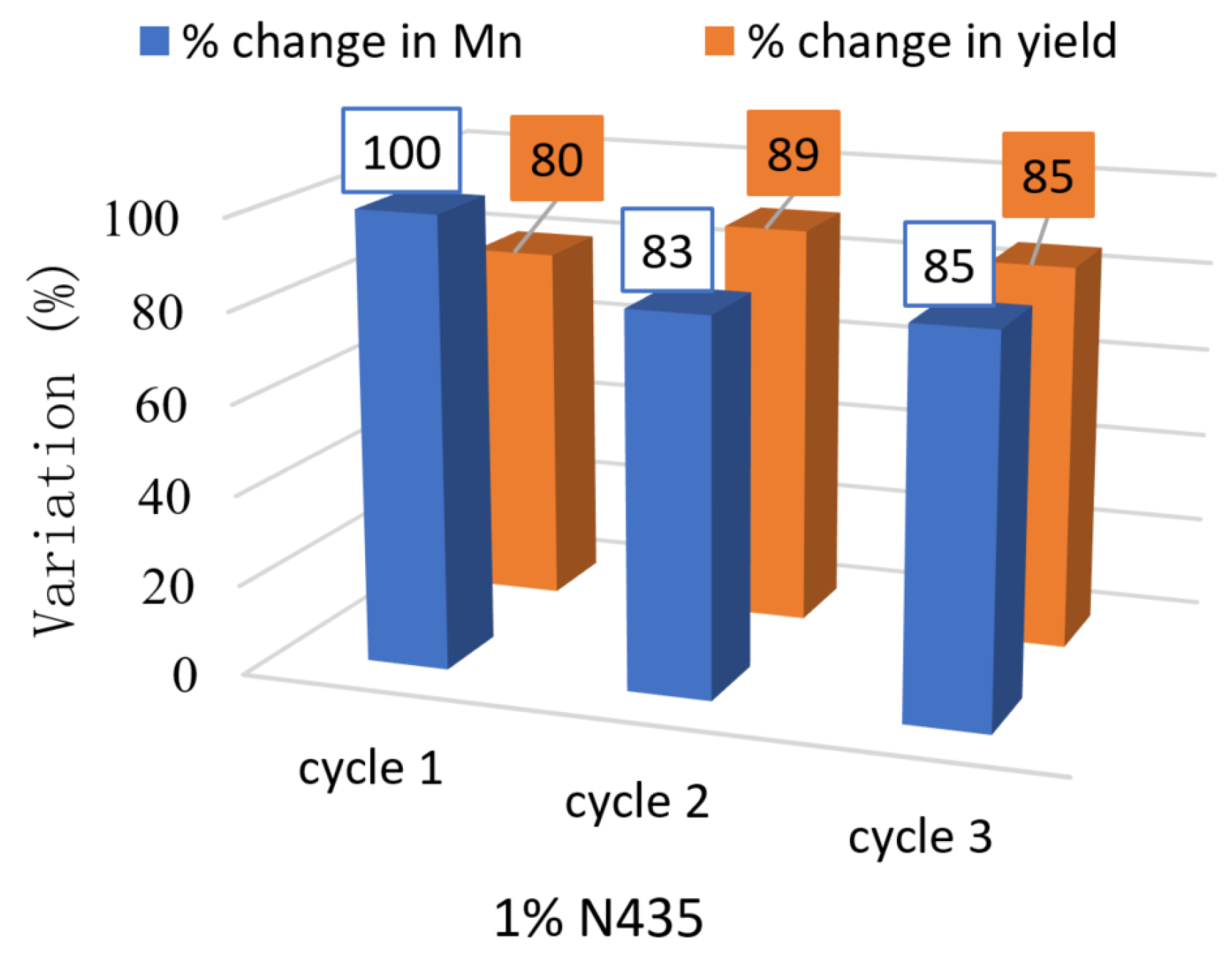
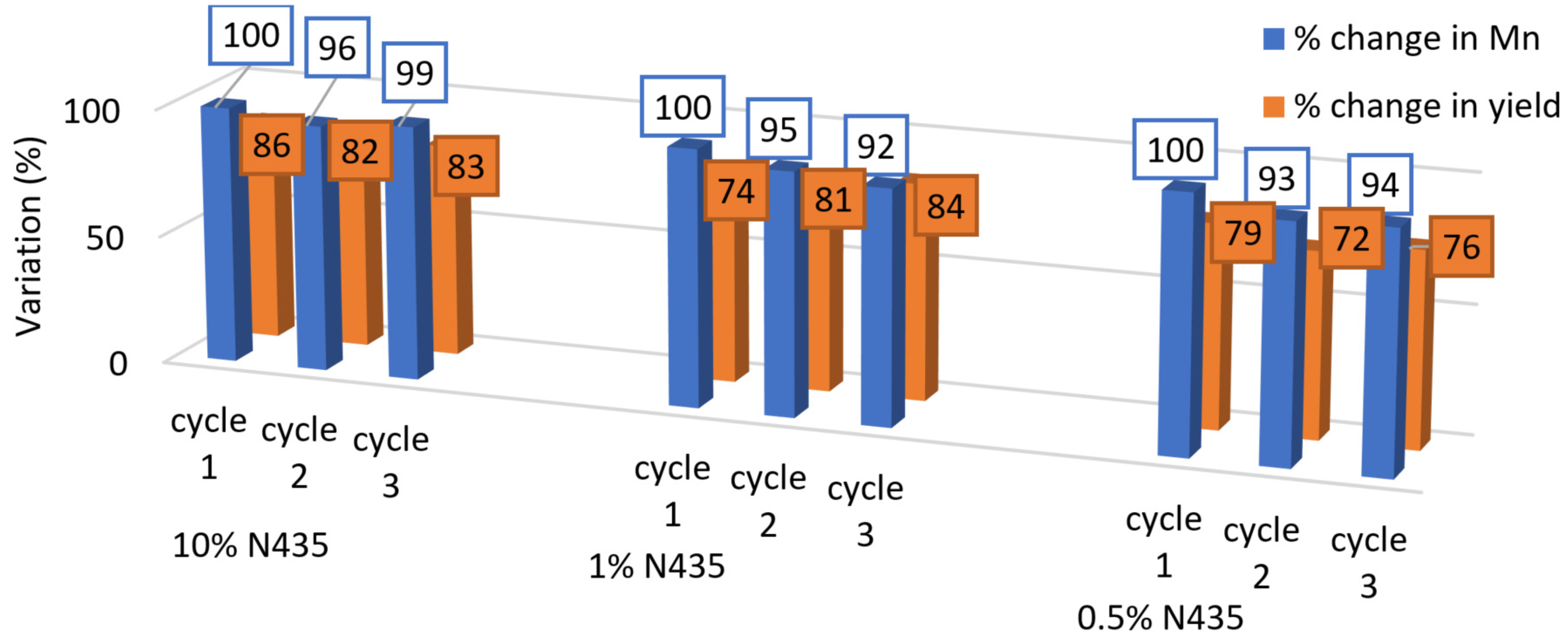
2.6. N435 Recyclability
2.7. Analytical Methods
2.7.1. H NMR Analysis
2.7.2. GPC Analysis
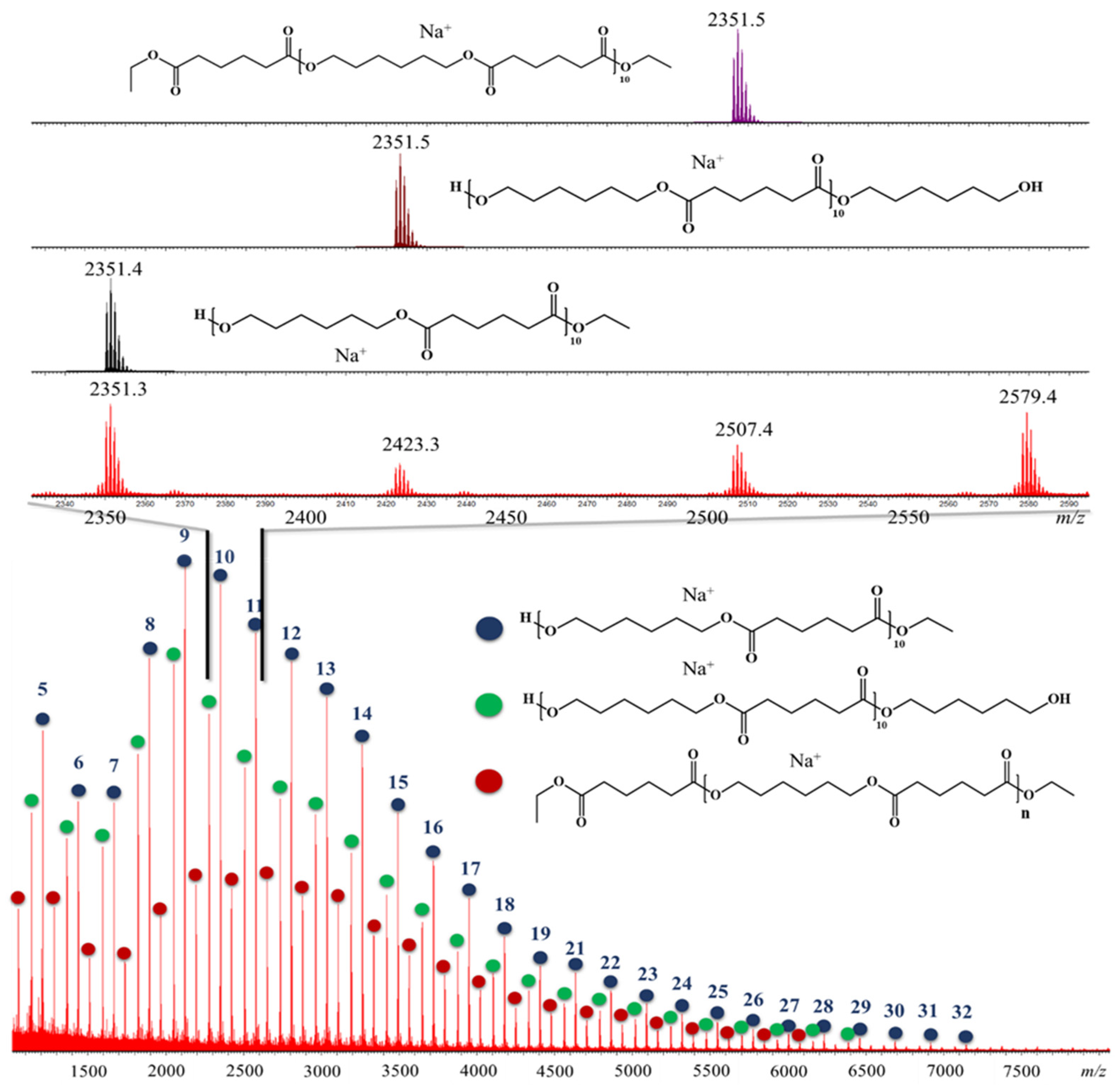
2.7.3. MALDI-MS Analysis
2.8. Statistical Analysis
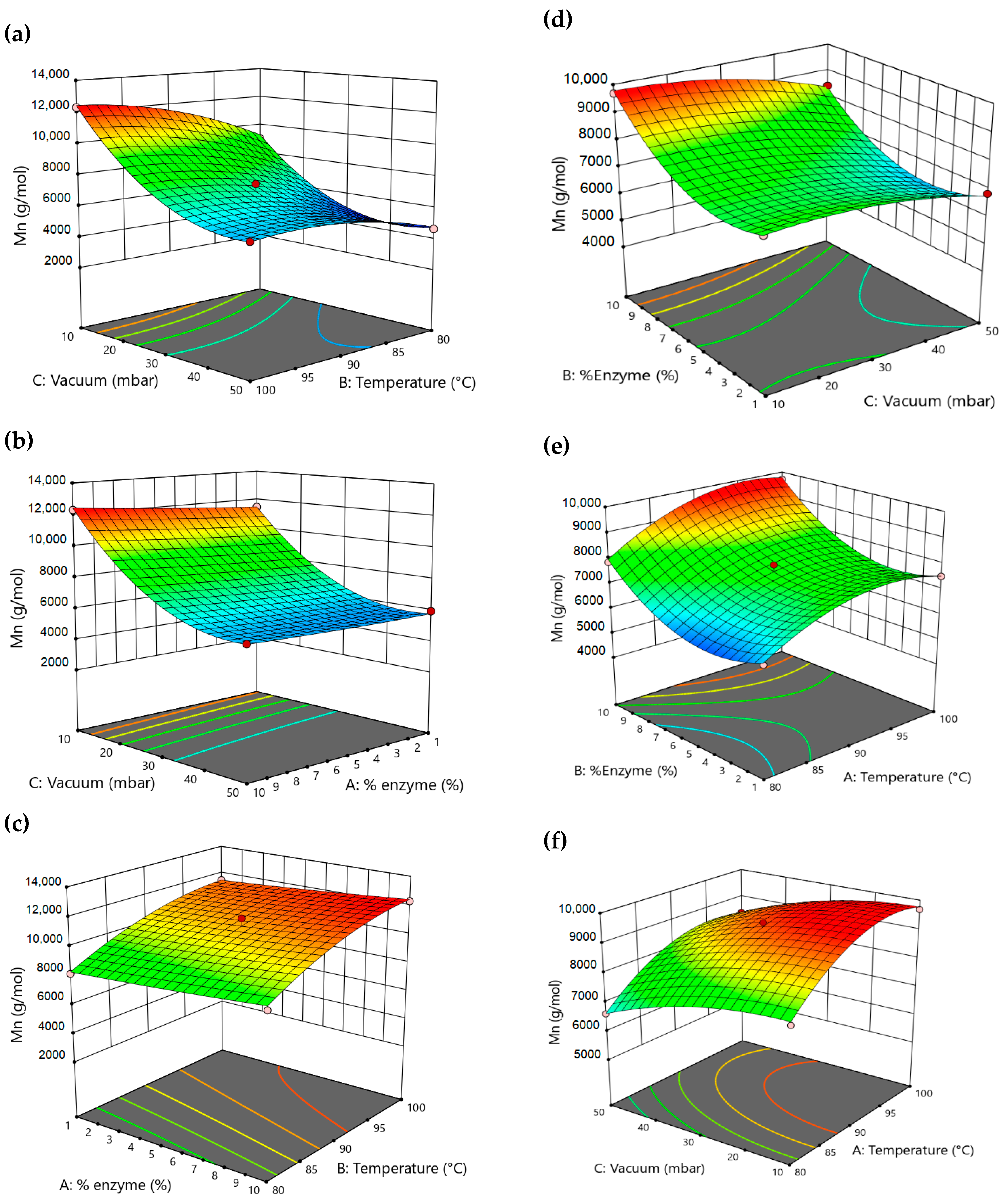
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Aeschelmann, F.; Carus, M. Biobased Building Blocks and Polymers in the World: Capacities, Production, and Applications–Status Quo and Trends Towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Hamaide, T.; Deterre, R.; Feller, J.-F. Environmental Impact of Polymers; ISTE Ltd.: London, UK; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.-M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Fodor, C.; Golkaram, M.; Woortman, A.J.J.; Dijken, J. van; Loos, K. Enzymatic approach for the synthesis of biobased aromatic–aliphatic oligo-/polyesters. Polym. Chem. 2017, 8, 6795–6805. [Google Scholar] [CrossRef]
- Zong, Z.; Xu, J.; Xue, W.; Zeng, Z. Kinetics of the Esterification Reaction of Adipic Acid with 1,6-Hexanediol Catalyzed by Tetrabutyl Titanate. Asian J. Chem. Sci. 2018, 1–11. [Google Scholar] [CrossRef]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Mochizuki, M.; Mukai, K.; Yamada, K.; Ichise, N.; Murase, S.; Iwaya, Y. Structural Effects upon Enzymatic Hydrolysis of Poly(butylene succinate-co-ethylene succinate)s. Macromolecules 1997, 30, 7403–7407. [Google Scholar] [CrossRef]
