Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes Tested
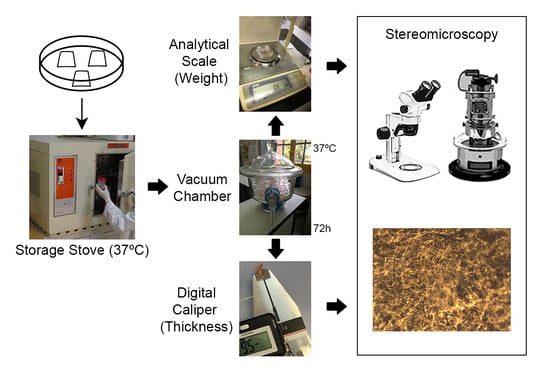
2.2. Degradation Assays
2.3. Statistical Analysis
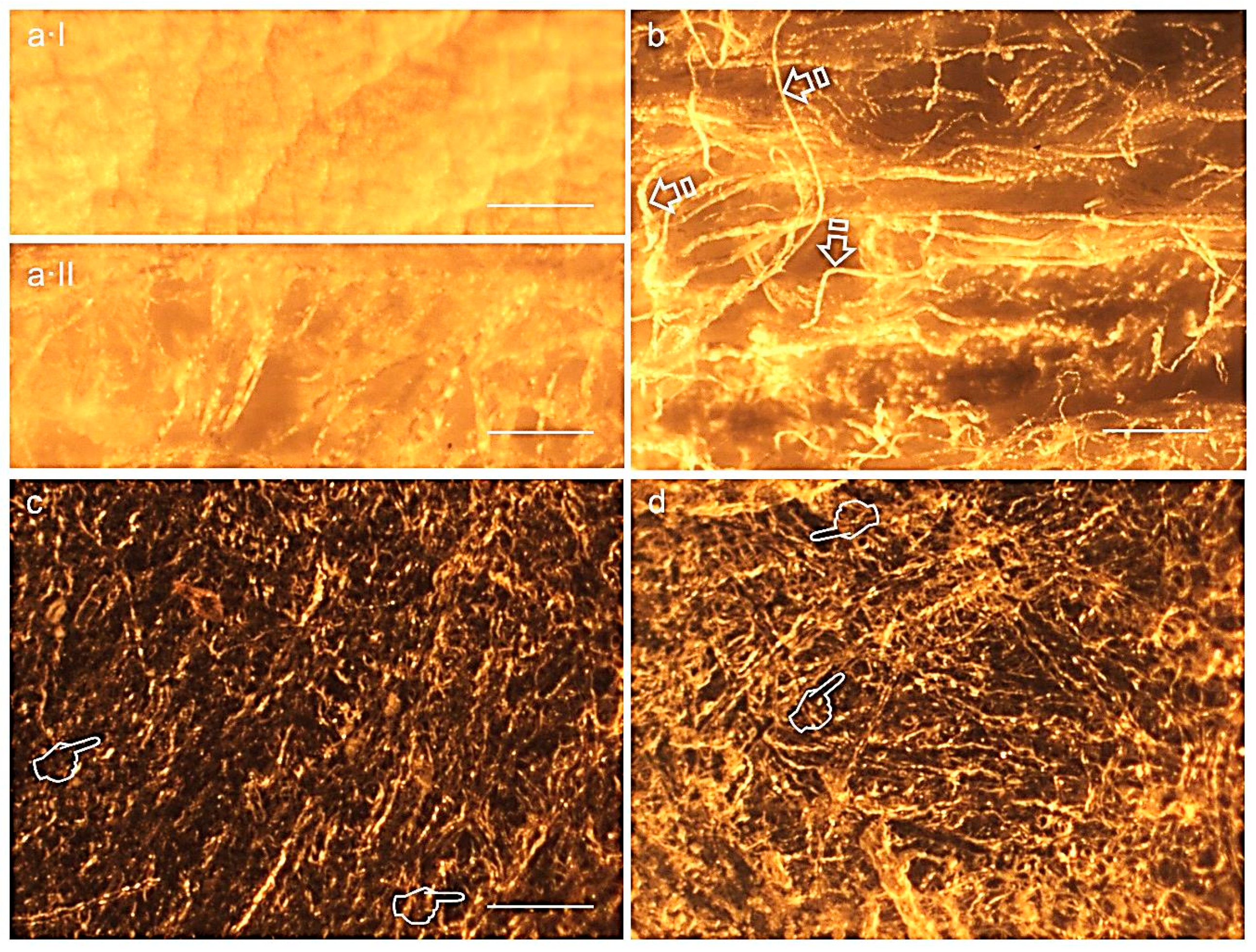
2.4. Light Microscopy Analysis
3. Results
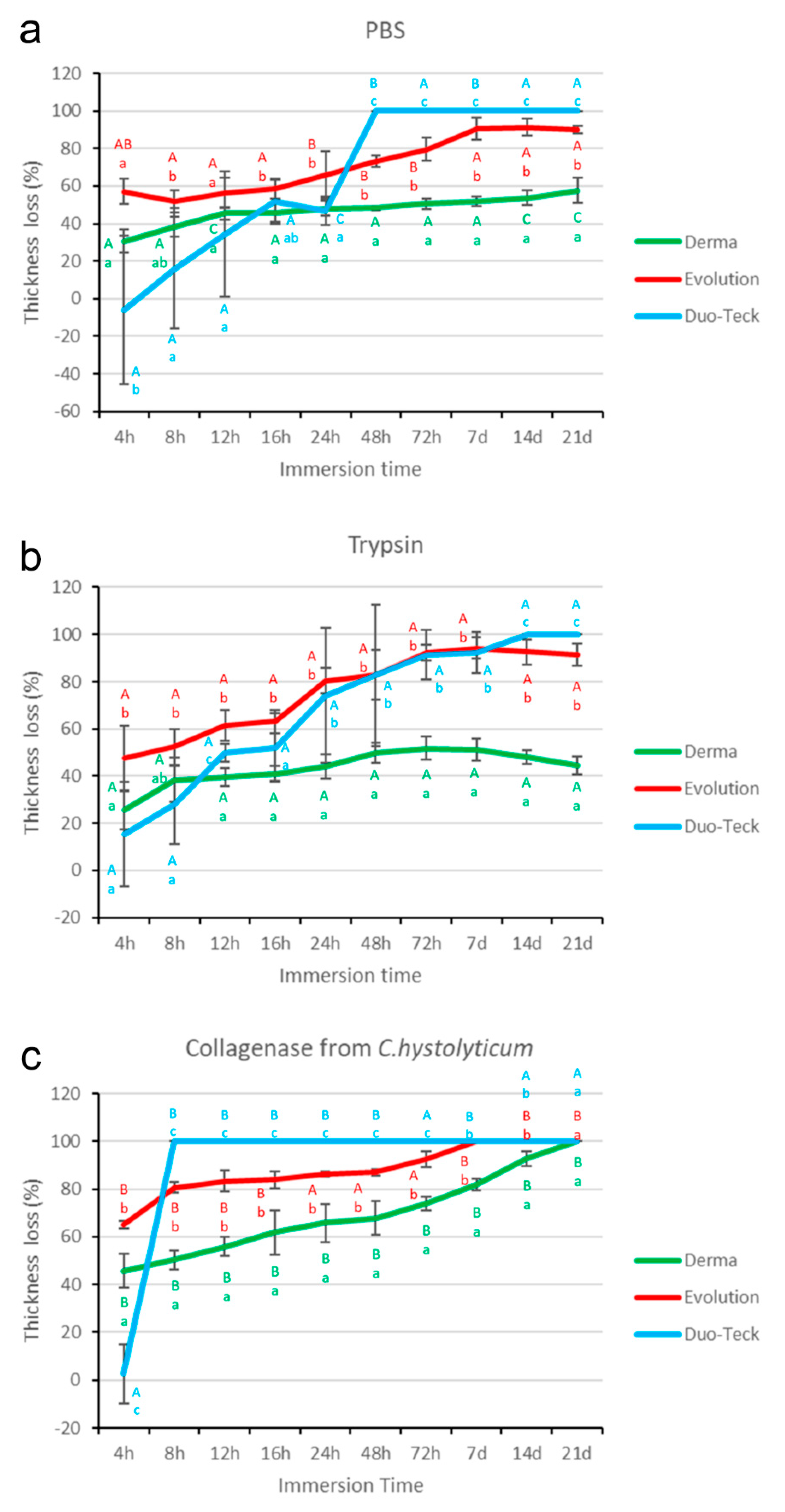
3.1. Thickness Evaluation after PBS Degradation Assay
3.2. Thickness Evaluation after Trypsin Degradation Assay
3.3. Thickness Evaluation after C. histolyticum Collagenase Degradation Assay
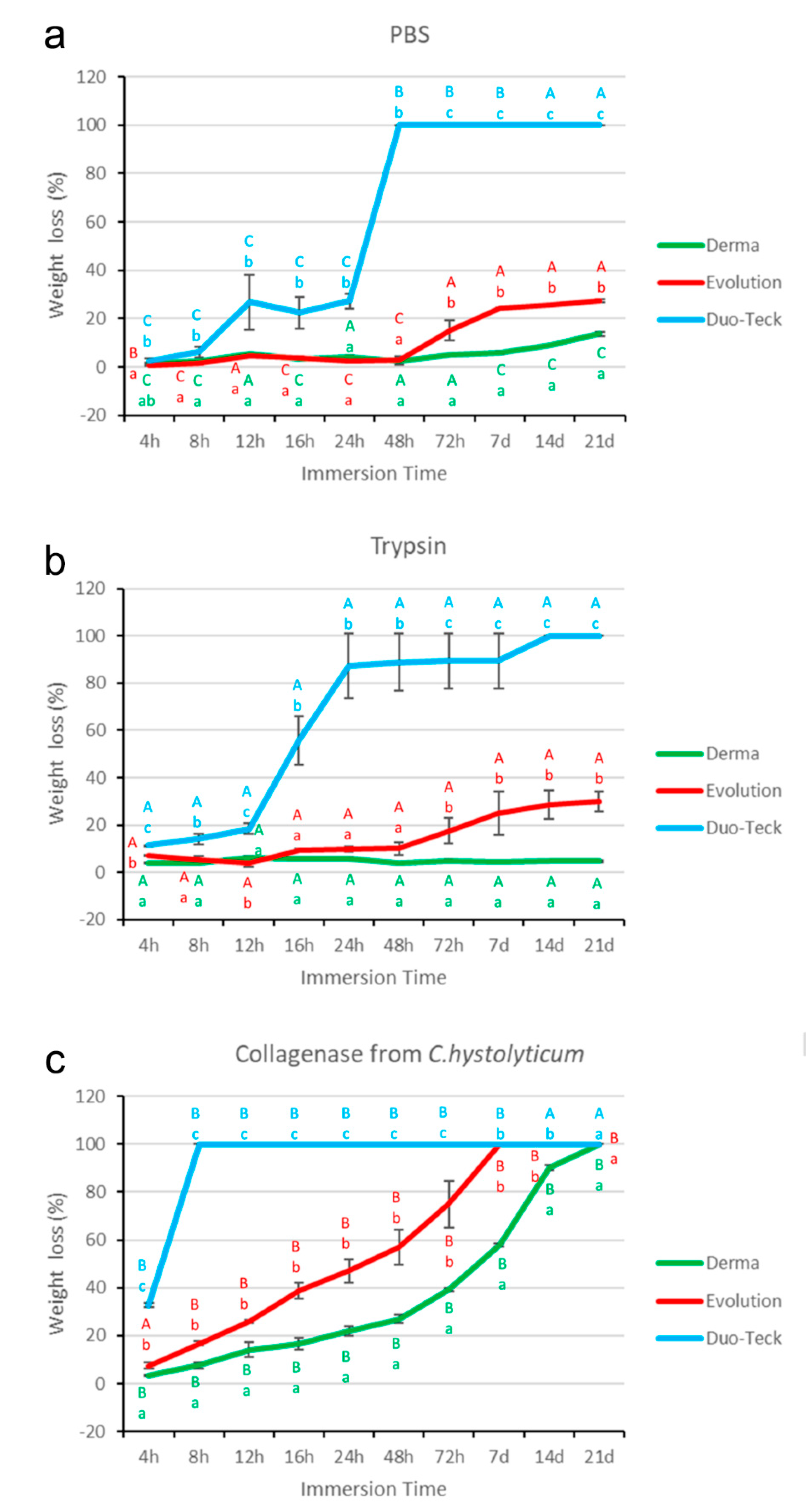
3.4. Weight Evaluation after PBS Degradation Assay
3.5. Weight Evaluation after Trypsin Degradation Assay
3.6. Weight Evaluation after C. histolyticum Collagenase Degradation Assay
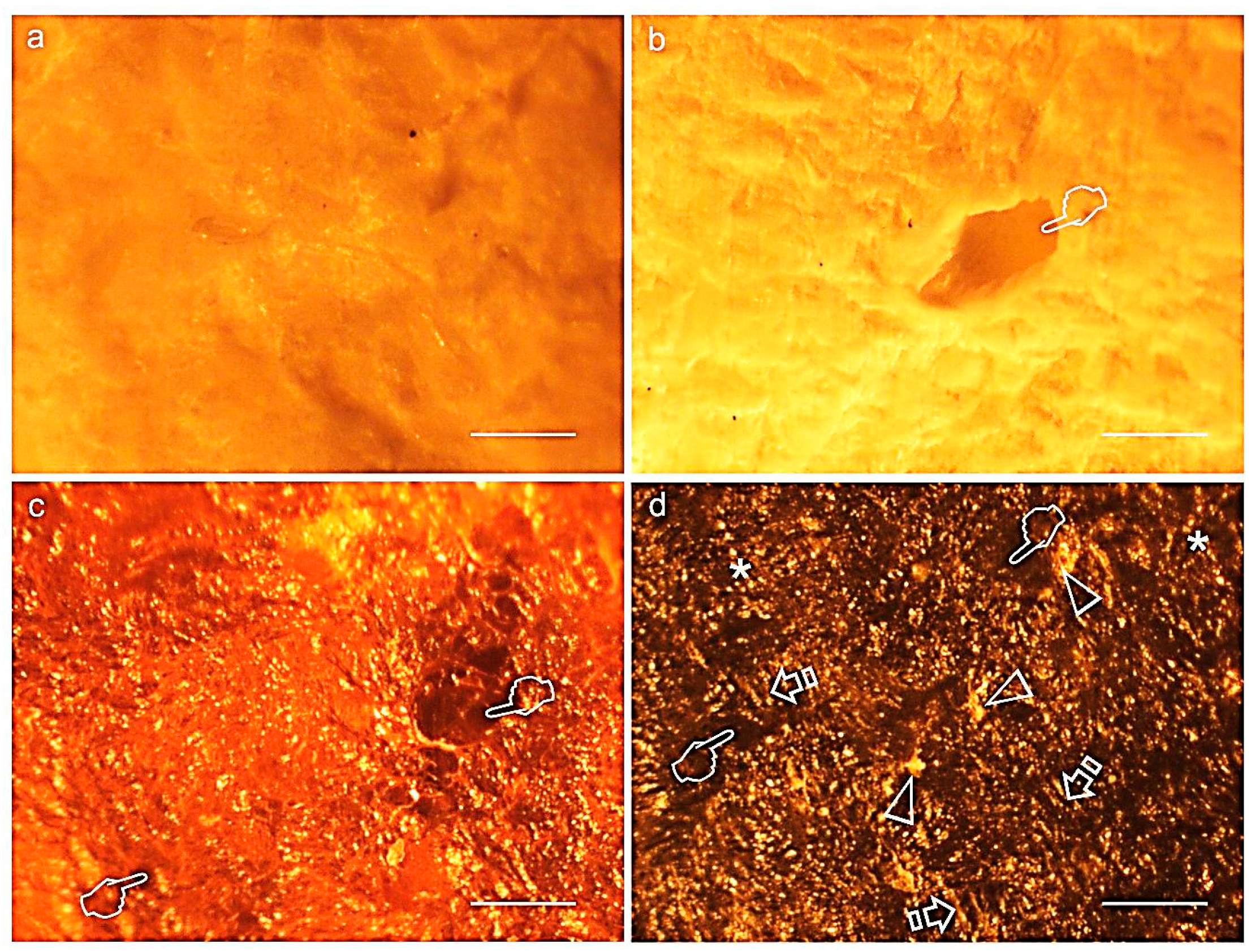
3.7. Membranes Morphological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Wong, C.; Yoganarasimha, S.; Carrico, C.; Madurantakam, P. Incorporation of Fibrin Matrix into Electrospun Membranes for Periodontal Wound Healing. Bioengineering 2019, 6, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Shi, B.; Miron, R. Membranes for guided tissue and bone regeneration. Oral. Maxillofac. Surg. 2013, 1, 1–10. [Google Scholar]
- Luz, E.P.; de Fátima Borges, M.; Andrade, F.K.; de Freitas Rosa, M.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Vieira, R.S. Strontium delivery systems based on bacterial cellulose and hydroxyapatite for guided bone regeneration. Cellulose 2018, 25, 6661–6679. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Aboodi, G.; Moses, O.; Tal, H.