Temperature and Time Dependence of the Solvent-Induced Crystallization of Poly(l-lactide)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
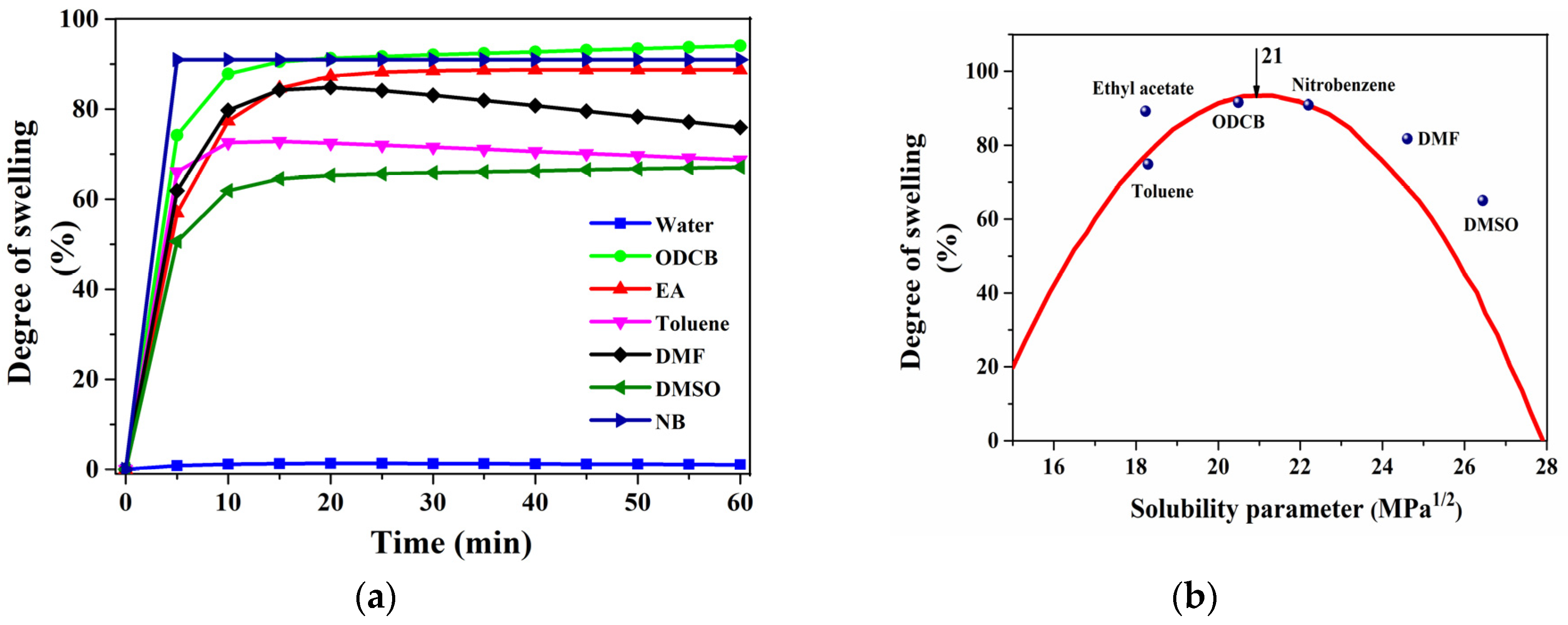
2.2. Solubility Test
2.3. Determination of Solubility Parameter of PLLA
2.4. Crystallization of PLLA
3. Characterization
4. Results and Discussion
4.1. Determination of Solubility Parameter (δ) of PLLA
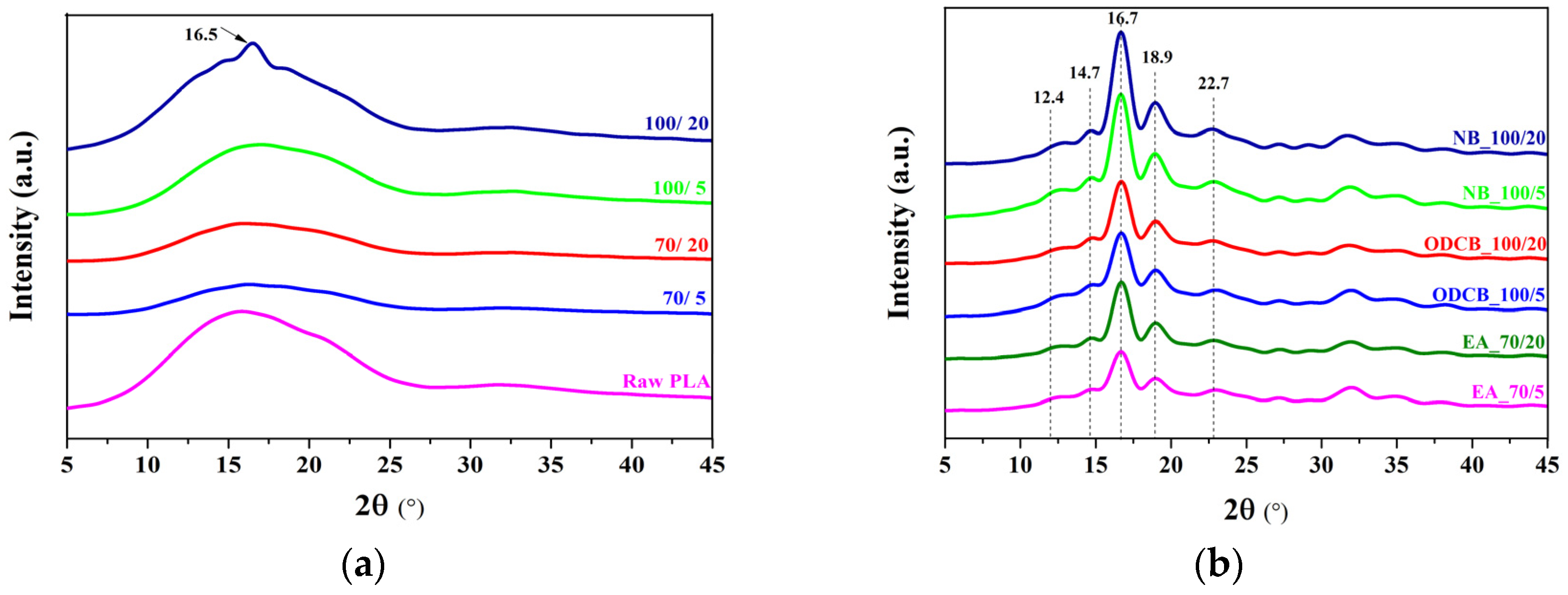
4.2. Crystallization Behaviour of PLLA Induced by Solvents
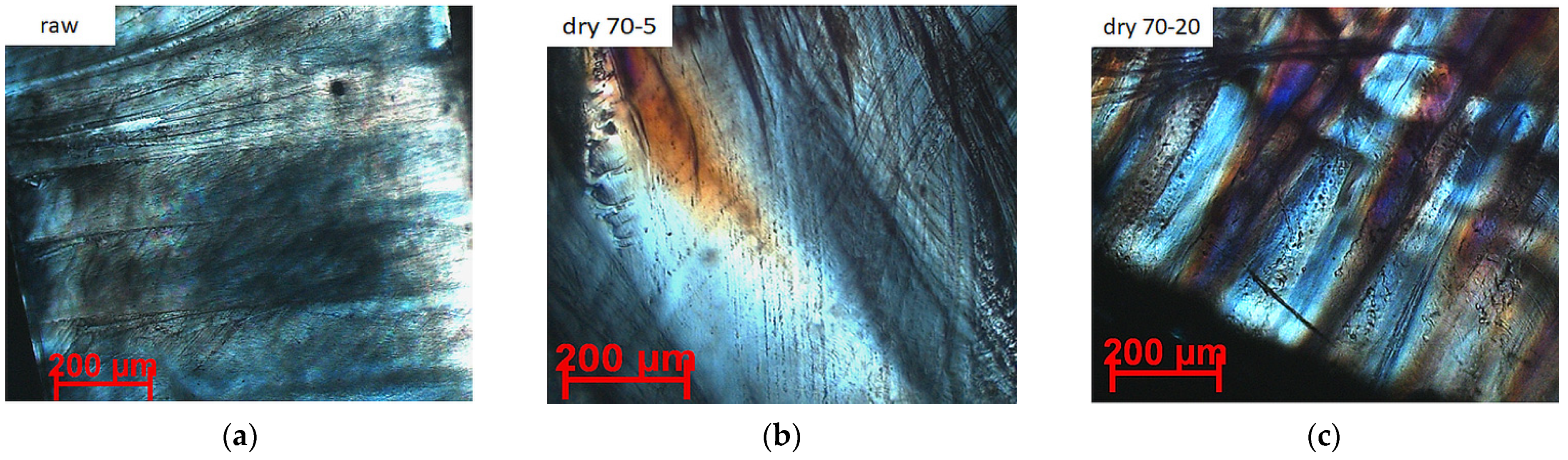
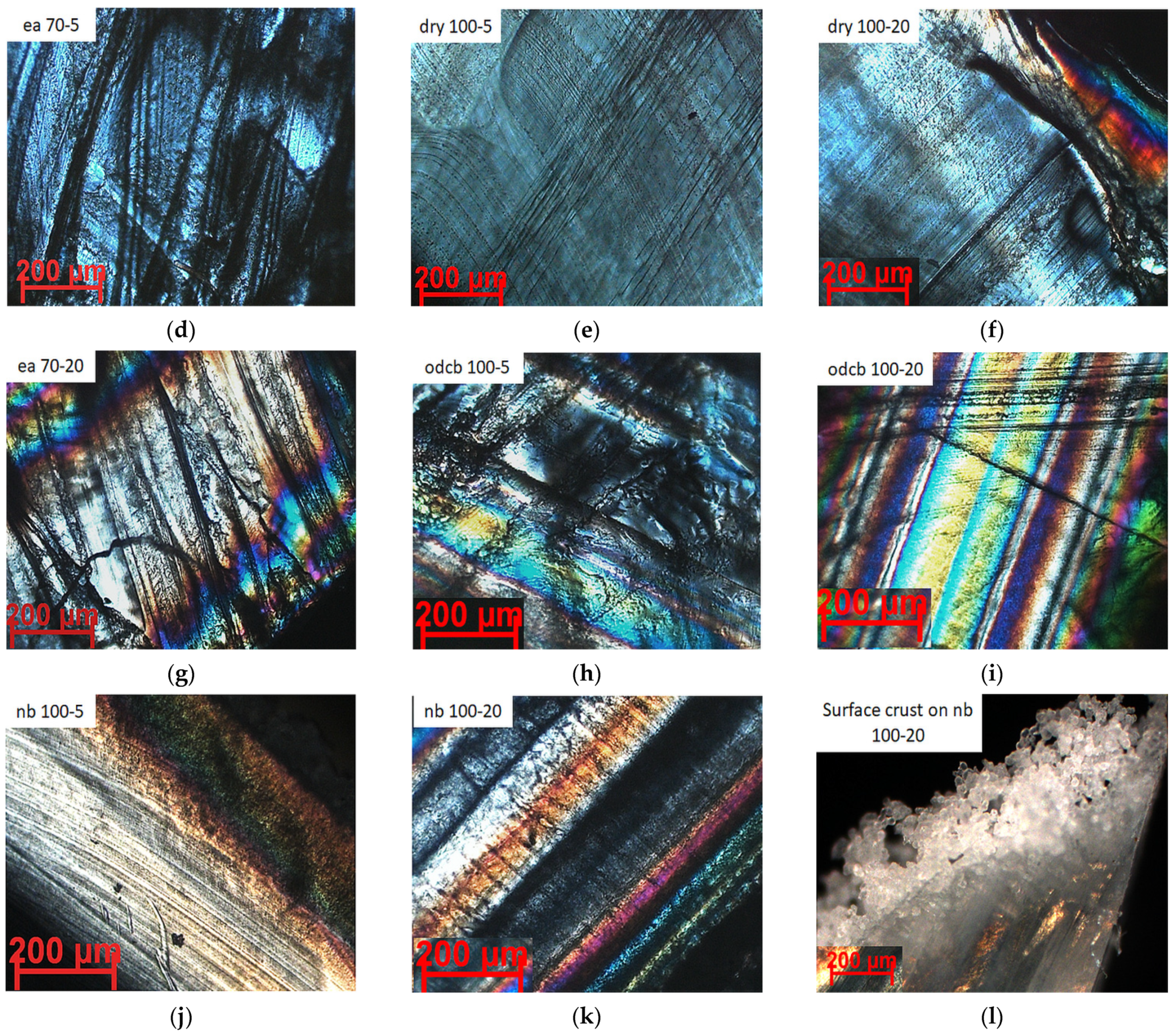
4.3. Crystal Morphological Evaluation of PLLA
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Plastics and the Environment; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003; pp. 1–69. [Google Scholar]
- Lackner, M. Bioplastics. In Kirk-Othmer Encyclopedia of Chemical Technology, 6th ed.; Othmer, K., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 1–41. ISBN 9780471238966. [Google Scholar]
- Clarinval, A.M.; Halleux, J. Classification of biodegradable polymers. In Biodegradable Polymers for Industrial Applications, 1st ed.; Smith, R., Ed.; Woodhead Publishing: Cambridge, UK, 2005; pp. 3–29. ISBN 1855739348. [Google Scholar]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.R.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, J.; Liu, T.-J.; Liu, J.F.; Yan, L.; Chen, X. Poly(lactic acid) Controlled Drug Delivery. In Fortschritte der Hochpolymeren-Forschung; Springer: Cham, Switzerland, 2017; Volume 112, pp. 109–138. [Google Scholar]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Henton, D.; Gruber, P.; Lunt, J.; Randall, J. Polylactic Acid Technology; CRC Press: Boca Raton, FL, USA, 2005; pp. 527–568. [Google Scholar]
