Abstract
From an economic point of view, the spoilage of food products during processing and distribution has a negative impact on the food industry. Lipid oxidation and deterioration caused by the growth of microorganisms are the main problems during storage of food products. In order to reduce losses and extend the shelf-life of food products, the food industry has designed active packaging as an alternative to the traditional type. In the review, the benefits of active packaging materials containing biopolymers (polysaccharides and/or proteins) and active compounds (plant extracts, essential oils, nanofillers, etc.) are highlighted. The antioxidant and antimicrobial activity of this type of film has also been highlighted. In addition, the impact of active packaging on the quality and durability of food products during storage has been described.
1. Introduction
Oxidative reactions and microbiological changes are the main processes causing unwanted changes to the quality of food products, thus affecting their safety. The current challenge is to design packaging materials that would extend the shelf life of foods. Active packaging with antimicrobial and/or antioxidant activity may control these processes, while extending the shelf life of a product. This type of packaging is defined as a packaging system containing ingredients that release or absorb substances into or out of packaged food or the surrounding food environment, to extend its shelf life or to improve the condition of the packaged food. Two aspects support the use of active biopolymer films, compared to the direct incorporation of antioxidant and/or antimicrobial components: (1) the diffusion of active compounds onto the food surface can be controlled, and (2) the amount of conservatives added to food can be reduced [1]. The use of packaging materials based on biopolymers obtained from natural sources, such as proteins and polysaccharides instead of synthetic materials, contributes to reducing the accumulation of non-degradable materials. Biopolymers can be obtained from many natural sources, such as fishery or cattle farming agricultural waste [2]. These types of materials are gaining more and more attention because they are renewable, biodegradable and inexpensive. Biopolymers from natural sources consist of macromolecules, including proteins and polysaccharides, which play an important role in improving the quality of food products. Polysaccharides (chitosan, starch, agar, cellulose, furcellaran, carrageenan, etc.) and proteins (gelatin, alginate, collagen, gluten, whey protein, etc.) are starting to become an interesting alternative to synthetic packaging. However, they have many disadvantages, associated with low strength parameters and a weak barrier against water vapour and oxygen. Nonetheless, these disadvantages may be minimised by combining different biopolymers with one another, including components such as plant extracts or the addition of nanofillers to the film structure, which complement defects in the matrix [3,4].
In this review, the current status of active packaging materials is presented on the basis of biopolymers (proteins and polysaccharides) and active ingredients (plant extracts, essential oils, nanofillers and other compounds). In the article, the antioxidant and antimicrobial properties of active packaging materials are summarised. Finally, the latest research results related to the practical use of active packaging materials in the food industry are reviewed (Figure 1).
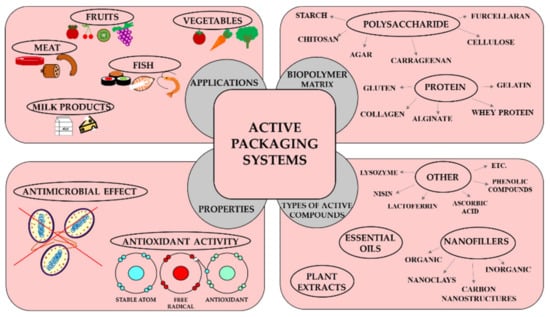
Figure 1.
The active properties of biopolymer films as the main compounds in active packaging materials.
2. Types of Active Compounds in Biopolymer Films and Functional Properties of Active Films
2.1. Antioxidant Agents
Lipid oxidation is one of the main causes of food spoilage. This is especially true in the case of products with high lipid content, such as nuts, fish and vegetable oils. Lipid oxidation results in the formation of toxic aldehydes and the loss of nutritional quality due to the degradation of polyunsaturated fatty acids [5]. Active packaging may assume antioxidant properties thanks to the presence of antioxidants, which prevent the oxidation of food. Antioxidants can further prevent lipid peroxidation via the following mechanisms:
- Preventing chain inhibition by scavenging initiating radicals
- Peroxide decomposition, so they cannot be reconverted into initiating radicals
- Breaking the chain reaction
- Reduction of localised oxygen concentrations and catalysts initiating chain bonds, such as metal ions [6,7].
The growing interest in the use of natural food additives is associated with the need to limit synthetic additives. Many synthetic antioxidants, such as organophosphate and thioester compounds, have been implemented, but due to their migration to food products and their potential toxicity, the use of such additives becomes questionable [5]. Natural antioxidants include active plant components called phenolic compounds, as well as their secondary metabolites [8]. Antioxidant packaging can work in two ways: by releasing antioxidants into a food, and by scavenging undesirable compounds that may appear during a given stage of the food oxidation process. Packaging materials which release antioxidants can discharge active substances into food in a controlled manner. In contrast, scavengers are substances that may react, modify or capture undesirable substances in the packaging environment, but these substances are not released into food [9]. In this section, the influence of adding active ingredients (plant extracts, essential oils, nanofillers and other compounds) on the antioxidant properties of the film is characterised.
2.1.1. Plant Extracts
Plant extracts may be successfully used as antioxidant components in packaging materials. By means of appropriate physical and chemical processing methods, they can be extracted from various types of plant stems, roots, leaves and fruits [10]. Antioxidant activity is determined by compounds such as polyphenols, flavonoids, alkaloids and terpene substances [11,12]. Pomegranate peel contains punicalagin, which is an active component contributing to the antioxidant capacity of this fruit [13]. This type of ingredient improves the antioxidant properties of fish gelatin films [14]. The main phenolic compounds responsible for the antioxidant properties of mango skins are, among others, mangiferin, quercetin, ellagic acid and gallic acid [15]. The addition of mango peel extract to fish gelatin films caused improvement in antioxidant activity. The presence of mangiferin in mango peel extract contributes to the inhibition of auto-oxidation. Additionally, the synergistic effect of bioactive compounds can significantly influence the antioxidant nature of gelatin films [8]. The phenolic compounds—for example, benzoic acid, p-toluic acid, p-salycyclic acid, ferulic acid and aloe emodin—which are present in aloe vera gel caused an increase of the antioxidant activity of gelatin films. Moreover, the presence of lipids, proteins, enzymes and DNA in aloe vera gel affects the strong scavenging effect [16]. Peanut shell and skin extracts were added to starch–chitosan films. The antioxidant capacity of peanut extracts was greater than that of peanut shells, which may be associated with the presence of polyphenols and flavonoids, in particular, linolenic acid, rutin and 4-O-caffeoulquinic acid. The inclusion of these compounds caused a high DPPH· (2,2-diphenyl-1-picryl-hydrazyl) and ABTS+ (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging rate [11]. Chinese hawthorn fruit extract was added to chitosan–gelatin films. The obtained films showed strong free radical scavenging ability (33.42–84.40%, measured using the DPPH· method), which is associated with the presence of polyphenols (epicatechin, chlorogenic acid and procyanidin B2) [17]. Lignocellulose agricultural products, such as rice or coffee skins, are rich in polyphenols, combined with hemicellulose or lignin fractions, which have antimicrobial or antioxidant activity. Collazo-Bigliardi et al. (2019) added cellulose fibres and active fractions extracted from rice and coffee husks to thermoplastic starch films. The extracts demonstrated antioxidant activity (5.37–5.29 mg extract solids/mg DPPH·). Starch films with the addition of coffee husk showed greater antioxidant activity than films with rice husks [18]. Sunflower husk extract was also added to the starch films and its addition caused an increase in antioxidant activity. What is noteworthy is that the process of obtaining the film did not reduce the antiradical activity of the extract. Phenolic compounds, in particular chlorogenic acid and small amounts of coumaric and ferulic acid derivates, mono-caffeoylquinic and dicaffeoylquinic acid derivates, are responsible for the antioxidant activity of the sunflower husk extract [19]. Jirdi et al. (2019) developed gelatin films with phenolic orange peel extracts and examined the effect of drying on the quality of orange (Citrus sinensis) peel extracts. In research, it has been shown that fresh extracts have higher antioxidant potential [1.38-Ferric (Fe3+) reducing power; 95.76%—β-carotene bleaching inhibition and 99.7%—DPPH· method] than thermally dried orange peel extracts. High antioxidant activity is associated with the presence of quinic acid, rutin, trans-ferulic acid, naringenin and 4,5-di-O-caffeoylquinate. Fresh extracts have antioxidant potential via acting as a hydrogen/electron donating agent, or by chelating metals, which interrupts the oxidative chain reaction [20].