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Bio-degradation of a resorbable collagen membrane (Bio-Gide) applied in a double-layer technique in rats. Clin. Oral. Implant. Res. 2009, 20, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E. The Effect of Membrane Exposure on the Outcome of Regenerative Procedures in Humans: A Meta-Analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.; Karring, T. New attachment and bone formation in periodontal defects following treatment of submerged roots with guided tissue regeneration. J. Clin. Periodontol. 1995, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Trisi, P.; Piattelli, A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int. J. Periodontics Restor. Dent. 1994, 14, 496–511. [Google Scholar]
- Calciolari, E.; Ravanetti, F.; Strange, A.; Mardas, N.; Bozec, L.; Cacchioli, A.; Kostomitsopoulos, N.; Donos, N. Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. J. Periodontal Res. 2018, 53, 430–439. [Google Scholar] [CrossRef]
- Kilinc, A.; Ataol, M. How effective is collagen resorbable membrane placement after partially impacted mandibular third molar surgery on postoperative morbidity? A prospective randomized comparative study. BMC Oral. Health 2017, 17, 126. [Google Scholar] [CrossRef]
- Rothamel, D.; Benner, M.; Fienitz, T.; Happe, A.; Kreppel, M.; Nickenig, H.-J.; Zöller, J.E. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices—An experimental study in the rat. Head Face Med. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- De Marco, P.; Zara, S.; De Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; Di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12, 055005. [Google Scholar] [CrossRef] [PubMed]