- Muller, A.J.; Avila, M.; Saenz, G.; Salazar, J. Crystallization of PLA-based materials. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 66–93. ISBN 978-1-84973-879-8. [Google Scholar]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization behavior of poly(l-lactic acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Register, R.A. Morphological Origin of the Multistep Relaxation Behavior in Semicrystalline Ethylene/Methacrylic Acid Ionomers. Macromolecules 2006, 39, 1079–1086. [Google Scholar] [CrossRef]
- Loo, Y.-L.; Register, R.A.; Ryan, A.; Dee, G.T. Polymer Crystallization Confined in One, Two, or Three Dimensions. Macromolecules 2001, 34, 8968–8977. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Lei, X.-X.; Lu, H.; Lu, L.; Xu, H.-Q.; Zhou, Y.; Zou, J. Improving the Thermal and Mechanical Properties of Poly(l-lactide) by Forming Nanocomposites with an in Situ Ring-Opening Intermediate of Poly(l-lactide) and Polyhedral Oligomeric Silsesquioxane. Nanomaterials 2019, 9, 748. [Google Scholar] [CrossRef]
- Ouyang, H.; Lee, W.-H.; Ouyang, W.; Shiue, S.-T.; Wu, T.-M. Solvent-Induced Crystallization in Poly(ethylene terephthalate) during Mass Transport: Mechanism and Boundary Condition. Macromolecules 2004, 37, 7719–7723. [Google Scholar] [CrossRef]
- Gao, J.; Duan, L.; Yang, G.; Zhang, Q.; Yang, M.; Fu, Q. Manipulating poly(lactic acid) surface morphology by solvent-induced crystallization. Appl. Surf. Sci. 2012, 261, 528–535. [Google Scholar] [CrossRef]