2.1.2. Essential Oils
Another aspect involves the incorporation of essential oils (EO) into the biopolymer matrix which can improve the antioxidant properties of packaging materials. The Food Drug Administration (FDA) considers EOs to be “generally recognized as safe” (GRAS) chemicals, which may be used as food additives in preservatives and flavouring. However, due to their intense aroma and potential side effects associated with EO sensitisation, they cannot be added directly to food. Therefore, the incorporation of essential oils into biopolymer films seems to be an interesting way to overcome these limitations [21]. There are many works in which the effects of plant essential oil addition on the antioxidant properties of biopolymer films have been investigated. Bonilla et al. (2018) incorporated ginger essential oil and eugenol into different film formulations (gelatin, chitosan, gelatin–chitosan). The gelatin–chitosan films with eugenol exhibited the best antioxidant activity (TEAC method). Eugenol has the ability to trap chain-carrying peroxy radicals (ROO·) by donation of the phenolic hydrogen atom reaction. The unsaturated double bond is responsible for the radical scavenging action of the eugenol radical [22]. In a different study, ginger essential oil was incorporated into gelatin and gelatin–montmorillonite films. The authors showed that the antioxidant potential of the films depended on the presence of the ginger essential oil [23]. Further, the biopolymer matrix plays the appropriate role of a carrier for active substances and, when properly designed, it allows for the most advantageous controlled release of the active compound. Xu et al. (2019) derived chitosan (CS) –gum arabic (GA) films with different biopolymer ratios (CS:GA—1:0; 1:0.25; 1:0.5; 1:1; 1:2; 1:4). Then, they added essential oil (8%) and assessed the effect of different biopolymer contents on the antioxidant activity of the film. The authors observed that the appropriate ratio of chitosan to gum arabic (from 1:0.25 to 1:2) results in higher retention and controlled release of the oil, which leads to the improved effect of oil as an antioxidant. In contrast, the design of the matrix in a ratio of 1:4 causes a decrease in antioxidant activity, which is associated with large losses of oil [24]. Similar conclusions were reached by Valizadeh et al. (2019), who added cinnamon essential oil (CEO), oleic acid (OA) and glutaraldehyde (GL) to chitosan–carboxymethyl cellulose (CS–CMC) composite films. Controlled release of the oil occurred with an antioxidant effect by trapping the essential oil droplets in the cross-linked matrix. Furthermore, it can be stated that cross-linking prevents the initial release of bioactive additives [25]. Echeverría et al. (2016) concluded that the addition of montmorillonite to soy protein films induces the polyphenols contained in clove essential oil to become more reactive against radical scavenging. In addition, the presence of nanoclay promotes the release of the essential oil’s active substances [26]. In Table 1, recent studies on biopolymer films with different antioxidant compounds are presented.

Table 1.
Recent studies on biopolymer films with antioxidant activity.
2.1.3. Nanofillers
The addition of nanostructures also has a positive effect on improving the antioxidant activity of biopolymer films. Roy et al. (2020) enriched chitosan films with the addition of melanin nanoparticles. Both synthetic and natural melanin have antioxidant potential. The incorporation of melanin into chitosan films caused a significant increase in antioxidant activity (by ~163% and by ~158%, measured via the DPPH· and ABTS+ methods, respectively). The antioxidant potential of melanin is associated with its ability to provide and receive an electron that can interact with free radicals through the process of electron transfer and, consequently, neutralise them [38]. Zhang and Zhao (2017) added rutin nanoparticles to zein films. Along with the increase in the concentration of rutin nanoparticles, the antioxidant properties of zein films also rose by ~320% (DPPH· method), by ~534% (ABTS+ method) and by ~86% (phosphomolybdenum method) [39]. In addition, some metal nanoparticles exhibit antioxidant properties. Boughriba et al. (2020) incorporated TiO2–Ag nanoparticles into Rhinobatos cemiculus gelatin films, and noted that the addition of nanostructures increased the antioxidant activity of the film evaluated via DPPH· radical scavenging activity (by ~167%) and ferrous chelating activity (by ~174%) [40].
2.1.4. Other Antioxidant Compounds
There are many antioxidant additives that can be incorporated into the biopolymer matrix to provide active properties to packaging materials. Kchaou et al. (2020) added cuttlefish (Sepia officinalis) skin protein isolate (CSPI) and hydrolysates (CSPH) to gelatin films. Free-form CSPI showed a higher radical scavenging activity than CSPH, which may be related to the difference in their molecular weights and solubility in the ethanol solution. The authors noted that CSPH demonstrated higher antioxidant activity (measured by the DPPH method) in the gelatin matrix than in the free form, which may be attributed to the presence of protein–protein interactions or hydrogen bonds between the gelatin matrix and the added peptides [41]. Tomadoni et al. (2019) incorporated vanillin into chitosan films, which caused a significant increase in antioxidant activity [42]. Moreover, Lu and Liu (2020) added a hexahydro-β-acid/2-O-methyl-β-cyclodextrin complex to chitosan films, and the noted increase in the scavenging effect was attributed to the good antioxidant activity of hexahydro-β-acid [43]. The addition of α-tocopherol in the form of a nanocapsule suspension improved the antioxidant activity of methylcellulose films [44]. Natamycin and/or nanoemulsioned α-tocopherol were added to the whey protein films. Only films with α-tocopherol exhibited antioxidant activity (measured by the ABTS and DPPH methods), which can be attributed to the properties of α-tocopherol. This antioxidant agent can donate a hydrogen atom to quench free radicals by creating α-tocopheroxyl radicals until decolourisation of ABTS and DPPH radicals. Films with natamycin do not have antioxidant properties, while films with natamycin and α-tocopherol have a lower activity than films with α-tocopherol. Such results may be attributed to the antagonistic effects of these two agents [45]. Analogous results were observed in the study of cassava starch–chitosan films with pitanga (Eugenia uniflora L.) leaf extract (PE) and/or natamycin (NA). Both the addition of PE and NA to the film caused an increase in antioxidant activity. On the other hand, the addition of PE and NA to cassava starch–chitosan films reduced scavenging activity, which may be attributed to the antagonistic effect associated with interactions between these active ingredients [36].