- Gross, R.A.; Ganesh, M.; Lu, W. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Hilker, I.; Rabani, G.; Verzijl, G.K.M.; Palmans, A.R.A.; Heise, A. Chiral Polyesters by Dynamic Kinetic Resolution Polymerization. Angew. Chem. Int. Ed. 2006, 45, 2130–2132. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Lopez, J.; Beckman, E.J.; Russell, A.J. Biocatalytic Solvent-Free Polymerization To Produce High Molecular Weight Polyesters. Biotechnol. Prog. 1997, 13, 318–325. [Google Scholar] [CrossRef]
- Kirk, O.; Christensen, M.W. Lipases from Candida antarctica: Unique Biocatalysts from a Unique Origin. Org. Process Res. Dev. 2002, 6, 446–451. [Google Scholar] [CrossRef]
- Kobayashi, S. Recent developments in lipase-catalyzed synthesis of polyesters. Macromol. Rapid Commun. 2009, 30, 237–266. [Google Scholar] [CrossRef]
- Lozano, P.; Diego, T. de; Carrié, D.; Vaultier, M.; Iborra, J.L. Lipase Catalysis in Ionic Liquids and Supercritical Carbon Dioxide at 150 °C. Biotechnol. Prog. 2003, 19, 380–382. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; van Ekenstein, G.O.R.A.; Loos, K. A biocatalytic approach towards sustainable furanic–aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies leading to the large scale synthesis of polyesters using enzymes. J. Chem. Soc. Perkin Trans. 1 1999, 2671–2676. [Google Scholar] [CrossRef]
- Azim, H.; Dekhterman, A.; Jiang, Z.; Gross, R.A. Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): Shorter chain building blocks also work. Biomacromolecules 2006, 7, 3093–3097. [Google Scholar] [CrossRef]
- Eriksson, M.; Fogelström, L.; Hult, K.; Malmström, E.; Johansson, M.; Trey, S.; Martinelle, M. Enzymatic one-pot route to telechelic polypentadecalactone epoxide: Synthesis, UV curing, and characterization. Biomacromolecules 2009, 10, 3108–3113. [Google Scholar] [CrossRef]