- Iwata, T.; Doi, Y. Morphology and Enzymatic Degradation of Poly(l-lactic acid) Single Crystals. Macromol. 1998, 31, 2461–2467. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. Sorption of ethyl acetate and d-limonene in poly(lactide) polymers. J. Sci. Food Agric. 2006, 86, 648–656. [Google Scholar] [CrossRef]
- Sawada, H.; Takahashi, Y.; Miyata, S.; Kanehashi, S.; Sato, S.; Nagai, K. Gas Transport Properties and Crystalline Structures of Poly(lactic acid) Membranes. Trans. Mater. Res. Soc. Jpn. 2010, 35, 241–246. [Google Scholar] [CrossRef]
- Gondo, D.; Wada, T.; Kanehashi, S.; Sato, S.; Nagai, K. Effects of alcohol solvent-induced crystallization on biodegradable poly(lactic) acid film. J. Packag. Sci. Technol. 2011, 20, 501–511. [Google Scholar]
- Naga, N.; Yoshida, Y.; Inui, M.; Noguchi, K.; Murase, S. Crystallization of amorphous poly(lactic acid) induced by organic solvents. J. Appl. Polym. Sci. 2010, 119, 2058–2064. [Google Scholar] [CrossRef]
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of various liquid organic solvents on solvent-induced crystallization of amorphous poly(lactic acid) film. J. Appl. Polym. Sci. 2012, 129, 1607–1617. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
- Barton, A.F.M. Determination of polymer cohesion parameters. In Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 405–434. ISBN 9780849301766. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters a User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA; Talylor Franics Group: Abingdon, UK, 2007; pp. 1–24. [Google Scholar]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 2007, 3, 71–80. [Google Scholar] [CrossRef]