2.2. Antimicrobial Agents
The incorporation of antimicrobial substances into the biopolymer matrix becomes a valuable strategy for extending the shelf life of various packaged food products. The potential mechanisms of the active ingredient in the antimicrobial action include: (1) membrane rupture with inhibition of ATPase activity; (2) leakage of necessary biomolecules from the cell; (3) disturbance of the proton motive force; (4) inactivation of the enzyme [46].
When designing this type of packaging, it is necessary to establish balance between the kinetics of microbial growth and the controlled release rate of antibacterial agents. There are four categories of antimicrobial packaging systems: (1) The incorporation of volatile antimicrobial substances into a sachet/pad within the packaging; (2) direct incorporation of an antimicrobial agent into the packaging film, for example, by co-extrusion of synthetic films with antimicrobials. However, heat treatment causes high loss of bioactive compounds, and thus other methods that do not use heat treatment, such as casting, electrospinning or solvent mixing, may be an alternative; (3) Packaging coating with a matrix, such as polysaccharides, that acts as a carrier for the antimicrobial agents, so that the active substances can be released onto the food surface by evaporation into free space (volatile substances) or migration to food (non-volatile substances) through diffusion; (4) The use of polymers that have inherent antimicrobial effects, e.g., chitosan [47,48]. In this section, the effects of active ingredients (plant extract, essential oils, nanofillers and other compounds) on the antimicrobial properties of films based on proteins and polysaccharides are described.
2.2.1. Plant Extract
Various plants may comprise a source of antimicrobial compounds to be used during microbial contamination [49]. Phenolic compounds are more effective against Gram-positive than Gram-negative bacteria [50]. Hannani et al. (2019) obtained fish gelatin films with pomegranate peel powder, which showed greater antimicrobial activity against S. aureus (7 mm of inhibition zone) and L. monocytogenes (5.13 mm of inhibition zone) than E. coli (4.13 mm of inhibition zone) [14]. Similar results were observed in gelatin–polyethylene bilayer films. The addition of pomegranate peel increased the antimicrobial activity against B. cereus (from 0 to 12.50 mm of inhibition zone), L. monocytogenes (from 0 to 16.50 mm), S. thyphi (from 0 to 14 mm) and E. coli (from 0 to 8 mm). In this work, other active ingredients were also added to this type of film: papaya and jackfruit. Only in the case of S. thyphi did this type of film show activity (papaya from 0 to 14 mm inhibition zone, and jackfruit from 0 to 14.50 mm). Pomegranate peel has polyphenols, in particular tannins, which have the ability to precipitate protein, and this causes leakage of the cell membrane and, consequently, cell lysis [51,52,53]. In many works, the positive effects of plant extract addition on the antimicrobial properties of the investigated films have been demonstrated [54]. The presence of flavonoids (mainly 7-O-β-glucoside luteolin and 7-O-β-glucoside) in Sonneratia caseolaris L. caused an increase in the antimicrobial effects of chitosan films against P. aerugino [55]. Nouri et al. (2018) noted that the addition of Rosmarinus officinalis L. extract to the biopolymer matrix increases the antimicrobial activity of the κ-carrageenan/nanoclay film. Compared to the control film, this type exhibits > 99% inhibition against B. cereus, E. coli, P. aeruginosa and S. aureus [21].
2.2.2. Essential Oils
Essential oils are a source of bioactive compounds, such as terpenoids and phenolic, that are recognised as antimicrobial agents. Essential oils attack microbial cells through a variety of mechanisms: by attacking the phospholipid bilayer cell membrane, disrupting enzymes, forming fatty acid hydroperoxidase caused by oxygenation of unsaturated fatty acids, and violating the genetic material of bacteria [56,57,58].The inclusion of essential oils in the structure of the film may be associated with a change in its functional properties, because this type of active ingredient can interact with both the polymer and the plasticiser, which reduces diffusion into the product. In addition, essential oils are quite volatile, and many processes, e.g., high temperature processing, can degrade them. A solution to this problem could be microencapsulation or film-forming processes that allow isolation, transport and controlled release. Benavides et al. (2012) noted that the inhibitory effect of oregano essential oil incorporated in alginate film was lower than that of pure essential oil. The reason for this may be the partial loss of volatile compounds during film preparation. In addition, interactions between the hydroxyl groups of phenolic compounds and polymer chains may be the reason for the slower diffusion of essential oil phenolic compounds [59]. The release of antimicrobial agents from the film depends on the electrostatic interactions between the antimicrobial agent and the biopolymer chains, structural changes caused by the presence of the microbial agent, osmosis, and environmental conditions [56,58]. dos Santos Paglione et al. (2019) compared the effects of adding free oregano essential oil and oregano oil in microcapsules on the functional properties of soy protein concentrate films. The results indicated that the soy protein concentrate films with microencapsulated essential oil showed better microbiological activity than the films with free essential oil. However, it should be noted that oregano essential oil, in the form of microcapsules among the film, exhibited lower antioxidant activity than the free oil in the soy protein concentrate film. The authors attribute this to the presence of a solvent, which is used to extract antioxidant compounds and modify the release profile of oils from the film to the release medium [60]. Rosemary essential oil, extracted from two Tunisian varieties, namely Zaghouan (ZG; North Tunisia; R. officinalis var. Typicus) and Chaab Tweel (CT; South Tunisia; R. officinalis var. Troglodiytarum), was added to the gelatin–chitosan–pectin film. Improvement in antimicrobial activity was observed against B. subtilis, S. aureus, E. aerogenes, E. faecalis and E. coli. These results indicated that the films with ZG essential oil had higher activity compared to films with CT [61].
2.2.3. Nanofillers
The nanostructures added to the biopolymer matrix had an influence on the antimicrobial activity of the films. Amjadi et al. (2019) prepared different types of gelatin films with zinc oxide nanoparticles (ZnONPs) and/or chitosan nanofiber. The gelatin films and those with chitosan nanofiber did exhibit any heavy antimicrobial activity. The addition of ZnONPs caused improvement in the antimicrobial effects of gelatin films against E. coli, S. aureus and P. aeruginosa. Higher values of the inhibition zone could be observed in gelatin films with ZnONPs + chitosan nanofiber, which proves the synergistic effect of ZnONPs and chitosan nanofiber on the antimicrobial properties of the gelatin matrix [62]. The type of nanofiber in the nanohybrid also affects the antimicrobial activity of the film. Almasi et al. (2018) noted that the addition of bacterial cellulose nanofiber decreased the antimicrobial activity of CuONPs, while chitosan nanofibres showed no synergistic effect on the antibacterial activity of CuONPs [63]. Peighambardoust et al. (2019) created active starch-based films, incorporating a combination of Ag, ZnO and CuO nanoparticles. The starch films with single nanoparticles (NPs) showed antimicrobial activity against S. aureus and E. coli, but starch films with a combination of NPs had better antimicrobial properties. Potential antimicrobial mechanisms are based on the synergistic action of NPs, and involve the destruction of the cell wall and bacterial DNA damage. Damage to DNA can lead to the disruption of microorganism replication and damage to the ribosome, thereby blocking the production of energy cycle enzymes [64]. The synergistic effect of NPs may consist in the use of various microbial mechanisms by specific NPs. When one type of NPs damages the cell membrane, the other type of NPs can affect the DNA of bacteria [65].