- Jiang, Z. Lipase-catalyzed synthesis of aliphatic polyesters via copolymerization of lactone, dialkyl diester, and diol. Biomacromolecules 2008, 9, 3246–3251. [Google Scholar] [CrossRef]
- Kulshrestha, A.S.; Gao, W.; Gross, R.A. Glycerol Copolyesters: Control of Branching and Molecular Weight Using a Lipase Catalyst. Macromolecules 2005, 38, 3193–3204. [Google Scholar] [CrossRef]
- Zeng, F.; Yang, X.; Li, D.; Dai, L.; Zhang, X.; Lv, Y.; Wei, Z. Functionalized polyesters derived from glycerol: Selective polycondensation methods toward glycerol-based polyesters by different catalysts. J. Appl. Polym. Sci. 2020, 137, 48574. [Google Scholar] [CrossRef]
- Linko, Y.-Y.; Lämsä, M.; Wu, X.; Uosukainen, E.; Seppälä, J.; Linko, P. Biodegradable products by lipase biocatalysis. J. Biotechnol. 1998, 66, 41–50. [Google Scholar] [CrossRef]
- Linko, Y.-Y.; Wang, Z.-L.; Seppälä, J. Lipase-catalyzed synthesis of poly(1,4-butyl sebacate) from sebacic acid or its derivatives with 1,4-butanediol. J. Biotechnol. 1995, 40, 133–138. [Google Scholar] [CrossRef]
- Kosugi, Y.; Kunieda, T.; Azuma, N. Continual conversion of free fatty acid in rice bran oil to triacylglycerol by immobilized lipase. J. Am. Oil Chem. Soc. 1994, 71, 445–448. [Google Scholar] [CrossRef]
- Poojari, Y.; Palsule, A.S.; Cai, M.; Clarson, S.J.; Gross, R.A. Synthesis of organosiloxane copolymers using enzymatic polyesterification. Eur. Polym. J. 2008, 44, 4139–4145. [Google Scholar] [CrossRef]