- Chanda, M. Characteristics of polymers and polymerization processes. In Plastics Technology Handbook, 5th ed.; CRC Press: Boca Raton, FL, USA; Talylor Franics Group: Abingdon, UK, 2017; pp. 1–157. ISBN 9781498786218. [Google Scholar]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Colloid Polym. Sci. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K.; Hanesaka, M.; Ohhara, T.; Kurihara, K.; Kuroki, R.; Tamada, T.; Ozeki, T.; Kanamoto, T. Crystal Structure Analysis of Poly(l-lactic Acid) α Form On the basis of the 2-Dimensional Wide-Angle Synchrotron X-ray and Neutron Diffraction Measurements. Macromolecules 2011, 44, 6441–6452. [Google Scholar] [CrossRef]
- Zander, A.T. Advanced Graphics Software. Anal. Chem. 1990, 62. [Google Scholar] [CrossRef]
- Gee, G. The interaction between rubber and liquids. IX. The elastic behaviour of dry and swollen rubbers. Trans. Faraday Soc. 1946, 42, 585. [Google Scholar] [CrossRef]
- Wu, N.; Lang, S.; Zhang, H.; Ding, M.; Zhang, J. Solvent-induced crystallization behaviours of PLLA ultrathin films investigated by RAIR spectroscopy and AFM measurements. J. Phys. Chem. B 2014, 118, 12652–12659. [Google Scholar] [CrossRef] [PubMed]
- Makrani, N.; Ammari, A.; Benrekaa, N.; Rodrigue, D.; Giroux, Y. Dynamics of the α-relaxation during the crystallization of PLLA and the effect of thermal annealing under humid atmosphere. Polym. Degrad. Stab. 2019, 164, 90–101. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal Modifications and Thermal Behavior of Poly(l-lactic acid) Revealed by Infrared Spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Lorenzo, M.L.D.; Androsch, R. Influence of α’-/α-crystal polymorphism on properties of poly(l-lactic acid). Polym. Int. 2018, 68, 320–334. [Google Scholar] [CrossRef]