There are two techniques for producing nanocomposite films. The first method (in situ) involves the use of a biopolymer matrix as a reaction medium to form nanostructures, acting on them as a stabilising agent. The second method (ex situ) is used as a dispersion and stabilising medium for separately pre-synthesized nanostructures [66]. Biopolymers can also constitute a matrix for obtaining stable nanostructures, while eliminating the use of environmentally harmful solvents and reducing agents. Such a matrix may be negatively charged furcellaran, which is obtained from Furcellaria lumbricalis red algae. This sulfated polysaccharide was used to obtain silver nanoparticles (AgNPs) with antibacterial activity against E. coli, E. facealis, P. aeruginosa, S. aureus and C. albicans. Moreover, nanocomposite films prepared using environmentally-friendly methods showed improved barrier properties against UV radiation and water vapour, as well as very good mechanical properties [67]. The use of nanofillers in films not only affects the active properties of packaging materials, but also has an influence on the controlled release of active substances from biopolymer films. The addition of halloysite to corn starch films, with peptides nisin and pediocin, caused the inhibition zone to be smaller than in the case of active films without halloysite. The presence of this type of nanofiller affects the controlled diffusion or increase of antimicrobial agents by biopolymer matrices [68,69].
In Table 2, information is presented regarding recent studies on active biopolymer films with antimicrobial activity.

Table 2.
A summary of biopolymer films with antimicrobial activity.
2.2.4. Other Antimicrobial Compounds
The cottonseed protein hydrolysate in alginate films has an inhibitory effect against S. aureus, but it does not exhibit any influence on E. coli [30]. The difficulty in accessing bacteria may result from their different morphology. Gram-negative bacteria have a cellular envelope consisting of lipopolysaccharide molecules that act as a barrier [30]. Oleoresin spices have resins in their compositions, and are used in food formulations [77]. Likewise, this type of natural active compound could be used as an antimicrobial agent in food packaging materials [78]. Oleoresin cloves have greater antimicrobial activity against E. coli and S. aureus than nutmeg and black pepper oleoresins when used in gelatin films [78]. Another interesting active additive to the biopolymer matrix is castor oil. Its addition to alginate films affects their antimicrobial properties. The authors reported that castor oil increases the antimicrobial properties of the film against Gram-positive bacteria (S. aureus—from 0 to 16.97 mm of inhibition zone; B. subtilis—from 0 to 17.30 mm), which is a result of the increased presence of hydroxyl groups affecting the hydrophilic nature of the films. This property facilitates the dissolving processes in the bacterial membrane, and then aids in the repair of damage. However, this type of film was unable to cope with the extra outer membrane of Gram-negative bacteria (E. coli and S. typhi) [79].
To provide the packaging with increased active properties, a mixture of antimicrobial additives is often used. Sani et al. (2019) developed packaging materials based on chitosan, melissa essential oil and ZnONPs. The presence of citronellal and geraniol compounds in the essential oil affects the antibacterial properties of the packaging material, because the compounds destroy the outer membrane of microorganisms and cause the release of liposaccharides, while increasing the permeability of the cytoplasmic membrane to ATP, which consequently causes cell death [80]. Cumin essential oil (CEO) and titanium dioxide (TiO2NPs) were added to the sodium caseinate–agar film in various concentrations and combinations. The activity of the biopolymer films varied depending on the used active additive and the type of bacteria. Films containing 1% TiO2NPs demonstrated better activity against E. coli, S. enteritidis, L. monocytogenes and S. aureus than films with 2% CEO. In contrast, films with 1% TiO2NPs and 2% CEO exhibited a synergistic effect on antimicrobial activity. This type of CEO activity is associated with the presence of phenolic compounds, such as 1,8-cineole, 4-dien-7-al, cumin aldehyde, γ-terpinene and β-pinene, which have hydrophobic and lipophilic functional groups. In contrast, the activity of TiO2NPs is assigned to the crystal structure, shape, size, and large area to volume ratio [81]. The presence of carvacrol and thymol in Ziziphora clinopodioide essential oil, and lavanols, procyanides and phenolic acid in grape seed extract, was the reason for the increase in the antimicrobial properties of chitosan and gelatin films against L. monocytogenes, S. aureus and B. cereus [82].
Furthermore, the incorporation of active ingredients improves the antifungal properties of biopolymer films. The addition of cottonseed protein hydrolysate to alginate films caused antifungal activity against C. gloeosporioides (14.4–27.02 mm of inhibition zone) and R. oligosporus (11.03–25.91 mm) [30]. The antibacterial mechanism of peptides is based on triggering changes in the biological membranes through the formation of ion channels. These changes cause deregulation of the replication, transcription and DNA sequence translation processes, with a disturbance to intracellular balance [30,83]. Dairi et al. (2019) obtained cellulose acetate/AgNPs–montmorillonite films with or without thymol. Antifungal tests against A. niger showed the best effects of ternary films. The presence of thymol caused a reduction in fungal colony density. The authors stated that nanoclay can control the release of thymol by retarding its vaporisation process [84]. Moreover, antifungal turmeric oil was added to the chitosan film, which showed an inhibitory effect against Aspergillus flavus. Essential oils can cause morphological changes in fungal hyphae. It is worth noting that the chitosan matrix slowed down the process of oil release due to an interaction between the oil and the biopolymer [85]. Adding chitin nanoparticles to gelatin films initiated an antifungal effect against Aspergillus niger. Sahraeet et al. (2017) assigned two potential mechanisms of antimicrobial activity to chitin nanoparticles. First, the nanoparticles penetrate the cell membrane, bind to DNA and block the synthesis of RNA and proteins. The second mechanism is based on the interaction of positively charged amino groups of chitin, chitosan and their derivatives with negatively charged groups on the microbial cell membrane, which disturbs its functionality [86].
3. Studied Systems in the Food Industry
The food industry develops various types of packaging, designed to reduce losses associated with spoilage of food products. In recent years, active packaging has become an alternative to synthetic packaging materials. There are various reports in the literature on the subject of the influence of active biopolymer coatings on food products. This type of packaging material demonstrated a protective function during the storage of fruit [87,88,89,90,91], meat [92,93,94,95], vegetables [96,97], cheese [98,99], fish and seafood [100,101,102,103].