- Braiuca, P.; Ebert, C.; Basso, A.; Linda, P.; Gardossi, L. Computational methods to rationalize experimental strategies in biocatalysis. Trends Biotechnol. 2006, 24, 419–425. [Google Scholar] [CrossRef]
- Sarotti, A.M.; Spanevello, R.A.; Suárez, A.G. An efficient microwave-assisted green transformation of cellulose into levoglucosenone. Advantages of the use of an experimental design approach. Green Chem. 2007, 9, 1137–1140. [Google Scholar] [CrossRef]
- Chang, S.-W.; Shaw, J.-F.; Yang, K.-H.; Shih, I.-L.; Hsieh, C.-H.; Shieh, C.-J. Optimal lipase-catalyzed formation of hexyl laurate. Green Chem. 2005, 7, 547–551. [Google Scholar] [CrossRef]
- Itabaiana, I.; Sutili, F.K.; Leite, S.G.F.; Gonçalves, K.M.; Cordeiro, Y.; Leal, I.C.R.; Miranda, L.S.M.; Ojeda, M.; Luque, R.; Souza, R.O.M.A. de Continuous flow valorization of fatty acid waste using silica-immobilized lipases. Green Chem. 2013, 15, 518–524. [Google Scholar] [CrossRef]
- Pellis, A.; Ferrario, V.; Cespugli, M.; Corici, L.; Guarneri, A.; Zartl, B.; Acero, E.H.; Ebert, C.; Guebitz, G.M.; Gardossi, L. Fully renewable polyesters via polycondensation catalyzed by Thermobifida cellulosilytica cutinase 1: An integrated approach. Green Chem. 2017, 19, 490–502. [Google Scholar] [CrossRef]
- Mahapatro, A.; Kalra, B.; Kumar, A.; Gross, R.A. Lipase-catalyzed polycondensations: Effect of substrates and solvent on chain formation, dispersity, and end-group structure. Biomacromolecules 2003, 4, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Carothers, W.H. Polymers and polyfunctionality. Trans. Faraday Soc. 1936, 32, 39–49. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules 2013, 3, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Reaction Mechanisms in Environmental Engineering: Analysis and Prediction; Butterworth-Heinemann: Oxford, UK, 2018; ISBN 978-0-12-800667-2. [Google Scholar]