- Sun, X.; Yan, S. Surface-induced Polymer Crystallization. In Polymer Morphology; Wiley: Hoboken, NJ, USA, 2016; pp. 204–241. [Google Scholar]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.-R. Infrared Spectrum of Poly(l-lactide): Application to Crystallinity Studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Zhang, J.; Tsuji, H.; Noda, I.; Ozaki, Y. Structural Changes and Crystallization Dynamics of Poly(l-lactide) during the Cold-Crystallization Process Investigated by Infrared and Two-Dimensional Infrared Correlation Spectroscopy. Macromolecules 2004, 37, 6433–6439. [Google Scholar] [CrossRef]
- Shaiju, P.; Murthy, N.S.; Gowd, E.B. Nonsolvent-induced morphological changes and nanoporosity in poly(l-lactide) films. Soft Matter. 2018, 14, 1492–1498. [Google Scholar] [CrossRef] [PubMed]




| Group | Number (n) | Molar Attraction, F (cal cm3)1/2 mol−1 | Fxn (cal cm3)1/2 mol−1 | Molar Mass (g/mol) |
|---|---|---|---|---|
| –CH3 | 1 | 214 | 214 | 15 |
| >CH- | 1 | 28 | 28 | 13 |
| –COO– | 1 | 310 | 310 | 44 |
| Σ | 552 | 72 | ||
| Solvents | Solubility Parameter (MPa1/2) [25,26] | (δp1 − δsolv)2 [32] | Swelling (%) |
|---|---|---|---|
| Toluene | 18.2 | 7.8 | 74.8 |
| Ethyl acetate | 18.2 | 7.8 | 89.0 |
| ODCB | 20.5 | 0.3 | 91.5 |
| nitrobenzene | 22.2 | 1.4 | 91.0 |
| DMF | 24.9 | 15.2 | 82.0 |
| DMSO | 26.7 | 32.5 | 65.4 |
| Water | 48.0 | 729.0 | 1.6 |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | ΔHc (J/g) | ΔHm (J/g) | %Xc |
|---|---|---|---|---|---|---|
| Raw polylactide (PLLA) | 65.3 | 124.1 | 150.0 | 2.6 | 12.1 | 10.2 |
| 50/5 | 59.0 | 118.8 | 148.8 | 7.6 | 14.5 | 7.4 |
| 50/20 | 59.9 | 125.0 | 150.9 | 3.8 | 11.1 | 7.8 |