3.1. Plant Extracts
One of the methods used for measuring oxidation level and oil degradation is the peroxide formation index, or peroxide value (PV), which is formed as a result of saturation of unsaturated fatty acids. Due to the presence of saturated hydrocarbons, aldehydes and ketones, physical and chemical changes in the food product occur, which result in an unpleasant smell and taste [104]. Rambabu et al. (2019) obtained chitosan films with mango leaf extract (MLE) in different concentrations (1%, 3% and 5%), and used them for the preservation of cashew nuts. Chitosan films with 3% and 5% MLE were characterised by a significant increase (56% higher than chitosan films without extract) in resistance to oxidation during the 28-day storage period of cashew nuts. A 62% increase of resistance was recorded for the minimal oxidation effects of films with 5% MLE [104]. Malherbiet al. (2019) added guabiroba pulp to corn starch/gelatin films, to be applied as packaging for extra-virgin olive oil. After 15 days of storage, the peroxide index for the samples wrapped in corn starch/gelatin and guabirola pulp films did not reach the maximum limit allowed by Brazil’s legislation, however, the addition of guabirola pulp did not have an additional effect on the oxidative stability of the extra virgin olive oil [105].
3.2. Essential Oils
Encapsulation of essential oils overcomes the problems associated with the oxidative stability of bioactive compounds and the provision of regulated or targeted transport. Lemon essential oil was encapsulated in chitosan nanoparticles, and was then placed in gelatin films in this form. The obtained active packaging materials effectively prevented the proliferation of microorganisms, inhibited lipid oxidation and delayed the deterioration of pork for 21 days at 4 °C [106]. Zhang et al. (2020) noticed that tarragon essential oil in the form of nanocapsules placed in chitosan–gelatin films caused a prolonged storage of pork slices in refrigerated conditions, compared to chitosan–gelatin films enriched with tarragon oil alone [107].
The content and type of biopolymers are of great importance when designing active packaging materials. Lian et al. (2020) obtained active packaging materials based on chitosan and two antimicrobial polysaccharides (xanthan and pullulan) or polysaccharides of plant origin (gum tragacanth and arabic gum). Then, they examined the effect of the polysaccharides used on the release effect of thyme essential oil film. The application of these types of film in the packaging of nectarine fruits was also investigated. After 60 h of storage, chitosan–gum arabic film had the best inhibitory effect. The addition of gum arabic to the chitosan–thyme oil film strengthened antifungal activity, and delayed the release of the oil by the electrostatic interaction between polysaccharides and the emulsifying effect of gum arabic on essential oil. Moreover, the release of essential oil can be controlled by regulating interactions between polysaccharides [108].
In Table 3, the impact of using active biopolymer films on the storage quality of various food products is presented.

Table 3.
Literature review regarding the use of active packaging materials on food products.
3.3. Nanofillers
One of the possibilities for improving the functional properties of biopolymer films is the addition of nanofillers. However, there is a tremendous fear that nanofillers will affect the quality of packaged food products. There are works that touch upon this aspect. Salarbashi et al. (2018) obtained soluble soybean polysaccharide films with the addition of TiO2NPs, and then checked whether TiO2NPs were transferred to the food products (in bread, for a 6-month storage period). The authors found that TiO2NPs were in concentrations acceptable by health requirements. In addition, a low incidence of TiO2NPs was found, as low concentrations of nanoparticles were detected in rat intestine epithelial cells [145]. Nonetheless, further research is needed to exclude the negative effect of TiO2NPs on human health. The influence of gelatin–carboxymethyl cellulose (Gel–CMC) films, with the addition of chitin nanofibers (CHNF) and Trachyspermum ammi essential oil (AJEO), on the quality of raw beef during 12 days of storage, at a temperature of 4 °C, was assessed. Active packaging materials caused delayed lipid oxidation and decomposition of proteins. The initial population, with a total viable count (TVC) in the control sample (without active film) of 3.1 log CFU/g, increased to 9.4 log CFU/g after 15 days of refrigerated storage. The lowest TVC values were recorded in samples wrapped in active films, which totaled 4.5 log CFU/g (in films with 4 wt % CHNF and 1% vol. AJEO) and 5.1 log CFU/g (in films with 2 wt % CHNF and 1% vol. AJEO). In addition, films with 2 wt % and 4 wt % CHNF, as well as 0.64% and 1% vol. AJEO, effectively inhibited the psychrotrophic bacterial count (PTC) in raw beef. After the storage period, the lowest increase in the total count of Pseudomonas spp. was recorded for beef wrapped in active films with 4 wt% CHNF and 1% vol. AJEO (from 1.94 to 3.05 log CFU/g). The presence of CHNF in the biopolymer matrix may act as a good oxygen barrier, which, in turn, can prevent the growth of aerobic bacteria. In addition, the presence of this type of nanofiller, due to its internal antimicrobial activity, reduces yeast and mould growth in packaged beef samples. Active Gel–CMC films reduced the TVB-N of raw beef, and after 15 days of storage, the content remained below the standard appropriate range, according to the TVB-N acceptance limit for beef samples (16.5 mg N/100 g). Gel–CMC films, with the addition of 4 wt% CHNF and 1% vol. AJEO, can extend the storage of beef, leading to minimised colour changes [146]. Wang et al. (2019) obtained anthocyanin nanocomplexes, produced using chitosan hydrochloride as well as carboxymethyl chitosan, and then incorporated them into gelatin films. The nanocomposite obtained in this way significantly delayed the deterioration of olive oil oxidation (21.2 meq O2/kg of peroxide value on the 56th day) compared to samples with gelatin films (24.3 meq O2/kg of peroxide value on day 56) [147].
3.4. Other Compounds
There are many scientific studies that focus on assessing the impact of active packaging enriched with antioxidant and/or antimicrobial agents on the properties of food products during storage. The addition of rosemary acid (RosA) to the gelatin film significantly affected the storage quality of Chinese bacon. After 60 days of storage, the difference in PV between the bacon in the gelatin film and the samples with the addition of 0.08% RosA was ~31%. Therefore, due to the presence of four phenolic-OH groups in RosA, lipid oxidation was delayed in the Chinese bacon [135]. Various bioactive additives were included in the starch films (gallic acid, chitosan and carvacrol), and their effect on ham was then examined during a 28-day storage period at 4 °C. The films with gallic acid inhibited the antimicrobial activity of ham the least. This was due to the presence of three hydroxyl groups in the given type of acid, while films with chitosan and carvacrol had an inhibitory effect on the growth of L. monocytogenes throughout the entire storage period. Furthermore, starch films enriched in chitosan or chitosan and carvacrol delayed the growth of the meat microflora of ham by 1 to 2 weeks. Antimicrobial activity of hydroxybenzoic acid decreases along with the increase in the number of hydroxyl groups, as hydroxyl groups increase polarity, and thus reduce membrane diffusion [148]. Zhang et al. (2020) studied the effect of a chitosan–zein film, with α-tocopherol, on the physicochemical properties and enzymatic activity of mushrooms (Agaricus bisporus) during storage at 4 °C for 12 days. The results of the study indicated a reduction in weight loss, maintaining firmness of the mushrooms wrapped in active films [149].
Biopolymer films without active additives can also be used as active packaging during the storage of food products. Martinez et al. (2018) used resveratrol as an active additive while storing processed smoked sea bass (Dicentrarchus labrax) products. The experiment was designed in several stages: (1) liquid resveratrol was superficially added to smoked fish; (2) resveratrol was suspended in liquid smoke, which was used for immersion of fillets; and (3) some fillets were coated with alginate or chitosan films. The fillets preserved by the first and second methods had reduced TBARS value. Active biopolymer films helped delay the oxidation process, however, only chitosan films were able to almost completely inhibit the growth of mesophilic, psychophilic and anaerobic bacteria. In a recent sensory evaluation, it was indicated that alginate films were conducive to limiting the deterioration of the fish’s condition [150].