- Speight, J.G. Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2016; ISBN 978-0-12-800668-9. [Google Scholar]
- Martin, K.; Spickermann, J.; Räder, H.J.; Müllen, K. Why Does Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry Give Incorrect Results for Broad Polymer Distributions? Rapid Commun. Mass Spectrom. 1996, 10, 1471–1474. [Google Scholar] [CrossRef]
- Spinella, S.; Ganesh, M.; Re, G.L.; Zhang, S.; Raquez, J.-M.; Dubois, P.; Gross, R.A. Enzymatic reactive extrusion: Moving towards continuous enzyme-catalysed polyester polymerisation and processing. Green Chem. 2015, 17, 4146–4150. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Secundo, F.; Carrea, G.; Soregaroli, C.; Varinelli, D.; Morrone, R. Activity of different Candida antarctica lipase B formulations in organic solvents. Biotechnol. Bioeng. 2001, 73, 157–163. [Google Scholar] [CrossRef]
- Chamouleau, F.; Coulon, D.; Girardin, M.; Ghoul, M. Influence of water activity and water content on sugar esters lipase-catalyzed synthesis in organic media. J. Mol. Catal. B Enzym. 2001, 11, 949–954. [Google Scholar] [CrossRef]
- Yadav, G.D.; Lathi, P.S. Kinetics and mechanism of synthesis of butyl isobutyrate over immobilised lipases. Biochem. Eng. J. 2003, 16, 245–252. [Google Scholar] [CrossRef]
- Zhao, H.; Nathaniel, G.A.; Merenini, P.C. Enzymatic ring-opening polymerization (ROP) of lactides and lactone in ionic liquids and organic solvents: Digging the controlling factors. RSC Adv. 2017, 7, 48639–48648. [Google Scholar] [CrossRef] [Green Version]