- An, Y.-Z.; Kim, Y.-K.; Lim, S.-M.; Heo, Y.-K.; Kwon, M.-K.; Cha, J.-K.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Physiochemical properties and resorption progress of porcine skin-derived collagen membranes: In vitro and in vivo analysis. Dent. Mater. J. 2018, 37, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Kohavi, D.; Krausz, E.; Steinberg, D.; Rosen, G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin. Oral. Implant. Res. 2003, 14, 263–268. [Google Scholar] [CrossRef]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: An in vitro and in vivo study. Clin. Oral. Implant. Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Jiang, H.B.; Ryu, J.-H.; Kang, H.; Kim, K.-M.; Kwon, J.-S. Comparing Properties of Variable Pore-Sized 3D-Printed PLA Membrane with Conventional PLA Membrane for Guided Bone/Tissue Regeneration. Materials 2019, 12, 1718. [Google Scholar] [CrossRef]
- Maurer, T.; Stoffel, M.H.; Belyaev, Y.; Stiefel, N.G.; Vidondo, B.; Küker, S.; Mogel, H.; Schäfer, B.; Balmer, J. Structural characterization of four different naturally occurring porcine collagen membranes suitable for medical applications. PLoS ONE 2018, 13, e0205027. [Google Scholar] [CrossRef]
- Sunandhakumari, V.J.; Vidhyadharan, A.K.; Alim, A.; Kumar, D.; Ravindran, J.; Krishna, A.; Prasad, M. Fabrication and in Vitro Characterization of Bioactive Glass/Nano Hydroxyapatite Reinforced Electrospun Poly(ε-Caprolactone) Composite Membranes for Guided Tissue Regeneration. Bioengineering 2018, 5, 54. [Google Scholar] [CrossRef]
- Silva, M.J.; Gonçalves, C.P.; Galvão, K.M.; D’Alpino, P.H.P.; Nascimento, F.D. Synthesis and Characterizations of a Collagen-Rich Biomembrane with Potential for Tissue-Guided Regeneration. Eur, J. Dent. 2019, 13, 295–302. [Google Scholar] [CrossRef]