| 70/5 | 65.0 | 124.8 | 150.6 | 3.1 | 11.4 | 8.9 |
| 70/20 | 65.2 | 123.5 | 150.6 | 5.3 | 13.8 | 9.1 |
| 80/5 | 63.6 | 124.7 | 151.5 | 2.6 | 10.5 | 8.5 |
| 80/20 | 62.4 | 123.3 | 150.4 | 5.2 | 13.9 | 9.3 |
| 100/5 | 62.4 | 124.2 | 150.1 | 2.9 | 16.6 | 14.7 |
| 100/20 | 62.3 | 112.2 | 148.7 | 6.1 | 21.1 | 16.1 |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | ΔHc (J/g) | ΔHm (J/g) | %Xc |
|---|---|---|---|---|---|---|
| EA_50/5 | 52.7 | 114 | 145 | 2.0 | 19.7 | 19.0 |
| EA_50/20 | 46.5 | - | 144.2 | 0 | 29.2 | 31.4 |
| EA_70/5 | 53.2 | - | 147.8 | 0 | 24.2 | 26.0 |
| EA_70/20 | 52.8 | - | 144.4 | 0 | 26.6 | 28.6 |
| ODCB_80/5 | 63.7 | - | 143.6 | 0 | 27.6 | 29.6 |
| ODCB_80/20 | 62.8 | - | 141.1 | 0 | 27.0 | 29.0 |
| ODCB_100/5 | 62.5 | - | 142.3 | 0 | 26.4 | 28.4 |
| ODCB_100/20 | 73.5 | - | 142.2 | 0 | 31.0 | 33.3 |
| NB_80/5 | 47.5 | - | 138.5 | 0 | 34.6 | 37.2 |
| NB_80/20 | 52.0 | - | 137.4 | 0 | 35.8 | 38.5 |
| NB_100/5 | 50.2 | - | 138.3 | 0 | 32.9 | 35.3 |
| NB_100/20 | 47.0 | - | 139.8 | 0 | 35.5 | 38.1 |
| Sample | Crystallinity (%) | Crystallite Size (nm) | Lattice Parameters (Å) | ||||
|---|---|---|---|---|---|---|---|
| DSC 1 | rel.c. 2 | LeBail 3 | a | b | c | ||
| Theoretical [30] | - | - | - | - | 10.683 | 6.170 | 28.860 |
| Raw PLA | 10.2 | 10.4 | 22.7 | 4 ± 1 | 10.125 | 5.927 | 31.345 |
| Annealed_70 ℃/5 min | 8.9 | 9.4 | 12.9 | 4 ± 1 | 9.986 | 5.911 | 32.044 |
| Annealed_70 ℃/20 min | 9.1 | 11.1 | 25.5 | 4 ± 1 | 10.050 | 5.914 | 31.953 |
| Annealed_100 ℃/5 min | 14.7 | 12.5 | 18.8 | 4 ± 1 | 10.124 | 5.922 | 31.353 |
| Annealed_100 ℃/20 min | 16.1 | 14.1 | 15.6 | 11 ± 3 | 10.025 | 5.957 | 31.600 |