- Mei, Y.; Kumar, A.; Gross, R. Kinetics and Mechanism of Candida antarctica Lipase B Catalyzed Solution Polymerization of ε-Caprolactone. Macromolecules 2003, 36, 5530–5536. [Google Scholar] [CrossRef]


| Entry 1 | Concentration (mol·L−1) | Yield (%) | Mn (g·mol−1) 2 | ĐM 3 |
|---|---|---|---|---|
| 1 | 4 | 88 | 12,300 | 1.44 |
| 2 | 2 | 86 | 10,700 | 1.49 |
| 3 | 1 | 74 | 8300 | 1.30 |
| 4 | 0.5 | 56 | 7400 | 1.18 |
| Entry | Reaction Time (h) 1 1st Step/2nd Step | Yield (%) | Mn (g·mol−1) 2 | ĐM 3 |
|---|---|---|---|---|
| 5 | 2/24 | 88 | 6700 | 1.38 |
| 6 | 4/24 | 86 | 7000 | 1.34 |
| 7 | 6/24 | 74 | 6700 | 1.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, K.; Meimoun, J.; Favrelle-Huret, A.; Winter, J.D.; Raquez, J.-M.; Zinck, P. Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk. Polymers 2020, 12, 1907. https://doi.org/10.3390/polym12091907
Nasr K, Meimoun J, Favrelle-Huret A, Winter JD, Raquez J-M, Zinck P. Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk. Polymers. 2020; 12(9):1907. https://doi.org/10.3390/polym12091907
Chicago/Turabian StyleNasr, Kifah, Julie Meimoun, Audrey Favrelle-Huret, Julien De Winter, Jean-Marie Raquez, and Philippe Zinck. 2020. "Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk" Polymers 12, no. 9: 1907. https://doi.org/10.3390/polym12091907
APA StyleNasr, K., Meimoun, J., Favrelle-Huret, A., Winter, J. D., Raquez, J.-M., & Zinck, P. (2020). Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk. Polymers, 12(9), 1907. https://doi.org/10.3390/polym12091907