- Hoornaert, A.; d’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef] [PubMed]
- Fickl, S.; Nannmark, U.; Schlagenhauf, U.; Hürzeler, M.B.; Kebschull, M. Porcine dermal matrix in the treatment of dehiscence-type defects—An experimental split-mouth animal trial. Clin. Oral. Implant. Res. 2015, 26, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Kumar, D.; Singh, S.K. Design, synthesis and collagenase inhibitory activity of some novel phenylglycine derivatives as metalloproteinase inhibitors. Int. J. Biol. Macromol. 2018, 107, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.W.; Yukna, R.A. Collagen membrane resorption in dogs: A comparative study. Implant. Dent. 2001, 10, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Pinholt, E.M.; Madsen, J.E.; Donath, K. Histological evaluation of different biodegradable and non-biodegradable membranes implanted subcutaneously in rats. J. Cranio-Maxillofac. Surg. 2000, 28, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Zohar, R.; Nemcovsky, C.E.; Kebudi, E.; Artzi, Z.; Tal, H.; Moses, O. Tetracycline impregnation delays collagen membrane degradation in vivo. J. Periodontol. 2004, 75, 1096–1101. [Google Scholar] [CrossRef] [PubMed]

| (a) | |||||||||
| Derma | Evolution | Duo-Teck | |||||||
| Trypsin | C. hystolyticum | PBS | Trypsin | C. hystolyticum | PBS | Trypsin | C. hystolyticum | PBS | |
| t0 | 1.02 (0.13) | 1.09 (0.17) | 1.22 (0.04) | 0.43 (0.14) | 0.39 (0.05) | 0.47 (0.01) | 0.15 (0.00) | 0.15 (0.01) | 0.17 (0.00) |
| 4 h | 0.75 (0.05) | 0.59 (0.05) | 0.85 (0.10) | 0.21 (0.02) | 0.14 (0.02) | 0.20 (0.03) | 0.13 (0.04) | 0.14 (0.01) | 0.18 (0.06) |