Combining active compounds in biopolymer films, and then performing their assessment as packaging materials for food products, was also tested. Grape seed extract (GSE) (1% and 2%) and Ziziphora clinopodioide (ZEO) essential oil (1% and 2%) were incorporated in the chitosan–gelatin film. Then, it was checked how active packaging affects the storage of minced trout fillet. For 11 days of refrigerated storage, the active film caused a delay in fish deterioration. The lowest PV and total volatile basic nitrogen (TVB-N) values were observed in fish wrapped with 2% ZEO and 2% GSE films, which exhibited the best organoleptic values [151]. Pomegranate peel extract and Thymus kotschyanus essential oil were added to chitosan–starch films, and it was then further examined how this type of active film affects the storage quality of beef during a period of 21 days at 4 °C. The films containing 1% extract and 2% essential oil were characterised by the best results of sensory evaluation, including smell, colour and general acceptability. In addition, after 21 days of storage, this type of film showed the best inhibitory effect against L. monocytogenes (from 5.04 to 8.11 log CFU/g), bacterial count (count values of lactic acid bacteria from 3.2 to 3.98 log CFU/g) and lipid oxidation (TBARS values from 0.9 to 1.02 mg malondialdehyde/kg) [152]. Ziziphora clinopodioide essential oil alone, or in combination with Ficus carica extract (FCH), was added to nanomontmorillonite–chitosan (MMT–CS) and nanomontmorillonite–carboxymethyl cellulose (MMT–CMC) films to improve the quality of camel minced meat during refrigerated storage. After storage, the active films (CMC–MMT + 2% ZEO + 1% FCH and CS–MMT + 2% ZEO +1% FCH) reduced the final meat microbial population by approximately 1–4 log CFU/g, compared to the control film. For meat wrapped in active films, there tended to be a retardation in the growth of TVB-N (from 8.1 to 20 mg of TVB-N/100 g), pH (from 5.9 to 6.1), PV values (from 0 to 0.55–0.63 meq peroxide oxygen/1000 g lipid) and TBARS values (from 0.32 to 1.1–2.91 mg MDA/kg), as well as protein carbonyl content (from 0.72 to 1.08–1.16 nmol/mg protein) [153]. Active films based on cassava starch, pumpkin extract (PRE) and oregano essential oil (OEO) were obtained. Then, the films were used to protect ground beef from oxidation. After 9 days of storage, films containing 2% OEO and 3% PRE demonstrated the lowest 2-thiobarbituric acid reactive substance (TBARS) values compared to the control films ( for those with active ingredients—from 1.9 to 6.1 mg MDA/kg sample; for those without active ingredients—from 1.9 to 8.9 mg MDA/kg sample; MDA = malondialdehyde) [154].
4. Concluding Remarks and Future Developments
Traditional packaging systems for food products come from non-renewable fossil resources and their disposal poses a problem. Biopolymers are a good alternative because they are biodegradable, available, non-toxic, and also reduce the use of fossil fuels. The combination of biopolymers with antioxidant and/or antimicrobial ingredients creates active and ecological packaging systems.
From the authors’ assessment, future research trends in the field of active packaging systems may include the following areas:
- Assessing the impact of active packaging on the sensory quality of tested food products. As discussed earlier in this paper, various substances are added to the film that, in addition to antimicrobial and/or antioxidant activity, affects the sensory quality of the products. Research in this area is essential for developing packaging with an optimal active ingredient content that does not adversely affect the sensory properties of the products.
- Enriching the biopolymer film with nanofillers is combined with the need to develop a mechanism to migrate these types of active additives from the film to the food product. It is also necessary to evaluate the optimal level of nanofillers that can be safely used as an additive to biopolymer films, without adversely affecting human health.
- More research is required that would focus on understanding the potential mechanisms for combining different active ingredients with various biopolymer matrices, which could help optimise the composition of active films.
- Another key aspect in further research is the stability of antioxidant and antimicrobial components during storage of active films, and their release during the storage of packaged food.
- To effectively implement active packaging systems, multi-level cooperation between scientists from various fields (e.g., microbiology, food technology, etc.) and the packaging industry is indispensable.
- The future of the food and packaging industry involves the development of ‘smart’ packaging systems, that have both active (extending the shelf life of food) and intelligent (conveying information about the quality of the food product) properties.
- Despite the fact that there is extensive literature concerning the impact of active biopolymer packaging on the quality of food in laboratory conditions, it is necessary to test this type of packaging material in large-scale research, and to develop potential commercial applications.
Author Contributions
Conceptualization, E.J. and P.K.; Resources, E.J.; Writing-Original Draft Preparation, E.J.; Writing-Review & Editing, E.J and P.K; Visualization, E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef] [PubMed]
- Luangapai, F.; Peanparkdee, M.; Iwamoto, S. Biopolymer films for food industries: Properties, applications, and future aspects based on chitosan. Rev. Agric. Sci. 2019, 7, 59–67. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Munteanu, S.B.; Vasile, C. Vegetable Additives in Food Packaging Polymeric Materials. Polymers (Basel) 2020, 12, 28. [Google Scholar] [CrossRef]
- Meng, W.; Shi, J.; Zhang, X.; Lian, H.; Wang, Q.; Peng, Y. Effects of peanut shell and skin extracts on the antioxidant ability, physical and structure properties of starch-chitosan active packaging films. Int. J. Biol. Macromol. 2020, 152, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Sánchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Sui Chin, S.; Han Lyn, F.; Nur Hanani, Z.A. Effect of Aloe vera (Aloe barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Packag. Shelf Life 2017, 12, 128–134. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of active packaging based on chitosan-gelatin blend films functionalized with Chinese hawthorn (Crataegus pinnatifida) fruit extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving properties of thermoplastic starch films by incorporating active extracts and cellulose fibres isolated from rice or coffee husk. Food Packag. Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Chiralt, A.; Vilaplana, F. Antioxidant starch films containing sunflower hull extracts. Carbohydr. Polym. 2019, 214, 142–151. [Google Scholar] [CrossRef]
- Jridi, M.; Boughriba, S.; Abdelhedi, O.; Nciri, H.; Nasri, R.; Kchaou, H.; Kaya, M.; Sebai, H.; Zouari, N.; Nasri, M. Investigation of physicochemical and antioxidant properties of gelatin edible film mixed with blood orange (Citrus sinensis) peel extract. Food Packag. Shelf Life 2019, 21, 100342. [Google Scholar] [CrossRef]
- Nouri, A.; Tavakkoli Yaraki, M.; Ghorbanpour, M.; Wang, S. Biodegradable κ-carrageenan/nanoclay nanocomposite films containing Rosmarinus officinalis L. extract for improved strength and antibacterial performance. Int. J. Biol. Macromol. 2018, 115, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; Sobral, P.J.d.A. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Structure, Functionality, and Active Release of Nanoclay–Soy Protein Films Affected by Clove Essential Oil. Food Bioprocess Technol. 2016, 9, 1937–1950. [Google Scholar] [CrossRef]