| EA_50 °C/20 min | 31.4 | 20.9 | 50.8 | 18 ± 4 | 9.790 | 6.021 | 31.608 |
| EA_70 ℃/5 min | 26.0 | 30.0 | 67.4 | 13 ± 3 | 9.833 | 6.026 | 31.714 |
| EA_70 ℃/20 min | 28.6 | 36.4 | 60.3 | 15 ± 4 | 9.785 | 6.036 | 31.686 |
| ODCB_100 ℃/5 min | 28.4 | 26.0 | 80.0 | 8 ± 2 | 10.653 | 6.096 | 28.845 |
| ODCB_100 ℃/20 min | 33.3 | 30.4 | 81.4 | 10 ± 2 | 10.628 | 6.099 | 28.778 |
| NB_100 ℃/5 min | 35.3 | 33.8 | 77.8 | 11 ± 3 | 10.638 | 6.115 | 28.804 |
| NB_100 ℃/20 min | 38.1 | 34.5 | 84.4 | 12 ± 3 | 10.642 | 6.114 | 28.801 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udayakumar, M.; Kollár, M.; Kristály, F.; Leskó, M.; Szabó, T.; Marossy, K.; Tasnádi, I.; Németh, Z. Temperature and Time Dependence of the Solvent-Induced Crystallization of Poly(l-lactide). Polymers 2020, 12, 1065. https://doi.org/10.3390/polym12051065
Udayakumar M, Kollár M, Kristály F, Leskó M, Szabó T, Marossy K, Tasnádi I, Németh Z. Temperature and Time Dependence of the Solvent-Induced Crystallization of Poly(l-lactide). Polymers. 2020; 12(5):1065. https://doi.org/10.3390/polym12051065
Chicago/Turabian StyleUdayakumar, Mahitha, Mariann Kollár, Ferenc Kristály, Máté Leskó, Tamás Szabó, Kálmán Marossy, Ildikó Tasnádi, and Zoltán Németh. 2020. "Temperature and Time Dependence of the Solvent-Induced Crystallization of Poly(l-lactide)" Polymers 12, no. 5: 1065. https://doi.org/10.3390/polym12051065
APA StyleUdayakumar, M., Kollár, M., Kristály, F., Leskó, M., Szabó, T., Marossy, K., Tasnádi, I., & Németh, Z. (2020). Temperature and Time Dependence of the Solvent-Induced Crystallization of Poly(l-lactide). Polymers, 12(5), 1065. https://doi.org/10.3390/polym12051065