| 8 h | 0.64 (0.07) | 0.54 (0.05) | 0.75 (0.08) | 0.20 (0.06) | 0.08 (0.01) | 0.23 (0.03) | 0.11 (0.03) | 0 (0) | 0.14 (0.05) |
| 12 h | 0.61 (0.06) | 0.48 (0.07) | 0.66 (0.03) | 0.16 (0.04) | 0.06 (0.01) | 0.21 (0.04) | 0.08 (0.01) | 0 (0) | 0.11 (0.05) |
| 16 h | 0.60 (0.05) | 0.41 (0.04) | 0.66 (0.05) | 0.16 (0.04) | 0.06 (0.01) | 0.20 (0.03) | 0.07 (0.02) | 0 (0) | 0.08 (0.02) |
| 24 h | 0.57 (0.02) | 0.36 (0.03) | 0.63 (0.04) | 0.08 (0.02) | 0.05 (0.01) | 0.16 (0.06) | 0.04 (0.04) | 0 (0) | 0.09 (0.01) |
| 48 h | 0.51 (0.04) | 0.34 (0.02) | 0.63 (0.03) | 0.06 (0.03) | 0.05 (0.01) | 0.13 (0.01) | 0.03 (0.03) | 0 (0) | 0 (0) |
| 72 h | 0.49 (0.05) | 0.28 (0.03) | 0.60 (0.05) | 0.03 (0.02) | 0.03 (0.01) | 0.10 (0.03) | 0.01 (0.02) | 0 (0) | 0 (0) |
| 7 d | 0.49 (0.02) | 0.20 (0.05) | 0.59 (0.03) | 0.03 (0.03) | 0 (0) | 0.05 (0.03) | 0.01 (0.01) | 0 (0) | 0 (0) |
| 14 d | 0.53 (0.07) | 0.08 (0.04) | 0.56 (0.04) | 0.04 (0.03) | 0 (0) | 0.04 (0.02) | 0 (0) | 0 (0) | 0 (0) |
| 21 d | 0.57 (0.07) | 0 (0) | 0.52 (0.08) | 0.04 (0.03) | 0 (0) | 0.05 (0.01) | 0 (0) | 0 (0) | 0 (0) |
| (b) | |||||||||
| Th0-Th1 | 0.002 | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | 0.152 | 0.602 | 0.323 |
| Th0-Th4h | 0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.009 | <0.001 | 0.46 |
| Th0- Th8h | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.02 | <0.001 | 0.052 |
| Th0-Th12h | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.035 | <0.001 | <0.001 |