- Rasid, N.A.M.; Nazmi, N.N.M.; Isa, M.I.N.; Sarbon, N.M. Rheological, functional and antioxidant properties of films forming solution and active gelatin films incorporated with Centella asiatica (L.) urban extract. Food Packag. Shelf Life 2018, 18, 115–124. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Nur Hanani, Z.A. Active packaging of fish gelatin films with Morinda citrifolia oil. Food Biosci. 2016, 16, 66–71. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Effect of melanin nanoparticles on the mechanical, water vapor barrier, and antioxidant properties of gelatin-based films for food packaging application. Food Packag. Shelf Life 2019, 21, 100363. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.d.; Rodrigues, J.M.; Valadares, A.C.F.; Almeida, A.B.d.; Lima, T.M.d.; Takeuchi, K.P.; Alves, C.C.F.; Sousa, H.A.d.F.; Silva, E.R.d.; Dyszy, F.H.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and characterization of chitosan-fungal extract films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Zhang, Z.-H.; Li, L.; Yuan, M.-L.; Fan, J.; Zhao, T.-R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kim, H.C.; Kim, J.W.; Zhai, L.; Zhu, Q.Y.; Kim, J. Incorporation of melanin nanoparticles improves UV-shielding, mechanical and antioxidant properties of cellulose nanofiber based nanocomposite films. Mater. Today Commun. 2020, 24, 100984. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Sirisha Nallan Chakravartula, S.; Lourenço, R.V.; Balestra, F.; Quinta Barbosa Bittante, A.M.; Sobral, P.J.d.A.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Roy, S.; Van Hai, L.; Kim, H.C.; Zhai, L.; Kim, J. Preparation and characterization of synthetic melanin-like nanoparticles reinforced chitosan nanocomposite films. Carbohydr. Polym. 2020, 231, 115729. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H. Preparation and properties of zein–rutin composite nanoparticle/corn starch films. Carbohydr. Polym. 2017, 169, 385–392. [Google Scholar] [CrossRef]
- Boughriba, S.; Souissi, N.; Jridi, M.; Li, S.; Nasri, M. Thermal, mechanical and microstructural characterization and antioxidant potential of Rhinobatos cemiculus gelatin films supplemented by titanium dioxide doped silver nanoparticles. Food Hydrocoll. 2020, 103, 105695. [Google Scholar] [CrossRef]
- Kchaou, H.; Jridi, M.; Benbettaieb, N.; Debeaufort, F.; Nasri, M. Bioactive films based on cuttlefish (Sepia officinalis) skin gelatin incorporated with cuttlefish protein hydrolysates: Physicochemical characterization and antioxidant properties. Food Packag. Shelf Life 2020, 24, 100477. [Google Scholar] [CrossRef]
- Tomadoni, B.; Ponce, A.; Pereda, M.; Ansorena, M.R. Vanillin as a natural cross-linking agent in chitosan-based films: Optimizing formulation by response surface methodology. Polym. Test. 2019, 78, 105935. [Google Scholar] [CrossRef]
- Lu, N.; Liu, Y. Structural, physicochemical, and functional (antioxidant-antimicrobial) properties of 2-O-methyl-β-cyclodextrin inclusion with hexahydro-β-acids in chitosan films. Colloids Surf. B Biointerfaces 2020, 191, 111002. [Google Scholar] [CrossRef]
- Noronha, C.M.; de Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef]
- Agudelo-Cuartas, C.; Granda-Restrepo, D.; Sobral, P.J.A.; Hernandez, H.; Castro, W. Characterization of whey protein-based films incorporated with natamycin and nanoemulsion of α-tocopherol. Heliyon 2020, 6, e03809. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity—A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Nouri, A.; Tavakkoli Yaraki, M.; Lajevardi, A.; Rahimi, T.; Tanzifi, M.; Ghorbanpour, M. An investigation of the role of fabrication process in the physicochemical properties of κ-carrageenan-based films incorporated with Zataria multiflora extract and nanoclay. Food Packag. Shelf Life 2020, 23, 100435. [Google Scholar] [CrossRef]
- Hamed, S.F.; Sadek, Z.; Edris, A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Inactivation of Gram-negative pathogens in refrigerated milk by reuterin in combination with nisin or the lactoperoxidase system. Eur. Food Res. Technol. 2008, 227, 77–82. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Dos Santos Paglione, I.; Galindo, M.V.; de Medeiros, J.A.S.; Yamashita, F.; Alvim, I.D.; Ferreira Grosso, C.R.; Sakanaka, L.S.; Shirai, M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life 2019, 22, 100419. [Google Scholar] [CrossRef]
- Yeddes, W.; Djebali, K.; Aidi Wannes, W.; Horchani-Naifer, K.; Hammami, M.; Younes, I.; Saidani Tounsi, M. Gelatin-chitosan-pectin films incorporated with rosemary essential oil: Optimized formulation using mixture design and response surface methodology. Int. J. Biol. Macromol. 2020, 154, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Emaminia, S.; Heyat Davudian, S.; Pourmohammad, S.; Hamishehkar, H.; Roufegarinejad, L. Preparation and characterization of gelatin-based nanocomposite containing chitosan nanofiber and ZnO nanoparticles. Carbohydr. Polym. 2019, 216, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Antonio Ortega-Rivera, O.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A.; et al. Synergistic Antimicrobial Effects of Silver/Transition-metal Combinatorial Treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications—A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran nanocomposite films: The effect of nanofillers on the structural, thermal, mechanical and antimicrobial properties of biopolymer films. Carbohydr. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Mascheroni, E.; Chalier, P.; Gontard, N.; Gastaldi, E. Designing of a wheat gluten/montmorillonite based system as carvacrol carrier: Rheological and structural properties. Food Hydrocoll. 2010, 24, 406–413. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocoll. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Development of antibacterial carboxymethyl cellulose/chitosan biguanidine hydrochloride edible films activated with frankincense essential oil. Int. J. Biol. Macromol. 2019, 139, 1162–1167. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, H.; Luan, Q.; Zheng, M.; Tang, H.; Huang, F. Fabrication of cellulose nanowhiskers reinforced chitosan-xylan nanocomposite films with antibacterial and antioxidant activities. Carbohydr. Polym. 2018, 184, 66–73. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Vargas, O.L. Spice oleoresins containing antimicrobial agents improve the potential use of bio-composite films based on gelatin. Food Packag. Shelf Life 2018, 17, 50–56. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E.; Sabaa, M.W. Biobased alginate/castor oil edible films for active food packaging. LWT 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Rhim, J.-W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Mora, L.; Escudero, E.; Vignolo, G.; Aznar, R.; Toldrá, F. Antilisterial peptides from Spanish dry-cured hams: Purification and identification. Food Microbiol. 2016, 59, 133–141. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef]
- Li, Z.; Lin, S.; An, S.; Liu, L.; Hu, Y.; Wan, L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019, 131, 420–434. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries—In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, J.; Cheng, F. Cross-linked starch-based edible coating reinforced by starch nanocrystals and its preservation effect on graded Huangguan pears. Food Chem. 2020, 311, 125891. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Salmieri, S.; Lacroix, M. Cellulose nanocrystals (CNCs) loaded alginate films against lipid oxidation of chicken breast. Food Res. Int. 2020, 132, 109110. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Tragacanth gum containing Zataria multiflora Boiss. essential oil as a natural preservative for storage of button mushrooms (Agaricus bisporus). Food Hydrocoll. 2017, 72, 202–209. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active edible furcellaran/whey protein films with yerba mate and white tea extracts: Preparation, characterization and its application to fresh soft rennet-curd cheese. Int. J. Biol. Macromol. 2019, 155, 1307–1316. [Google Scholar] [CrossRef]
- Lotfi, M.; Tajik, H.; Moradi, M.; Forough, M.; Divsalar, E.; Kuswandi, B. Nanostructured chitosan/ monolaurin film: Preparation, characterization and antimicrobial activity against Listeria monocytogenes on ultrafiltered white cheese. LWT 2018, 92, 576–583. [Google Scholar] [CrossRef]
- Mohebi, E.; Shahbazi, Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: A novel functional wrapping design. LWT Food Sci. Technol. 2017, 76, 108–116. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT 2020, 117, 108633. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-Caballero, M.E.; Martínez-Bartolomé, M.Á.; de Lacey, A.M.L.; Gómez-Guillen, M.C.; Montero, M.P. The effect of the combined use of high pressure treatment and antimicrobial edible film on the quality of salmon carpaccio. Int. J. Food Microbiol. 2018, 283, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, S.; Li, S.; Liu, Y. Evaluation of the preservation effect of gelatin-water soluble chitosan film incorporated with maillard peptides on bluefin tuna (Thunnus thynnus) slices packaging. LWT 2019, 113, 108294. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Malherbi, N.M.; Schmitz, A.C.; Grando, R.C.; Bilck, A.P.; Yamashita, F.; Tormen, L.; Fakhouri, F.M.; Velasco, J.I.; Bertan, L.C. Corn starch and gelatin-based films added with guabiroba pulp for application in food packaging. Food Packag. Shelf Life 2019, 19, 140–146. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Lian, H.; Shi, J.; Zhang, X.; Peng, Y. Effect of the added polysaccharide on the release of thyme essential oil and structure properties of chitosan based film. Food Packag. Shelf Life 2020, 23, 100467. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. The effects of Chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Joanne Kam, W.-Y.; Mirhosseini, H.; Abas, F.; Hussain, N.; Hedayatnia, S.; Florence Chong, H.-L. Antioxidant activity enhancement of biodegradable film as active packaging utilizing crude extract from durian leaf waste. Food Control 2018, 90, 66–72. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Effects of bio-nanocomposite films from tilapia and squid skin gelatins incorporated with ethanolic extract from coconut husk on storage stability of mackerel meat powder. Food Packag. Shelf Life 2015, 6, 42–52. [Google Scholar] [CrossRef]
- Baek, S.-K.; Kim, S.; Song, K.B. Cowpea starch films containing maqui berry extract and their application in salmon packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Xu, S.; Wang, L. Developing a simultaneously antioxidant and pH-responsive κ-carrageenan/hydroxypropyl methylcellulose film blended with Prunus maackii extract. Int. J. Biol. Macromol. 2019, 155, 1393–1400. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef]
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf Life 2014, 2, 7–16. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Sumpavapol, P.; Nirmal, N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012, 155, 171–178. [Google Scholar] [CrossRef]
- Ugalde, M.L.; de Cezaro, A.M.; Vedovatto, F.; Paroul, N.; Steffens, J.; Valduga, E.; Backes, G.T.; Franceschi, E.; Cansian, R.L. Active starch biopolymeric packaging film for sausages embedded with essential oil of Syzygium aromaticum. J. Food Sci. Technol. 2017, 54, 2171–2175. [Google Scholar] [CrossRef]
- Radha krishnan, K.; Babuskin, S.; Rakhavan, K.R.; Tharavin, R.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Potential application of corn starch edible films with spice essential oils for the shelf life extension of red meat. J. Appl. Microbiol. 2015, 119, 1613–1623. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S.; Ibrahim, O.A.; Youssef, A.M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating. Carbohydr. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevic, V.; Makarewicz, M. Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.-H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Ben Amara, C.; Oulahal, N.; Gharsallaoui, A.; Joly, C.; Tongdeesoontorn, W.; Rawdkuen, S.; Degraeve, P. Gelatin films with nisin and catechin for minced pork preservation. Food Packag. Shelf Life 2018, 18, 173–183. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T.; de la Caba, K. Fish gelatin monolayer and bilayer films incorporated with epigallocatechin gallate: Properties and their use as pouches for storage of chicken skin oil. Food Hydrocoll. 2019, 89, 783–791. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Gonzaga, M.L.C.; Bastos, M.S.R.; Magalhães, H.C.R.; Benevides, S.D.; Furtado, R.F.; Zambelli, R.A.; Garruti, D.S. Packaging with cashew gum/gelatin/essential oil for bread: Release potential of the citral. Food Packag. Shelf Life 2020, 23, 100431. [Google Scholar] [CrossRef]
- Choi, I.; Lee, S.E.; Chang, Y.; Lacroix, M.; Han, J. Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. LWT 2018, 93, 427–433. [Google Scholar] [CrossRef]
- Moreno, O.; Gil, À.; Atarés, L.; Chiralt, A. Active starch-gelatin films for shelf-life extension of marinated salmon. LWT 2017, 84, 189–195. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Dai, H.; Zhang, Y. Physico-mechanical and antioxidant properties of gelatin film from rabbit skin incorporated with rosemary acid. Food Packag. Shelf Life 2019, 19, 121–130. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Julian McClements, D. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite- clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A.; Zunabovic-Pichler, M.; Fatehi, S. Nanocomposite films with CMC, okra mucilage, and ZnO nanoparticles: Extending the shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 21, 100330. [Google Scholar] [CrossRef]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F. Soluble soybean polysaccharide/TiO2 bionanocomposite film for food application. Carbohydr. Polym. 2018, 186, 384–393. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti khiabani, M.; Akhondzadeh basti, A.; Abdulkhani, A. The effects of gelatin-CMC films incorporated with chitin nanofiber and Trachyspermum ammi essential oil on the shelf life characteristics of refrigerated raw beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging films formulated with gelatin and anthocyanins nanocomplexes: Physical properties, antioxidant activity and its application for olive oil protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Zhao, Y.; Teixeira, J.S.; Saldaña, M.D.A.; Gänzle, M.G. Antimicrobial activity of bioactive starch packaging films against Listeria monocytogenes and reconstituted meat microbiota on ham. Int. J. Food Microbiol. 2019, 305, 108253. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Combined antioxidant and sensory effects of active chitosan/zein film containing α-tocopherol on Agaricus bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Martínez, O.; Salmerón, J.; Epelde, L.; Vicente, M.S.; de Vega, C. Quality enhancement of smoked sea bass (Dicentrarchus labrax) fillets by adding resveratrol and coating with chitosan and alginate edible films. Food Control 2018, 85, 168–176. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Caetano, K.; Almeida Lopes, N.; Haas Costa, T.M.; Brandelli, A.; Rodrigues, E.; Hickmann Flôres, S.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).