| Th0-Th16h | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| Th0-Th24h | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0-Th48h | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0- Th72h | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0-Th7d | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0-Th14d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0-Th21d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| (a) | |||||||||
| Derma | Evolution | Duo-Teck | |||||||
| Trypsin | C. hystolyticum | PBS | Trypsin | C. hystolyticum | PBS | Trypsin | C. hystolyticum | PBS | |
| t0 | 55.47 (4.04) | 57.49 (1.54) | 59.69 (1.13) | 15.52 (3.19) | 14.78 (0.70) | 16.04 (0.13) | 8.83 (0.40) | 9.35 (0.52) | 8.9 (0.28) |
| 4 h | 53.25 (3.71) | 55.58 (1.61) | 58.62 (1.17) | 14.42 (2.96) | 13.67 (0.44) | 15.89 (0.10) | 7.83 (0.35) | 6.28 (0.31) | 8.70 (0.17) |
| 8 h | 53.19 (3.68) | 53.02 (0.68) | 58.34 (1.33) | 14.67 (2.78) | 12.30 (0.46) | 15.8 (0.04) | 7.60 (0.54) | 0 (0) | 8.34 (0.09) |
| 12 h | 51.95 (3.60) | 49.32 (0.39) | 56.45 (1.11) | 14.89 (2.86) | 10.94 (0.47) | 15.27 (0.12) | 7.20 (0.14) | 0 (0) | 6.53 (1.20) |
| 16 h | 52.42 (3.64) | 47.90 (0.15) | 57.64 (1.24) | 14.08 (2.98) | 9.04 (0.07) | 15.41 (0.08) | 3.88 (0.75) | 0 (0) | 6.92 (0.79) |
| 24 h | 52.21 (3.62) | 44.87 (0.04) | 57.11 (1.47) | 14.04 (3.06) | 7.79 (0.35) | 15.67 (0.07) | 1.16 (1.27) | 0 (0) | 6.47 (0.45) |
| 48 h | 53.27 (3.79) | 41.93 (0.06) | 58.25 (1.78) | 14.02 (3.27) | 6.33 (0.79) | 15.6 (0.17) | 1.02 (1.12) | 0 (0) | 0 (0) |
| 72 h | 52.73 (3.60) | 34.89 (0.49) | 56.66 (1.02) | 12.95 (3.45) | 3.63 (1.27) | 13.58 (0.55) | 0.97 (1.06) | 0 (0) | 0 (0) |
| 7 d | 52.96 (3.64) | 24.34 (1.00) | 56.24 (0.68) | 11.87 (3.81) | 0 (0) | 12.13 (0.13) | 0.98 (1.07) | 0 (0) | 0 (0) |
| 14 d | 52.88 (3.69) | 5.67 (0.80) | 54.33 (1.20) | 11.26 (3.23) | 0 (0) | 11.93 (0.07) | 0 (0) | 0 (0) | 0 (0) |
| 21 d | 52.89 (3.70) | 0 (0) | 51.51 (1.55) | 10.99 (2.88) | 0 (0) | 11.66 (0.06) | 0 (0) | 0 (0) | 0 (0) |
| (b) | |||||||||
| W0-W4h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 |
| W0-W8h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | 0.044 |
| W0-W12h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
| W0-W16h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0-W24h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0-W48h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.015 | <0.001 | <0.001 | <0.001 |
| W0-W72h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0-W7d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0-W14d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0-W21d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. https://doi.org/10.3390/polym12061290
Toledano M, Asady S, Toledano-Osorio M, García-Godoy F, Serrera-Figallo M-A, Benítez-García JA, Osorio R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers. 2020; 12(6):1290. https://doi.org/10.3390/polym12061290
Chicago/Turabian StyleToledano, Manuel, Samara Asady, Manuel Toledano-Osorio, Franklin García-Godoy, María-Angeles Serrera-Figallo, José A. Benítez-García, and Raquel Osorio. 2020. "Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration" Polymers 12, no. 6: 1290. https://doi.org/10.3390/polym12061290
APA StyleToledano, M., Asady, S., Toledano-Osorio, M., García-Godoy, F., Serrera-Figallo, M.-A., Benítez-García, J. A., & Osorio, R. (2020). Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers, 12(6), 1290. https://doi.org/10.3390/polym12061290